Abstract
Background
This study assessed the incremental healthcare costs and resource utilization (HRU) associated with generalized myasthenia gravis (gMG), as well as variability in these outcomes among patients with gMG and common comorbidities and acute MG-related events.
Methods
Adults with gMG and without MG were identified from a large US database (2017–2021). The index date was the first MG diagnosis (gMG cohort) or random date (non-MG cohort). Cohorts were propensity score matched 1:1. The gMG cohort included subgroups of patients with a 12-month pre-index (baseline) cardiometabolic or psychiatric comorbidity, or a post-index MG exacerbation/crisis. Monthly healthcare costs (2021 USD) and HRU were compared post-index between gMG and non-MG cohorts.
Results
The gMG and matched non-MG cohorts each contained 2,739 patients. Mean incremental healthcare costs associated with MG were $4,155 (gMG: $5,567; non-MG: $1,411), with differences driven by incremental inpatient costs of $2,166 (gMG: $2,617; non-MG: $452); all p < 0.001. The gMG versus non-MG cohort had 4.36 times more inpatient admissions and 2.26 times more outpatient visits; all p < 0.001. Among patients with gMG in cardiometabolic (n = 1,859), psychiatric (n = 1,308), and exacerbation/crisis (n = 419) subgroups, mean monthly healthcare costs were $6,660, $7,443, and $17,330, respectively.
Conclusions
gMG is associated with substantial incremental costs and HRU, with inpatient costs driving the total incremental costs. Costs increased by 20% and 34% among patients with cardiometabolic and psychiatric conditions, respectively, and over three times among those with acute MG-related events. gMG is a complex disease requiring management of comorbidities and treatment options that can prevent acute symptomatic events.
PLAIN LANGUAGE SUMMARY
Generalized myasthenia gravis (gMG) is a rare long-standing condition that affects the junctions between nerves and muscles, causing them to be weak. In a serious case, the diaphragm – a muscle that helps with breathing – becomes so weak that a patient will need a machine to breathe for them. This is called MG exacerbation or crisis. In this study, we used a large insurance database in the United States to look at how much money healthcare payers paid for gMG patients on average and what healthcare resources patients with gMG used. We compared these findings with patients without gMG. Also, among patients with gMG, we reported these findings specifically for patients who also had heart, blood, or blood vessel disease; patients who had a mental illness; and patients who had MG exacerbation or crisis later on. We found that patients with gMG used $5,567 per month on average ($4,155 more than patients without gMG), mostly from overnight hospital stays. Patients with gMG also had four times more overnight hospital stays and two times more hospital day visits when we compared them to patients without gMG. Patients with gMG and other health conditions used even more money and resources per month. Patients with MG exacerbation or crisis used $17,330 per month on average. Our results showed that gMG led to higher healthcare cost and resource use. In order to reduce cost and resources, doctors also need to control for other health conditions as they treat patients with gMG, and to prevent patients from having MG exacerbation or crisis later on.
Introduction
Myasthenia gravis (MG) is a rare neurologic autoimmune disease that causes fluctuating muscle weaknessCitation1–3. In 2021, the overall prevalence of MG in the US was estimated to be 37 per 100,000 personsCitation4. Pure ocular MG, where symptoms are limited to the eyes, occurs in an estimated 20% of cases of MG, while the remaining patients have generalized MG (gMG)Citation5. Initially, ocular MG may develop into gMG within 2 years of symptom onset in up to 80% of patientsCitation1. Most patients with gMG experience generalized weakness that can affect neck and proximal limb muscle movement, the ability to swallow, as well as the respiratory musclesCitation2,Citation5. Approximately 15% to 20% of patients experience myasthenic crisis, an exacerbation of gMG characterized by respiratory failure requiring intensive care and respiratory supportCitation1,Citation2.
