Abstract
Cardiovascular disease (CVD) remains the most prevalent cause of premature death worldwide. It had been suspected for decades that increased activity of the sympathetic nervous system (SNS) might play a pathogenetic role in the development and progression of hypertension, heart failure (HF) and CVD. The use of microneurographic techniques to directly assess the SNS has allowed this field to advance considerably in recent years. We now have compelling evidence for a key role of sympathetic overactivity in the pathogenesis and progression of hypertension and associated hypertension-mediated organ damage (such as endothelial dysfunction, arterial stiffness and left ventricular hypertrophy), HF (with or without reduced left ventricular ejection fraction). Sympathetic overactivity also drives increased cardiovascular risk in the settings of obesity, metabolic syndrome, chronic kidney disease and obstructive sleep apnoea, among other conditions. Thus, sympathetic overactivity is an important factor that drives patients through the CVD continuum, from the early appearance of cardiovascular risk factors, to impairments of the structure and function of components of the heart and arteries, to established CVD, and ultimately to a life-threatening cardiovascular event. A deeper understanding of the role of sympathetic overactivity in the pathogenesis of CVD and HF will support the optimization of therapeutic interventions for these conditions.
Introduction
We have been aware of a role for the sympathetic nervous system (SNS) in increasing blood pressure (BP) since the nineteenth century. Our understanding of the nuances of this relationship has evolved in line with the precision of the techniques available to investigate itCitation1. The demonstration of increased levels of noradrenaline and its metabolites in the urine of people with hypertension by von Euler and colleagues in 1954 was perhaps the first clue that SNS activity was increased in this conditionCitation2. The development of techniques to measure noradrenaline overflow from the central nervous system (CNS) in plasma in the following decade added to the study of the relationships between the activity of the SNS and various aspects of cardiovascular status, although both measurements essentially provided an average of SNS activity across the whole bodyCitation3,Citation4. Moreover, measures of plasma noradrenaline are also confounded by variations in the rate of its clearance.
At about the same time, Hagbarth and Vallbo published on microneurography, a new method for making direct measurements of the level of activity in sympathetic nerves in real time in awake humansCitation5. This new technique revolutionized research in this area and has provided greater precision in defining the relationships between SNS activity, hypertension and hypertension-mediated organ damage, compared with measurements of plasma noradrenaline aloneCitation6. In the 1990s the rate of spillover of radiolabelled noradrenaline into the plasma from specific vascular beds, and the measurement of muscle sympathetic nerve traffic (MSNA) by microneurography brought together the advantages of these techniquesCitation4. Early studies using microneurography measured MSNA that was effectively averaged across multiple nerves; subsequent measurement of sympathetic nerve activity from single functional firing units within sympathetic nerves, with defined physiologic effects on BP when stimulated via the electrodeCitation7,Citation8. Microneurography has allowed more precise associations to be made between the level of SNS activity and hypertensive states than was possible using catecholamine overflow aloneCitation9.
Much of the research cited below in this review has been gained using this invaluable method. These studies have identified overactivity of the SNS as an important mediator of hypertension and related damage to the cardiovascular system that subsequently leads to hypertension-mediated organ damage, major adverse cardiovascular events, and premature death (). This review focuses on the potential pathogenetic role of sympathetic overactivity at different stages of the cardiovascular disease (CVD) continuum.
Figure 1. Overview of the cardiorenal pathophysiology associated with overactivity of the sympathetic nervous system.
aInitially, may be followed by myocardial pump failure as adverse cardiac remodelling progresses. Abbreviations: LVH, left ventricular hypertrophy; MACE, major adverse cardiovascular events; OSA, obstructive sleep apnoea; RAAS, renin-angiotensin-aldosterone system; SNS, sympathetic nervous system. See text for references.
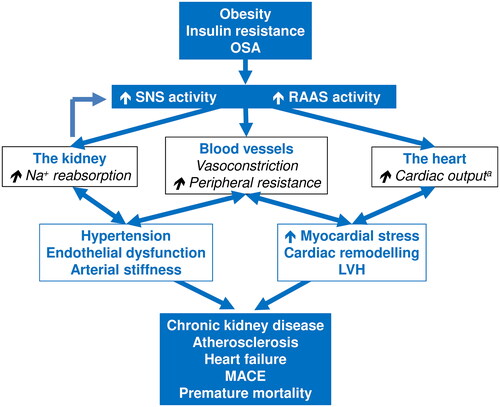
Sympathetic overactivity at different stages of the CVD continuum
Hypertension
Sympathetic overactivity and hypertension
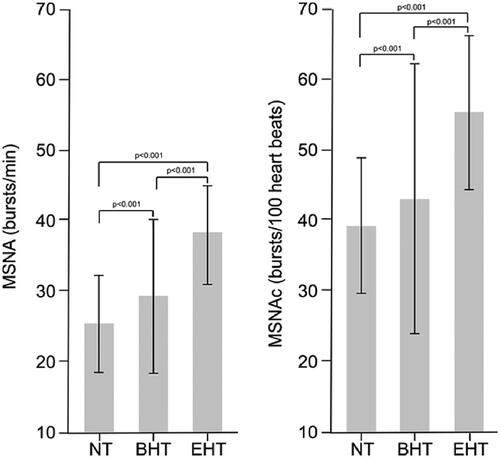
Research during the 1990s, enabled by the development of sensitive and specific techniques for measuring sympathetic nerve activity, first established a clear pathogenetic link between sympathetic overactivity and hypertension. Importantly, these early studies showed that this phenomenon was associated strongly with hypertension, and that the degree of sympathetic overactivity paralleled the severity of hypertensionCitation10. For example, a study that used single-unit microneurography to measure single-unit MSNA was able to distinguish between subjects with high-normal BP and subjects with normal BP, in addition to groups with established hypertensionCitation9. Many studies in this area have been conducted since then and space here does not allow individual review of them. However, a large and recent meta-analysis (2018, 63 studies) has confirmed the significant associations between increased MSNA (sympathetic overactivity) and hypertension of any severity (borderline or established essential hypertension []) and phenotype (masked, “white coat”, non-dipping, dipping, etc.) and irrespective of whether the hypertension was treated, or whether clinic or ambulatory blood pressure measurement was usedCitation6. Increased SNS activity has been identified as a reason for failure of antihypertensive therapy to control BP in patients with resistant hypertensionCitation11. Sympathetic overactivity may also contribute to BP variability in people with hypertension, another parameter associated with an adverse long-term outcomeCitation12.
Figure 2. Demonstration of the presence of sympathetic overactivity, as shown by increased muscle sympathetic nerve activity (MSNA) in patients with borderline or established essential hypertension from a meta-analysis of 63 studies.
Columns are means, bars are SD. MSNAc: MSNA corrected for heart rate. Abbreviations: NT, normotensive; BHT, borderline hypertension; EHT, essential hypertension. Reproduced with permission from reference 6.
Sympathetic overactivity and hypertension-mediated organ damage
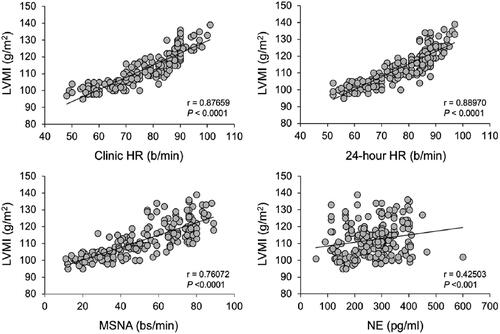
Uncontrolled hypertension leads to the development of adverse structural changes to the heart and vasculature, such as increased a loss of endothelium-dependent vasodilatation, increased arterial stiffness (which promotes the isolated systolic hypertension often seen in the elderly) and left ventricular hypertrophy (LVH)Citation13. summarizes some of the key neurohumoral and structural cardiac pathology associated with the progression through sympathetic overactivity, to LVH or other hypertension-mediated organ damage, to the failing heartCitation14. Activation of the renin-angiotensin-aldosterone system (RAAS) and SNS in the early stages of heart failure (HF) activates baroreceptors, increases MSNA and enhances cardiac preload and afterload. Initially, myocardial contractility may be increased, as β1-adrenoceptor stimulation mobilizes intracellular Ca2+ in cardiomyocytes. However, this also increases myocardial O2 consumption; this, together with the onset of LVH and a range of adverse structural changes in the left ventricular wall, tends to exacerbate further deterioration of myocardial performance in a vicious circle. The close correlations between increasing left ventricular mass index and increasing MSNA, plasma noradrenaline, and heart rate illustrate the central role of sympathetic overactivity in these pathological processes ()Citation15.
Figure 3. Overview of key steps in the pathology of the failing heart.
Drawn from information presented in reference 14.
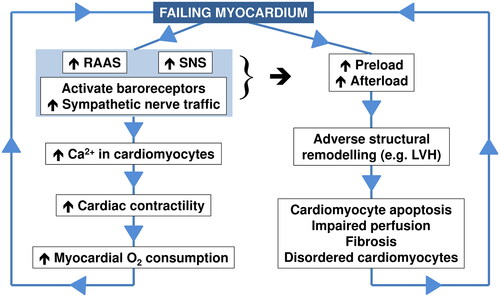
Figure 4. Association of left ventricular mass index (LVMI) with indices of sympathetic overactivity in patients with hypertension.
Reproduced with permission from reference 15. Abbreviation: NE, norepinephrine (noradrenaline).
These manifestations of hypertension-mediated organ damage are key steps in the CVD continuum between hypertension and other risk factors and major adverse cardiovascular events (). Sympathetic stimulation or overactivity reduces endothelial function in several clinical settings associated with increased cardiovascular riskCitation7,Citation16, Measures of arterial stiffness (e.g. augmentation index, arterial velocity pulse index or pulse wave velocity) increase with increasing MSNA in healthy subjects and in patients with hypertension or existing CVDCitation17–23, independently of BPCitation23, with a greater effect in older individualsCitation18–21. Conversely, pulse wave velocity has been described as a method for estimating sympathetic nervous system activityCitation24. Sympathetic overactivity has also been implicated in the development of LVHCitation25. Finally, inhibiting SNS activity by nephrectomy during renal transplantation or by renal denervation reversed various aspects of hypertension-mediated organ damage in patients with hypertensionCitation26.