Long-term treatment is necessary for almost all patients to control symptoms associated with gMG, the choice of which is based on disease severity and autoantibody subtypeCitation2,Citation6. Standard initial therapies to improve muscle weakness include acetylcholinesterase inhibitors, corticosteroids, and non-steroidal immunosuppressive treatmentsCitation2,Citation7. Thymectomy may be used for patients that do not respond to initial therapy or to reduce the dose or duration of immunotherapyCitation7. Rituximab is recommended as an alternative to steroids and azathioprine for initial treatment in newly diagnosed patients, as well as in patients with treatment-refractory symptomsCitation8. Eculizumab may be considered in patients with severe, refractory gMGCitation9. Intravenous immunoglobulin (IVIg) or plasmapheresis (PLEX) should be used in patients with myasthenic crisisCitation7. Current treatment strategies have variable effectiveness, and long-term exposure is associated with side-effects and cumulative toxicitiesCitation6. Newer therapies or those under investigation for the treatment of gMG include immunotherapies that target cytokines, complement proteins, B cells, and neonatal Fc receptorsCitation6.
Management of gMG is associated with considerable healthcare costsCitation10,Citation11, which vary based on disease severity and the required treatments, as well as the presence of specific comorbidities and demographic characteristicsCitation12,Citation13. A 2020 review on cost drivers of gMG found that the literature is scarce, dated, and subject to non-trivial variabilityCitation12. Furthermore, little is known about the variability of healthcare resource utilization (HRU) and costs among patients with gMG with common comorbidities and those experiencing acute MG-related events. Therefore, the current study aimed to (1) estimate the incremental healthcare costs and healthcare resource utilization (HRU) associated with gMG and (2) among patients with gMG, describe the variability in costs and HRU, specifically in those with common comorbidities and acute MG-related events.
Methods
Data source
De-identified administrative claims data were obtained from the IQVIA PharMetricsFootnotei Plus database (1 January 2017 to 31 December 2021). The insurance database contains fully adjudicated claims data of over 215 million enrollees across all US census regions and contains information on patient demographics, plan type, payer type, and the start and stop dates of patients’ health plan enrollment. Integrated medical claims include information on diagnoses and procedures codes, provider specialty, and place of service, while integrated pharmacy claims include information on medication dispensed. Both integrated medical and pharmacy claims data contain service dates, the patients’ pharmacy and medical benefits (copayment, deductible), as well as the actual amount paid by health plans to the provider for all services rendered. Data are de-identified and comply with the Health Insurance Portability and Accountability Act (HIPAA) regulations; therefore, no institutional review board exemption was required. The IQVIA PharMetrics Plus database has been widely accepted and used in a number of prior real-world studiesCitation14–18.
For this study, data on all patients with ≥1 diagnosis of MG between 1 January 2017 and 31 December 2021 and data for a randomly selected control cohort of patients without a diagnosis of MG (ratio 2:1 to the cohort of patients with MG) during the same period of time were obtained from the IQVIA PharMetrics Plus database.
Study design
A retrospective cohort study design was used to analyze patients with gMG (gMG cohort) and without gMG (non-gMG cohort) during the index window, spanning from 1 January 2018 to 31 December 2021. For the gMG cohort, the index date was defined as the date of the first diagnosis of gMG, while for the non-MG cohort, the index date was defined as a random date within a continuous health plan eligibility in the same index window. The 12-month baseline period before the index date was used to describe patient characteristics, including demographics, comorbidities, treatments, HRU, and characteristics of gMG on the index date for the gMG cohort. The follow-up period, spanning from the index date until the end of health plan eligibility or data availability, whichever came first, was used to measure HRU and healthcare costs.