Sympathetic overactivity associated with dysfunction of the vasculature and myocardium has also been observed in survivors of severe Covid-19 infectionCitation27. Left ventricular diastolic dysfunction is another potential consequence of hypertension, and the relationships between this outcome and sympathetic overactivity are discussed below in the section on HF. Experimental data from animal models suggested that sympathetic overactivity may overcome the ability of endothelium-derived hyperpolarising factor to oppose vasoconstriction in the setting of chronic kidney disease (CKD)Citation28 or metabolic syndromeCitation29 (see below for separate discussion of these common comorbidities of hypertension). Recent data suggest that small, non-coding microRNAs mediate relationships between the SNS, renin-angiotensin-aldosterone system and LVH in the setting of hypertension, although the precise mechanisms of these relationships have yet to be elucidatedCitation30.
Heart rate as an indicator of sympathetic overactivity
The current European guidelines for the management of hypertension recommend the use of a cut-off value for heart rate of >80 beats per minute within its algorithm for identifying patients at elevated risk of CVDCitation31. Observational data from 193 subjects with untreated essential hypertension of moderate severity supports this recommendationCitation15. Stratifying the study population for those with heart rate above or below 80 beats per minute revealed that the higher heart rate group had higher MSNA and venous plasma noradrenaline (indicative related to sympathetic overactivity) and greater left ventricular mass index (a manifestation of hypertension-mediated organ damage that predicts increased risk of adverse cardiovascular outcomesCitation31). Mean BP was similar in magnitude between the two groups, suggesting that sympathetic overactivity may contribute to hypertension-mediated organ damage in hypertension over and above that seen with elevated BP per se.
Heart failure
HF was identified as a hyperadrenergic state more than a decade agoCitation32 and continued clinical research has strengthened this association ( and )Citation33,Citation34. A meta-analysis (2016), which pooled 106 studies using MSNA, demonstrated a 1.9-fold increase in MSNA among people with HF, compared with healthy controlsCitation35. The level of MSNA was correlated significantly with heart rate and venous plasma noradrenaline, and also with cardiac function.
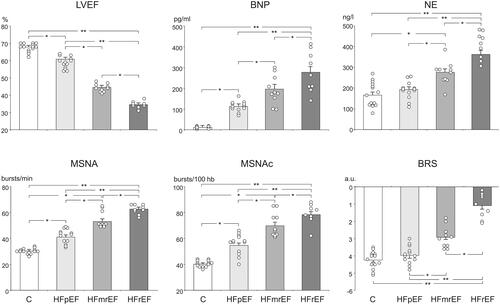
Current European guidelines for the management of HF include a classification according to LVEF, where LVEF ≤40% represents HF with reduced LVEF (HFrEF), LVEF 40–49% represents heart failure with mildly reduced LVEF (HFmrEF) and higher LVEF represents HF with preserved LVEF (HFpEF)Citation36. Data from a recent (2019) study in 32 patients with HF of mixed aetiology showed that MSNA was increased significantly in patients who fell within either category of LVEF, compared with control subjects ()Citation37. The increase in MSNA was also significant in patients with HFpEF, broadly in parallel with baroreflex sensitivity and markers of the severity/aetiology of HF, i.e. LVEF and circulating B-type natriuretic peptide (BNP)Citation37. It is notable in this study that circulating levels of BNP were lower for people with HFpEF vs. HFmrEF or HFrEF. HF was diagnosed according to European guidelines current at the timeCitation38 and all patients in this study had elevated BNP. A number of additional factors can affect the actual prevailing level of natriuretic peptides in HFpEFCitation39,Citation40. Another recent (2021) observational study in 23 patients demonstrated increased MSNA in subjects with LVEF consistent with the new guideline classifications, irrespective of whether the underlying aetiology was ischaemic or non-ischaemicCitation41. Sympathetic overactivity has also been shown to promote left ventricular diastolic dysfunction in people with hypertension, independently of the level of BP elevationCitation42. A study from 2014 demonstrated for the first time that patients with Takotsubo cardiomyopathy demonstrated increased sympathetic nerve activity, measured in the peroneal nerveCitation43. This was also associated with reduced baroreflex control of the SNS. Sympathetic overactivity is now recognized as an important risk factor for this reversible presentation of cardiomyopathyCitation44.
Figure 5. Sympathetic overactivity in heart failure: data from a study in 32 patients with treated heart failure and 14 age-matched healthy control subjects.
Parameters measured: LVEF: left ventricular ejection fraction; BNP: B-type natriuretic peptide; NE: plasma norepinephrine (noradrenaline); MSNA: muscle sympathetic nerve activity; MSNAc: MSNA corrected for heart rate; BRS: spontaneous baroreflex sensitivity. Reproduced with permission from reference 37.
Other conditions relevant to the CVD continuum
Metabolic syndrome
The presence of the metabolic syndrome, a constellation of cardiovascular risk factors associated with insulin resistance, predicts an increased long-term risk of both CVD and type 2 diabetesCitation45. A study from 2017 in 65 subjects with metabolic syndrome confirmed the association of increased heart rate with sympathetic overactivity (increased MSNA) in this population, although MSNA appeared to be a more precise indicator than heart rate for the presence of sympathetic over activityCitation46. A more recent (2022) cross-sectional study of 70 individuals with metabolic syndrome found that a resting heart rate of at least 80 beats per minute, compared with subjects with metabolic syndrome and heart rates of 70–79 beats per minute or <70 beats per minute, was predictive of sympathetic overactivity, as measured using MSNA or plasma noradrenalineCitation47. The diagnostic cut-off of 80 beats per minute for heart rate in people with the metabolic syndrome was therefore consistent with that used to identify sympathetic overactivity in people with hypertension, as described above. A meta-analysis (2020, 16 studies) estimated that MSNA was increased by about 30% in populations with vs. without the metabolic syndromeCitation48.
A single-nucleotide polymorphism of the ADRA1A gene (rs17055869) may predict both increased risk of metabolic syndrome and increased sympathetic nervous activity in those who have itCitation49.
Obstructive sleep apnoea
Obstructive sleep apnoea (OSA) is characterized by repeated obstruction of the upper airways during sleepCitation50. Typically, people with OSA endure a vicious cycle where the airway obstruction causes them to partially wake multiple times each night, at which point the obstruction partially reverses, and a resumption of deeper sleep leads to a repeat of the obstruction. Risk factors for OSA and CVD overlap (e.g. higher age, obesity, male gender) and people with this condition are at markedly increased risk of all-cause and CVD mortality (). Patients may report daytime sleepiness due to their inability to achieve a sustained period of restorative sleep and as a result are also at increased risk of other adverse outcomes, such as road traffic accidents.
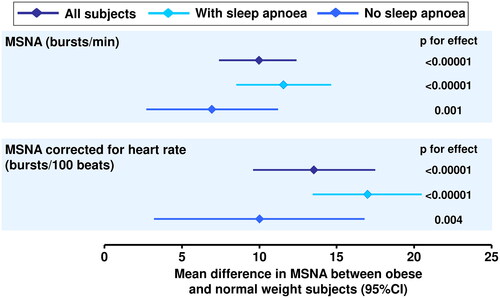
A recent (2022) meta-analysis of 14 studies that used microneurography to measure MSNA in patients without comorbid CVD found that this parameter increased in parallel with the severity of OSACitation51. The associations found across the individual studies were highly consistent, adding strength to the likely validity of the findings. Interestingly, MSNA was correlated significantly with the apnoea-hypopnea index, a key marker of OSA severity, but not with body mass index. Another meta-analysis found that the presence of OSA exacerbated the increase in sympathetic overactivity associated with obesity ()Citation52. Accordingly, these data suggested that the association between OSA and sympathetic overactivity was independent of the presence or absence of obesity. A further meta-analysis (2021, 26 studies) reported similar findings and suggested the use of elevated heart rate (also significantly associated with sleep apnoea-related sympathetic overactivity) as a surrogate marker for diagnosing this conditionCitation53.
Figure 6. Associations of sympathetic overactivity, measured as increased muscle sympathetic nerve activity (MSNA), with overweight or obesity with or without comorbid obstructive sleep apnoea.
A positive mean difference means higher MSNA in obese vs. normal weight subjects. Mean (95%CI) differences in MSNA in overweight vs. normal weight subjects (not shown on the figure) were 3.3 (0.3 to 6.3) bursts/min and 7.9 (0.9 to 15.0) bursts/100 beats. The presence or absence of sleep apnoea was self-declared. Drawn from data presented in reference 52.
The presence of OSA exacerbates sympathetic overactivity in people with metabolic syndrome (see above), apparently due a reflex activation of the sympathetic nervous system caused by hypoxia during airway obstructionCitation54.
Obesity
Increased MSNA was associated significantly with overweight and (to a greater extent) obesity in a 2019 meta-analysis of 45 studies that employed MSNA ()Citation52. Here, the presence of sympathetic overdrive predicted the presence of increased overall or central adiposity, higher BP and an adverse lipid profile, although there was no correlation with levels of plasma glucose or insulin, insulin resistance, heart rate or venous plasma noradrenaline. Sympathetic nervous activity is increased in muscle but not skin in people with overweight or obesity, indicating that sympathetic overactivity is directed to particular organ systems rather than generally throughout the bodyCitation55. The development of overweight or obesity is a strong predictor of future hypertension, and sympathetic overactivity (mainly directed towards muscle and the kidney) and obesity combine to increase the risk of adverse cardiovascular outcomes as overweight/obese individuals grow older ()Citation56.
Chronic kidney disease
A diagnosis of CKD confers increased risk of adverse cardiovascular outcomes and progression of renal dysfunction, especially in patients with comorbid conditions such as type 2 diabetesCitation57. A recent (2021) study of 82 individuals with CKD found that heart rate, MSNA and plasma noradrenaline were elevated, compared with 24 age-matched healthy control subjectsCitation58. MSNA and plasma noradrenaline were significantly and inversely related to estimated glomerular filtration rate (eGFR); however, the correlation between heart rate and eGFR did not achieve statistical significance, suggesting limited utility if this parameter as a surrogate marker of functionally significant sympathetic overactivity in the population with CKD. These findings support earlier studies that identified sympathetic overactivity as an important contributor to the pathophysiology of CKD (reviewed elsewhereCitation59,Citation60). Finally, sympathetic overactivity and increased asymmetric dimethylarginine (ADMA, an endogenous inhibitor of nitric oxide synthase and a known risk factor for CKD progression) may form part of a common pathogenetic mechanism in the progression of renal dysfunction and adverse cardiac remodelling in the setting of CKDCitation61.