Selection criteria
gMG and non-MG cohorts
Adult patients (≥18 years on the index date) with ≥12 months of continuous health plan eligibility before the index date were included in this study. Given that there was no specific diagnostic code for gMG, patients were identified based on a combination of diagnoses of MG and physician specialty – an algorithm suggested by Phillips et al. that was further refined in this studyCitation13. Patients included in this study had (i) ≥1 claim with a principal diagnosis of MG (International Classification of Diseases, 10th Revision, Clinical Modification [ICD-10-CM]: G70.00, G70.01, or G70.2) in an inpatient setting or ≥2 claims on separate days with a diagnosis of MG in any position in an outpatient, emergency, or other setting and (ii) ≥1 diagnosis of MG by a neurologist. In addition, patients in the non-MG cohort must not have had a diagnosis of MG during the study period.
gMG subgroups by comorbidity and acute MG-related event occurrence
Three non-mutually exclusive subgroups were created to analyze patients with gMG and commonly observed comorbidities, as well as those with acute MG-related events. Subgroups included (i) the cardiometabolic subgroup, where patients had ≥1 diagnosis of acute myocardial infarction, coronary artery disease, diabetes, heart failure and cardiomyopathy, hypertension, hypothyroidism, obesity, or received a coronary artery bypass graft surgery during the baseline period; (ii) the psychiatric subgroup, where patients had ≥1 diagnosis of conditions in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) during the baseline period; and (iii) the exacerbation/crisis subgroup, where patients had ≥1 MG exacerbation-related hospitalization and/or crisis (defined as ≥1 endotracheal intubation or tracheostomy in the inpatient or intensive care settings) during the follow-up period.
Study outcome measures
Study outcomes were all-cause and MG-related healthcare costs and HRU. All-cause healthcare costs and HRU were comprehensive of all medical services and pharmacy prescription fills received for any medical reason. MG-related healthcare costs and HRU were defined as medical claims with an MG diagnosis or pharmacy claims for gMG-related medications (i.e. acetylcholinesterase inhibitors, corticosteroids, immunoglobulin, neonatal Fc receptor antagonists, non-steroidal immunosuppressants, and monoclonal antibodies).
Medical costs and HRU were reported by following components: inpatient (days, admissions, costs), emergency room (days, costs), outpatient (days, costs), and other services (i.e. durable medical equipment services, home-care services, dental or vision care; days with services and costs). Pharmacy costs included costs for prescription medications (over-the-counter medications and samples were not captured). Total healthcare costs were the sum of pharmacy and medical costs. Healthcare costs were reported in 2021 US dollars from the payer’s perspective; both healthcare costs and HRU were reported per-patient-per-month.
Outcomes did not include costs or resources incurred outside of the healthcare setting (e.g. sick leave, loss of productivity).
Statistical analyses
To compare outcomes between the two cohorts, the gMG and non-MG cohorts were exactly matched using a 1:1 ratio based on the index year and propensity scores that considered age, sex, payer type, region, index quarter, and the Quan-Charlson Comorbidity Index (excluding rheumatic diseases). The balance of baseline characteristics after matching was assessed using standardized differences, where <10% was considered well balanced. Means, standard deviations (SDs), and medians were used to describe continuous variables, while frequencies and proportions were used to describe categorical variables for all study groups.
All comparative analyses between the gMG and non-MG cohorts accounted for matched design using generalized estimating equations. Cost variables were compared between cohorts as the mean cost difference (MCD) using ordinary least squares regressions. Of note, in cost evaluations designed to have an impact on payer policies, it is the estimate of the arithmetic mean that is informative, as measures other than arithmetic mean do not provide information about the costs of treating all patients and may result in misleading conclusionsCitation19. Count variables (i.e. number of inpatient days) were compared as rate ratio (RR) using Poisson regressions. To account for non-normal distributions, a non-parametric bootstrap procedure with 500 replications was used to produce 95% confidence intervals (95% CI) and p values. All analyses were conducted using SAS EnterpriseFootnoteii Guide version 7.1.
Results
Patient characteristics
After matching, a total of 2,739 patients were included in each of the gMG (mean [SD] age = 56.2 [14.4] years; 50.6% female) and non-MG (mean [SD] age = 56.7 [14.3] years; 51.5% female) cohorts (). The most common comorbidities in both cohorts were hypertension (gMG cohort: 48.7%; non-MG cohort: 43.1%), obesity (gMG cohort: 27.2%; non-MG cohort: 19.2%), and diabetes (gMG cohort: 21.5%; non-MG cohort: 22.7%). The most common psychiatric conditions were sleep–wake disorders (gMG cohort: 22.6%; non-MG cohort: 14.0%) and depressive disorders (gMG cohort: 14.3%; non-MG cohort: 10.1%). Based on the ICD-10-CM diagnosis codes, most patients in the gMG cohort presented on index date without exacerbation (83.7%).