Relevance of sympathetic overactivity to the management of patients at elevated cardiovascular risk
Observations of the prognostic importance of sympathetic overactivity in people with hypertension may help guide treatment for this condition, either by consideration of pharmacologic approaches that may reduce hypertension-mediated damage to the vasculature and other organs (e.g. β1-adrenoceptor blockade in patients with HFCitation36, or in selected hypertensive patientsCitation31) or by non-pharmacologic approaches (e.g. renal denervation or carotid baroreceptor stimulation)Citation62–65. Inhibition of sympathetic nervous system activity has also been proposed as a potential mechanism for the cardiorenal protection observed following treatment of high-risk patients with sodium-glucose cotransporter-2 (SGLT2) inhibitorsCitation66,Citation67. Other articles in this collection address the role of pharmacotherapy (and β-blockade in particular) in addressing the adverse effects of sympathetic overactivity on the cardiovascular systemCitation68–72.
Conclusions
Activation of the SNS has been described as a “natural physiological consequence to life stressors but also to any condition that may harm our body”Citation33. The evidence summarized above demonstrates conclusively that an over application of this intended protective mechanism does more harm than good in the setting of hypertension, HF and other conditions associated with increased cardiovascular risk, including obesity, CKD and obstructive sleep apnoea. Indeed, sympathetic overactivity promotes and exacerbates hypertension, and drives the patient along the CVD continuum towards hypertension-mediated organ damage and an eventual catastrophic cardiovascular event (). This phenomenon has been observed consistently across many individual studies, and in meta-analyses. Consideration of the possibility of sympathetic overactivity should play a part in the clinical evaluation of patients presenting with increased cardiovascular risk or established CVD.
Transparency
Declaration of funding
Merck Healthcare KGaA, Darmstadt, Germany, funded open access publication of this collection of articles and editorial assistance. No other funding applied.
Declaration of financial/other relationships
GG has received honoraria for lectures from Servier and Menarini unrelated to this article. LFD received honoraria for lectures from Biolab, Daiichi Sankyo and Merck. No conflict of interest is declared for writing this article.
Author contributions
Both authors contributed equally to the development of this article.
Supplement statement
This article is part of a supplement sponsored by Merck Healthcare KGaA, Darmstadt, Germany. All articles within this supplement have been rigorously peer reviewed by at least two experts in the field, as per CMRO’s peer review policy. Any conflicts of interest are stated in the “Declaration of financial/other relationships” section.
Artificial intelligence (AI)
No AI-related technology was used in the preparation of this article.
Acknowledgements
Dr Mike Gwilt (GT Communications) provided editorial assistance.
References
- Esler M. The sympathetic nervous system through the ages: from Thomas Willis to resistant hypertension. Exp Physiol. 2011;96(7):611–622. doi:10.1113/expphysiol.2010.052332.
- Von Euler US, Hellner S, Purkhold A. Excretion of noradrenaline in urine in hypertension. Scand J Clin Lab Invest. 1954;6(1):54–59. doi:10.3109/00365515409134833.
- Engelman K, Portnoy B, Lovenberg W. A sensitive and specific double-isotope derivative method for the determination of catecholamines in biological specimens. Am J Med Sci. 1968;255(4):259–268. doi:10.1097/00000441-196804000-00007.
- Grassi G, Esler M. How to assess sympathetic activity in humans. J Hypertens. 1999;17(6):719–734. doi:10.1097/00004872-199917060-00001.
- Hagbarth KE, Vallbo AB. Pulse and respiratory grouping of sympathetic impulses in human muscle-nerves. Acta Physiol Scand. 1968;74(1–2):96–108. doi:10.1111/j.1365-201X.1968.tb10904.x.
- Grassi G, Pisano A, Bolignano D, et al. Sympathetic nerve traffic activation in essential hypertension and its correlates: systematic reviews and meta-analyses. Hypertension. 2018;72(2):483–491. doi:10.1161/HYPERTENSIONAHA.118.11038.
- Macefield VG. Recording and quantifying sympathetic outflow to muscle and skin in humans: methods, caveats and challenges. Clin Auton Res. 2021;31(1):59–75. doi:10.1007/s10286-020-00700-6.
- Shoemaker JK, Klassen SA, Badrov MB, et al. Fifty years of microneurography: learning the language of the peripheral sympathetic nervous system in humans. J Neurophysiol. 2018;119(5):1731–1744. doi:10.1152/jn.00841.2017.
- Greenwood JP, Stoker JB, Mary DA. Single-unit sympathetic discharge: quantitative assessment in human hypertensive disease. Circulation. 1999;100(12):1305–1310. doi:10.1161/01.cir.100.12.1305.
- Grassi G. Role of the sympathetic nervous system in human hypertension. J Hypertens. 1998;16(12 Pt 2):1979–1987. doi:10.1097/00004872-199816121-00019.
- Dudenbostel T, Acelajado MC, Pisoni R, et al. Refractory hypertension: evidence of heightened sympathetic activity as a cause of antihypertensive treatment failure. Hypertension. 2015;66(1):126–133. doi:10.1161/HYPERTENSIONAHA.115.05449.
- Grassi G, Bombelli M, Brambilla G, et al. Total cardiovascular risk, blood pressure variability and adrenergic overdrive in hypertension: evidence, mechanisms and clinical implications. Curr Hypertens Rep. 2012;14(4):333–338. doi:10.1007/s11906-012-0273-8.
- Quarti-Trevano F, Dell’Oro R, Cuspidi C, et al. Endothelial, vascular and sympathetic alterations as therapeutic targets in chronic heart failure. Biomedicines. 2023;11(3):803. doi:10.3390/biomedicines11030803.
- Schwinger RHG. Pathophysiology of heart failure. Cardiovasc Diagn Ther. 2021;11(1):263–276. doi:10.21037/cdt-20-302.
- Grassi G, Quarti-Trevano F, Seravalle G, et al. Association between the European Society of cardiology/European society of hypertension heart rate thresholds for cardiovascular risk and neuroadrenergic markers. Hypertension. 2020;76(2):577–582. doi:10.1161/HYPERTENSIONAHA.120.14804.
- Lee MGY, Hemmes RA, Mynard J, et al. Elevated sympathetic activity, endothelial dysfunction, and late hypertension after repair of coarctation of the aorta. Int J Cardiol. 2017;243:185–190. doi:10.1016/j.ijcard.2017.05.075.
- Egan B, Grassi G. Sympathetic activation and endothelial dysfunction as therapeutic targets in obesity-related hypertension. J Hypertens. 2013;31(2):259–260. doi:10.1097/HJH.0b013e32835d0dcf.
- Holwerda SW, Luehrs RE, DuBose LE, et al. Sex and age differences in the association between sympathetic outflow and Central elastic artery wall thickness in humans. Am J Physiol Heart Circ Physiol. 2019;317(3):H552–H560. doi:10.1152/ajpheart.00275.2019.
- Harvey RE, Barnes JN, Hart EC, et al. Influence of sympathetic nerve activity on aortic hemodynamics and pulse wave velocity in women. Am J Physiol Heart Circ Physiol. 2017;312(2):H340–H346. doi:10.1152/ajpheart.00447.2016.
- Okada Y, Galbreath MM, Shibata S, et al. Relationship between sympathetic baroreflex sensitivity and arterial stiffness in elderly men and women. Hypertension. 2012;59(1):98–104. doi:10.1161/HYPERTENSIONAHA.111.176560.
- Casey DP, Curry TB, Joyner MJ, et al. Relationship between muscle sympathetic nerve activity and aortic wave reflection characteristics in young men and women. Hypertension. 2011;57(3):421–427. doi:10.1161/HYPERTENSIONAHA.110.164517.
- Bruno RM, Ghiadoni L, Seravalle G, et al. Sympathetic regulation of vascular function in health and disease. Front Physiol. 2012;3:284. doi:10.3389/fphys.2012.00284.
- Nardone M, Incognito AV, Millar PJ. Evidence for pressure-independent sympathetic modulation of Central pulse wave velocity. J Am Heart Assoc. 2018;7(3):e007971. doi:10.1161/JAHA.117.007971.
- Xu Z, Sakagawa T, Furui A, et al. Beat-to-beat estimation of peripheral arterial stiffness from local PWV for quantitative evaluation of sympathetic nervous system activity. IEEE Trans Biomed Eng. 2022;69(9):2806–2816. doi:10.1109/TBME.2022.3154398.
- Grassi G. Sympathetic overdrive as an independent predictor of left ventricular hypertrophy: prospective evidence. J Hypertens. 2006;24(5):815–817. doi:10.1097/01.hjh.0000222748.37078.2d.
- Bruno RM, Taddei S, Borghi C, et al. Italian Society of Arterial Hypertension (SIIA) position paper on the role of renal denervation in the management of the difficult-to-treat hypertensive patient. High Blood Press Cardiovasc Prev. 2020;27(2):109–117. doi:10.1007/s40292-020-00367-0.
- Faria D, Moll-Bernardes RJ, Testa L, et al. Sympathetic neural overdrive, aortic stiffening, endothelial dysfunction, and impaired exercise capacity in severe COVID-19 survivors: a mid-term study of cardiovascular sequelae. Hypertension. 2023;80(2):470–481. doi:10.1161/HYPERTENSIONAHA.122.19958.
- Cao W, Wu L, Zhang X, et al. Sympathetic overactivity in CKD disrupts buffering of neurotransmission by endothelium-derived hyperpolarizing factor and enhances vasoconstriction. J Am Soc Nephrol. 2020;31(10):2312–2325. doi:10.1681/ASN.2020030234.
- Battault S, Meziat C, Nascimento A, et al. Vascular endothelial function masks increased sympathetic vasopressor activity in rats with metabolic syndrome. Am J Physiol Heart Circ Physiol. 2018;314(3):H497–H507. doi:10.1152/ajpheart.00217.2017.