Table 1. Patient demographic and clinical characteristics during the baseline period.Table Footnotea
Mean baseline all-cause monthly total healthcare (SD) costs were $1,633 ($3,439) in the gMG cohort and $1,220 ($4,043) in the non-MG cohort.
Economic burden of gMG
The mean duration of follow-up period was 18.5 months in the gMG cohort and 13.3 months in the non-MG cohort.
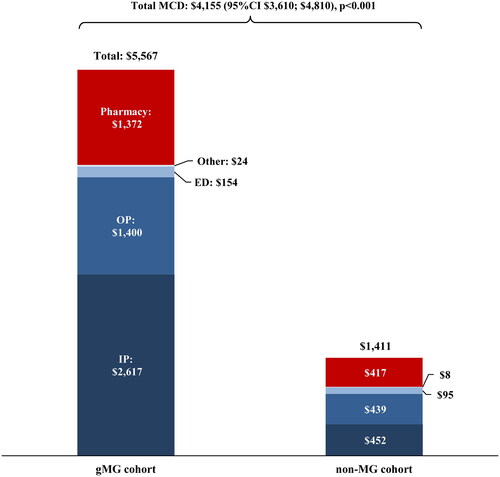
During the follow-up period, mean (SD) all-cause monthly total healthcare costs in the gMG cohort were $5,567 ($15,360), on average $4,155 higher relative to the non-MG cohort (p < 0.001; ). Inpatient costs were the primary driver, accounting for 52.1% of the mean total monthly cost difference, followed by outpatient costs accounting for 23.1% and pharmacy costs accounting for 23.0%.
Figure 1. Mean all-cause healthcare costs (2021 USD) during the follow-up period, per-patient-per-month. Abbreviations: ED, emergency department; gMG, generalized myasthenia gravis; IP, inpatient; MCD, mean cost difference; MG, myasthenia gravis OP, outpatient; USD, US dollars.
In the gMG cohort, more than half (56.9%) of total mean all-cause medical costs were MG-related. MG-related inpatient costs accounted for 63.4% of all-cause inpatient costs. MG-related pharmacy costs accounted for 66.3% of all-cause pharmacy costs. Among all patients in the cohort, 2.1% and 38.5% of patients experienced MG crisis and exacerbation, respectively. The mean (SD) cost related to MG crisis was $454 ($5,210); the mean (SD) cost of MG exacerbation was $1,782 ($8,033). Among all MG exacerbation-related costs, 77.1% ($1,374) occurred during an inpatient admission.
In terms of high mean pharmacy costs in the gMG cohort, 53.6% of these costs were due to IVIg therapy costs; 14.0% of the gMG cohort used IVIg therapy during the follow-up period.
Higher inpatient and outpatient costs in the gMG cohort were consistent with higher use of respective healthcare resources. Specifically, compared to the non-MG cohort, patients in the gMG cohort had over four times the rate of inpatient admission and number of inpatient days and over twice the number of outpatient visits; they also had almost twice the number of emergency visits (all p < 0.001; ).
Figure 2. Healthcare resource utilization among the gMG and non-MG cohorts during the follow-up period, per-patient-per-month.
Abbreviations: ED, emergency department; gMG, generalized myasthenia gravis; IP, inpatient; MG, myasthenia gravis; OP, outpatient; RR, rate ratio.
Notes:
[1] Forest plot is presented on the logarithmic scale.
[2] RR is reported based on univariate Poisson regression models; bootstrap procedure is used to produce 95% CIs and p values. RR >1 indicates higher HRU in the gMG cohort.
![Figure 2. Healthcare resource utilization among the gMG and non-MG cohorts during the follow-up period, per-patient-per-month.Abbreviations: ED, emergency department; gMG, generalized myasthenia gravis; IP, inpatient; MG, myasthenia gravis; OP, outpatient; RR, rate ratio.Notes:[1] Forest plot is presented on the logarithmic scale.[2] RR is reported based on univariate Poisson regression models; bootstrap procedure is used to produce 95% CIs and p values. RR >1 indicates higher HRU in the gMG cohort.](/cms/asset/8f9e1d8a-26ee-420d-98f3-ab4f4ea48a86/icmo_a_2353381_f0002_c.jpg)
Subgroups of patients with gMG by comorbidity and acute MG-related events
In the gMG cohort, patients were stratified into cardiometabolic (n = 1,859; mean follow-up length: 17.9 months), psychiatric (n = 1,308; mean follow-up length: 17.7 months), and exacerbation/crisis (n = 419; mean follow-up length: 17.9 months) subgroups ().
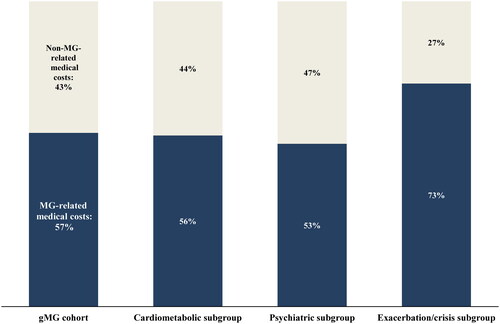
Across all subgroups over the follow-up period, inpatient costs were the main driver of total healthcare costs, accounting for 49.9%, 48.2%, and 63.1% of costs in the cardiometabolic, psychiatric, and exacerbations/crisis subgroups, respectively (). MG-related medical costs accounted for over half of the total medical costs in the cardiometabolic (56.0%), psychiatric (53.3%), and exacerbation/crisis subgroups (73.1%; ). Costs of IVIg drove all-cause pharmacy costs across cohorts, accounting from 52.4% to 53.6% of these costs in the cardiometabolic and psychiatric subgroups to 63.6% in the exacerbation/crisis subgroup. Proportions of patients using IVIg were 15.0% among the cardiometabolic subgroup, 16.4% among the psychiatric subgroup, and 35.8% among the exacerbation/crisis subgroup. Among the exacerbation/crisis subgroup, exacerbation-related and crisis-related mean (SD) medical costs were $10,239 ($17,640) and $2,968 ($13,051), respectively.
Figure 3. Mean all-cause healthcare costs (2021 USD) during the follow-up period, per-patient-per-month by comorbidity and disease profile subgroup.
Abbreviations: ED, emergency department; gMG, generalized myasthenia gravis; IP, inpatient; OP, outpatient.
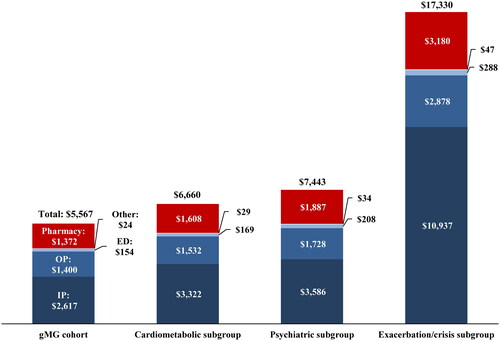
Figure 4. Proportion of MG-related costs in all-cause medical costs during the follow-up period by comorbidity and disease profile subgroup.
Abbreviations: gMG, generalized myasthenia gravis; MG, myasthenia gravis.