- Improta-Caria AC, Aras MG, Nascimento L, et al. MicroRNAs regulating renin-angiotensin-aldosterone system, sympathetic nervous system and left ventricular hypertrophy in systemic arterial hypertension. Biomolecules. 2021;11(12):1771. doi:10.3390/biom11121771.
- Mancia G, Kreutz R, Brunström M, et al. ESH guidelines for the management of arterial hypertension. The task force for the management of arterial hypertension of the European society of hypertension endorsed by the European renal association (ERA) and the international society of hypertension (ISH). J Hypertens. 2023;41(12):1874–2071. doi:10.1097/HJH.0000000000003480.
- Grassi G, Seravalle G, Quarti-Trevano F, et al. Sympathetic activation in congestive heart failure: evidence, consequences and therapeutic implications. Curr Vasc Pharmacol. 2009;7(2):137–145. doi:10.2174/157016109787455699.
- Grassi G, Mancia G, Esler M. Central and peripheral sympathetic activation in heart failure. Cardiovasc Res. 2022;118(8):1857–1871. doi:10.1093/cvr/cvab222.
- Grassi G, Quarti-Trevano F, Esler MD. Sympathetic activation in congestive heart failure: an updated overview. Heart Fail Rev. 2021;26(1):173–182. doi:10.1007/s10741-019-09901-2.
- Grassi G, D’Arrigo G, Pisano A, et al. Sympathetic neural overdrive in congestive heart failure and its correlates: systematic reviews and meta-analysis. J Hypertens. 2019;37(9):1746–1756. doi:10.1097/HJH.0000000000002093.
- McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726. doi:10.1093/eurheartj/ehab368.
- Seravalle G, Quarti-Trevano F, Dell’Oro R, et al. Sympathetic and baroreflex alterations in congestive heart failure with preserved, midrange and reduced ejection fraction. J Hypertens. 2019;37(2):443–448. doi:10.1097/HJH.0000000000001856.
- Ponikowski P, Voors AA, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC). Eur Heart J. 2016;37(27):2129–2200.)doi:10.1093/eurheartj/ehw128.
- Januzzi JL, Jr, Myhre PL. The challenges of NT-proBNP testing in HFpEF: shooting arrows in the wind. JACC Heart Fail. 2020;8(5):382–385. doi:10.1016/j.jchf.2020.03.003.
- Shah SJ. BNP: biomarker not perfect in heart failure with preserved ejection fraction. Eur Heart J. 2022;43(20):1952–1954. doi:10.1093/eurheartj/ehac121.
- Urbancsek R, Csanádi Z, Forgács IN, et al. Sympathetic activation in heart failure with reduced and mildly reduced ejection fraction: the role of aetiology. ESC Heart Fail. 2021;8(6):5112–5120. doi:10.1002/ehf2.13580.
- Grassi G, Seravalle G, Quarti-Trevano F, et al. Sympathetic and baroreflex cardiovascular control in hypertension-related left ventricular dysfunction. Hypertension. 2009;53(2):205–209. doi:10.1161/HYPERTENSIONAHA.108.121467.
- Vaccaro A, Despas F, Delmas C, et al. Direct evidences for sympathetic hyperactivity and baroreflex impairment in Tako Tsubo cardiopathy. PLoS One. 2014;9(3):e93278. doi:10.1371/journal.pone.0093278.
- Naegele M, Flammer AJ, Enseleit F, et al. Endothelial function and sympathetic nervous system activity in patients with Takotsubo syndrome. Int J Cardiol. 2016;224:226–230. doi:10.1016/j.ijcard.2016.09.008.
- Guembe MJ, Fernandez-Lazaro CI, Sayon-Orea C, et al. Risk for cardiovascular disease associated with metabolic syndrome and its components: a 13-year prospective study in the RIVANA cohort. Cardiovasc Diabetol. 2020;19(1):195. doi:10.1186/s12933-020-01166-6.
- Quarti Trevano F, Seravalle G, Macchiarulo M, et al. Reliability of heart rate as neuroadrenergic marker in the metabolic syndrome. J Hypertens. 2017;35(8):1685–1690. doi:10.1097/HJH.0000000000001370.
- Seravalle G, Vanoli J, Molisano C, et al. Heart rate thresholds for cardiovascular risk and sympathetic activation in the metabolic syndrome. Acta Diabetol. 2022;59(11):1429–1435. doi:10.1007/s00592-022-01945-5.
- Quarti Trevano F, Dell’Oro R, Biffi A, et al. Sympathetic overdrive in the metabolic syndrome: meta-analysis of published studies. J Hypertens. 2020;38(4):565–572. doi:10.1097/HJH.0000000000002288.
- Grassi G, Padmanabhan S, Menni C, et al. Association between ADRA1A gene and the metabolic syndrome: candidate genes and functional counterpart in the PAMELA population. J Hypertens. 2011;29(6):1121–1127. doi:10.1097/HJH.0b013e328346d72c.
- Abbasi A, Gupta SS, Sabharwal N, et al. A comprehensive review of obstructive sleep apnea. Sleep Sci. 2021;14:142–154.