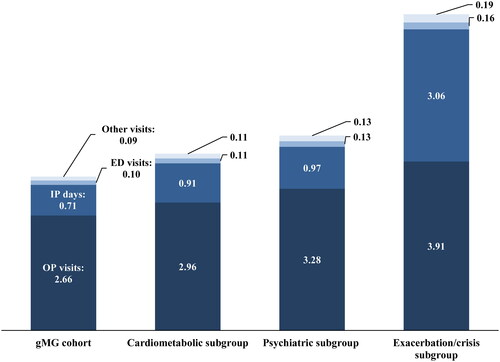
The mean (SD) monthly number of outpatient visits was 2.66 (2.49) in the overall gMG cohort, 2.96 (2.65) in the cardiometabolic subgroup, 3.28 (2.75) in the psychiatric subgroup, and 3.91 (2.90) in the exacerbation/crisis subgroup (). The mean (SD) monthly number of inpatient days was 0.71 (2.70) for the overall gMG cohort, 0.91 (3.02) for the cardiometabolic subgroup, 0.97 (3.27) for the psychiatric subgroup, and 3.06 (4.98) for the exacerbation/crisis subgroup.
Discussion
This retrospective cohort study evaluated the incremental healthcare costs and HRU associated with gMG, and cost and HRU variability among patients with common comorbidities and acute MG-related events. gMG was associated with a significant economic burden, including higher costs and HRU, compared to patients without MG. Overall, inpatient costs drove incremental healthcare costs associated with gMG. Within the subgroups of patients with gMG, healthcare costs were highest among those with exacerbation or crisis. Patients with these acute events also had higher proportions of IVIg use and costs compared to the overall gMG cohorts and other comorbidity subgroups.
The cost of managing gMG varies according to disease progression, where patients with more severe disease incur greater healthcare costsCitation12. In general, patients with gMG experience weakness that can affect the limbs, facial muscles, and even respiratory musclesCitation5. As a result, patients may have difficulty with everyday activities, including climbing stairs, speaking, swallowing, or breathingCitation1,Citation5. While milder forms of gMG can be treated with medications, such as acetylcholinesterase inhibitors and corticosteroids, patients with severe, refractory gMG or myasthenic crisis require more intensive treatments, such as eculizumab, IVIg, PLEX, and mechanical ventilationCitation2,Citation7,Citation9. A systematic literature search revealed that the main drivers of medical costs associated with treatment of gMG were IVIg and PLEX, myasthenic crisis, mechanical ventilatory support, and hospitalizationCitation12. In addition, patients requiring assistance for daily activities incurred higher costs than those who did notCitation12. Similarly, a 2022 study based on charged amounts from a provider perspective showed that HRU was greater in patients with exacerbation events, compared to those withoutCitation13. In this study, with a focus on paid amounts from a payer’s perspective, the mean per-patient cost per hospitalization ranged from $2,550 to $164,730. Among patients with gMG, the highest costs were incurred by the exacerbation/crisis subgroup, who also had the highest mean number of IP days. This suggests that while patients who experience exacerbation or myasthenic crisis represent only 15% to 20% of patients with gMG, they require more intensive and costly medical interventions, and are responsible for driving up the rates of HRU and healthcare costsCitation5,Citation11.
Consistent with previous findingsCitation20, inpatient care was the primary driver of overall healthcare costs observed among patients with gMG in this study, despite most patients receiving treatment in an outpatient settingCitation21. This finding was observed across all comorbidity and disease profile subgroups, particularly among patients who experienced MG exacerbation or myasthenic crisis. Patients who present with MG exacerbation or myasthenic crisis require more intensive and costly medical treatments, such as mechanical ventilatory supportCitation12, and are more likely to have an extended length of hospital stay, as well as more frequent hospitalization and emergency department visitsCitation13. Furthermore, a previous study revealed that in the year following MG exacerbation or myasthenic crisis, mean hospitalization, length of stay, and emergency department visits had risen by 2 to3 times compared with the preceding yearCitation13, suggesting a higher risk of readmission. As a result, this subset of patients may develop side-effects resulting from recurrent treatment, become more susceptible to comorbidities, and experience reduced quality-of-lifeCitation6.
Management of gMG in patients with pre-existing comorbidities, particularly psychiatric, may be associated with both higher gMG and non-gMG-related HRU and costs than in patients without comorbidities. This may result from the additional complexity and heterogeneity in treating both gMG and comorbid conditions. In particular, medication used to treat pre-existing comorbid conditions, such as beta blockers for hypertension and statins for dyslipidemia – common among patients with cardiometabolic conditions, may worsen symptoms of gMG, which can lead to MG exacerbation or myasthenic crisisCitation22. Moreover, patients with serious comorbidities may not be able to tolerate certain medications used to treat gMG, such as immunotherapy and steroids, and long-term exposure can lead to unwanted side-effects, toxicities, infection, and malignancyCitation6. Furthermore, compared with patients who have gMG alone, patients with comorbidities are more likely to require inpatient treatment for MG exacerbationsCitation23. Given the current treatment approach of comedication and use of intensive treatments, such as IVIg, in the treatment of severe MGCitation7,Citation24, particularly in those with comorbidities, there is a need for new and improved treatments to reduce not only the costs associated with gMG, but treatment complications and potential drug interactions. Taken together, this highlights the clinical and economic necessity to identify and manage comorbidities and prevent acute symptomatic events associated with gMG.
Limitations
The findings of this study are subject to certain limitations. First, given that there is no specific diagnosis code, gMG was identified based on a refined version of an algorithm suggested by Phillips et al.Citation13. Second, residual confounding may exist due to unmeasured confounders (e.g. patient socioeconomic status, quality of insurance coverage that may influence patient out-of-pocket costs). Third, for the gMG subgroups, the observational and descriptive nature of the data precludes definitive statements about causation. Fourth, the use of administrative claims data only included those with specific types of insurance, namely Medicare, Medicaid, or self-insured plans. Therefore, the results of this study may not be generalizable to patients without or with health insurance other than those mentioned. Finally, analyses of administrative claims data depend on correct diagnosis, procedure, and drug codes, and coding inaccuracies may lead to case misidentification and misclassification.
Conclusions
This retrospective study demonstrated that gMG is associated with substantial incremental costs and HRU. Furthermore, comorbidities and acute MG-related events were shown to escalate these costs and HRU. Together, results of this study highlight opportunities to improve management of gMG with new treatments targeting different pathomechanisms of the disease.
Transparency
Declaration of funding
This study was funded by Johnson & Johnson Innovative Medicine, LLC. The study sponsor was involved in several aspects of the research, including the study design, interpretation of data, and writing of the manuscript.
Declaration of financial/other relationships
MZ, PB, DP, and PL are employees of Analysis Group, Inc., a consulting company that has provided paid consulting services to Johnson & Johnson Innovative Medicine, LLC, which funded the development and conduct of this study and manuscript. JP, QC, and ZC are employees and stockholders of Johnson & Johnson Innovative Medicine, LLC. NS has no disclosures. Peer reviewers on this manuscript have received an honorarium from CMRO for their review work but have no other relevant financial relationships to disclose.
Author contributions
MZ, PB, and DP contributed to the conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, software, visualization, original draft preparation, and review and editing of the draft. JP, QC, ZC, and NS contributed to the formal analysis, investigation, data interpretation and analysis, original draft preparation, review and editing of the draft.
Ethics statement
Data were de-identified and comply with the patient requirements of the Health Insurance Portability and Accountability Act (HIPAA) of 1996; therefore, no review by an institutional review board was required per Title 45 of CFR, Part 46.101(b)(4) (https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/#46.101).
Previous presentations
Part of the material in this manuscript was presented at the American Association of Neuromuscular & Electrodiagnostic Medicine Conference held November 1–4, 2023 in Phoenix, AZ as a poster presentation.
CMRO-2024-FT-0259 - Supplemental Material - 04-24-2024.docx
Download MS Word (51.8 KB)Acknowledgements
Medical writing assistance was provided by professional medical writer, Roxanne Wosu, BEng, MASc, an employee of Analysis Group, Inc., a consulting company that has provided paid consulting services to Johnson & Johnson Innovative Medicine, LLC, which funded the development and conduct of this study and manuscript.
Data availability statement
The data that support the findings of this study are available from IQVIA. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors upon reasonable request with the permission of IQVIA.