- Biffi A, Quarti-Trevano F, Bonzani M, et al. Neuroadrenergic activation in obstructive sleep apnoea syndrome: a new selected meta-analysis – revisited. J Hypertens. 2022;40(1):15–23. doi:10.1097/HJH.0000000000003045.
- Grassi G, Biffi A, Seravalle G, et al. Sympathetic neural overdrive in the obese and overweight state. Meta-analysis of published studies. Hypertension. 2019;74(2):349–358. doi:10.1161/HYPERTENSIONAHA.119.12885.
- Quarti-Trevano F, Biffi A, Bonzani M, et al. Neuroadrenergic activation in obstructive sleep apnea syndrome: a systematic review and meta-analysis. J Hypertens. 2021;39(11):2281–2289. doi:10.1097/HJH.0000000000002934.
- Grassi G, Seravalle G, Quarti-Trevano F, et al. Reinforcement of the adrenergic overdrive in the metabolic syndrome complicated by obstructive sleep apnea. J Hypertens. 2010;28(6):1313–1320. doi:10.1097/HJH.0b013e328337a9fd.
- Grassi G, Seravalle G, Brambilla G, et al. Regional differences in sympathetic activation in lean and obese normotensive individuals with obstructive sleep apnoea. J Hypertens. 2014;32(2):383–388. doi:10.1097/HJH.0000000000000034.
- Balasubramanian P, Hall D, Subramanian M. Sympathetic nervous system as a target for aging and obesity-related cardiovascular diseases. Geroscience. 2019;41(1):13–24. doi:10.1007/s11357-018-0048-5.
- Gavina C, Carvalho DS, Dias DM, et al. Premature mortality in type 2 diabetes mellitus associated with heart failure and chronic kidney disease: 20 years of real-world data. J Clin Med. 2022;11(8):2131. doi:10.3390/jcm11082131.
- Dell’Oro R, Quarti-Trevano F, Seravalle G, et al. Limited reliability of heart rate as a sympathetic marker in chronic kidney disease. J Hypertens. 2021;39(7):1429–1434. doi:10.1097/HJH.0000000000002763.
- Grassi G, Quarti-Trevano F, Seravalle G, et al. Early sympathetic activation in the initial clinical stages of chronic renal failure. Hypertension. 2011;57(4):846–851. doi:10.1161/HYPERTENSIONAHA.110.164780.
- Grassi G, Bertoli S, Seravalle G. Sympathetic nervous system: role in hypertension and in chronic kidney disease. Curr Opin Nephrol Hypertens. 2012;21(1):46–51. doi:10.1097/MNH.0b013e32834db45d.
- Grassi G, Seravalle G, Ghiadoni L, et al. Sympathetic nerve traffic and asymmetric dimethylarginine in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(11):2620–2627. doi:10.2215/CJN.06970711.
- Pathak A, Mrabeti S. β-blockade for patients with hypertension, ischemic heart disease or heart failure: Where are we now? Vasc Health Risk Manag. 2021;17:337–348. doi:10.2147/VHRM.S285907.
- Grassi G, Seravalle G, Brambilla G, et al. The sympathetic nervous system and new nonpharmacologic approaches to treating hypertension: a focus on renal denervation. Can J Cardiol. 2012;28(3):311–317. doi:10.1016/j.cjca.2011.11.005.
- Grassi G, Seravalle G, Esler M. Sympathomodulation in congestive heart failure: from drugs to devices. Int J Cardiol. 2020;321:118–125. doi:10.1016/j.ijcard.2020.07.027.
- Sharp TE, 3rd, Lefer DJ. Renal denervation to treat heart failure. Annu Rev Physiol. 2021;83(1):39–58. doi:10.1146/annurev-physiol-031620-093431.
- Raza S, Osasan S, Sethia S, et al. A systematic review of sodium-glucose cotransporter 2 (SGLT2) inhibitors and sympathetic nervous system inhibition: an underrated mechanism of cardiorenal protection. Cureus. 2022;14(6):e26313. doi:10.7759/cureus.26313.
- Sano M. A new class of drugs for heart failure: SGLT2 inhibitors reduce sympathetic overactivity. J Cardiol. 2018;71(5):471–476. doi:10.1016/j.jjcc.2017.12.004.
- Taddei S, Tsabedze N, Tan R-S. β-blocker are not all the same: pharmacologic similarities and differences, potential combinations and clinical implications. Curr Med Res Opin. 2024. In press, doi:10.1080/03007995.2024.2318058.
- Mahfoud F, Wang J, Ray S. The current position of β-blockers in hypertension: guidelines and clinical practice. Curr Med Res Opin. 2024. In press, doi:10.1080/03007995.2024.2318003.
- Palatini P, Faria-Neto JR, Santos RD. The clinical value of β-blockers in patients with stable angina. Curr Med Res Opin. 2024. In press, doi:10.1080/03007995.2024.2317443.
- de Oliveira MT, Jr Baptista R, Chavez-Leal SA, et al. Heart failure management with β-blockers: can we do better? Curr Med Res Opin. 2024. In press, doi:10.1080/03007995.2024.2318002.
- Marti H-P, Pavía López AA, Schwartzmann P. Safety and tolerability of β-blockers. Curr Med Res Opin. 2024. In press, doi:10.1080/03007995.2024.2317433.