Notes
i IQVIA PharMetrics, Durham, NC, USA
ii SAS Enterprise, SAS institute, Cary, NC, USA
References
- Dresser L, Wlodarski R, Rezania K, et al. Myasthenia gravis: epidemiology, pathophysiology and clinical manifestations. J Clin Med. 2021;10(11): 1–17.
- Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol. 2015;14(10):1023–1036. doi: 10.1016/S1474-4422(15)00145-3.
- Jayam Trouth A, Dabi A, Solieman N, et al. Myasthenia gravis: a review. Autoimmune Dis. 2012;2012:874680.
- Rodrigues E, Umeh E, Uday A, et al. Incidence and prevalence of myasthenia gravis in the United States: a claims-based analysis (S19.007). Neurology. 2023;100(17_supplement_2):2994. doi: 10.1212/WNL.0000000000202945.
- Hehir MK, Silvestri NJ. Generalized myasthenia gravis: classification, clinical presentation, natural history, and epidemiology. Neurol Clin. 2018;36(2):253–260. doi: 10.1016/j.ncl.2018.01.002.
- Nair SS, Jacob S. Novel immunotherapies for myasthenia gravis. Immunotargets Ther. 2023;12:25–45. doi: 10.2147/ITT.S377056.
- Sanders DB, Wolfe GI, Benatar M, et al. International consensus guidance for management of myasthenia gravis: executive summary. Neurology. 2016;87(4):419–425.
- Gilhus NE, Andersen H, Andersen LK, et al. Generalized myasthenia gravis with acetylcholine receptor antibodies: a guidance for treatment. Eur J Neurol. 2024;31(5):e16229. doi: 10.1111/ene.16229.
- Narayanaswami P, Sanders DB, Wolfe G, et al. International consensus guidance for management of myasthenia gravis: 2020 update. Neurology. 2021;96(3):114–122. doi: 10.1212/WNL.0000000000011124.
- Guptill JT, Marano A, Krueger A, et al. Cost analysis of myasthenia gravis from a large U.S. insurance database. Muscle Nerve. 2011;44(6):907–911.
- Guptill JT, Sharma BK, Marano A, et al. Estimated cost of treating myasthenia gravis in an insured U.S. population. Muscle Nerve. 2012;45(3):363–366. doi: 10.1002/mus.22327.
- Landfeldt E, Pogoryelova O, Sejersen T, et al. Economic costs of myasthenia gravis: a systematic review. Pharmacoeconomics. 2020;38(7):715–728. doi: 10.1007/s40273-020-00912-8.
- Phillips G, Abreu C, Goyal A, et al. Real-World healthcare resource utilization and cost burden assessment for adults with generalized myasthenia gravis in the United States. Front Neurol. 2022;12:809999. doi: 10.3389/fneur.2021.809999.
- Batt K, Schultz BG, Caicedo J, et al. A real-world study comparing pre-post billed annualized bleed rates and total cost of care among non-inhibitor patients with hemophilia a switching from FVIII prophylaxis to emicizumab. Curr Med Res Opin. 2022;38(10):1685–1693.
- Desai PC, Chen CC, McGuiness CB, et al. Real-world characteristics of patients with sickle cell disease who initiated crizanlizumab therapy. Curr Med Res Opin. 2023;39(4):555–565.
- Hanna ML, Singer D, Valdecantos WC. Economic burden of generalized pustular psoriasis and palmoplantar pustulosis in the United States. Curr Med Res Opin. 2021;37(5):735–742. doi: 10.1080/03007995.2021.1894108.
- Sah J, Teeple A, Muser E, et al. Treatment persistence and maintenance dose titration among ulcerative colitis patients on biologics: a pooled study of three United States claim databases. Curr Med Res Opin. 2022;38(7):1093–1101.
- Xie J, Wu A, Liao L, et al. Characteristics and treatment patterns of relapsed/refractory diffuse large B-cell lymphoma in patients receiving >/=3 therapy lines in post-CAR-T era. Curr Med Res Opin. 2021;37(10):1789–1798.
- Thompson SG, Barber JA. How should cost data in pragmatic randomised trials be analysed? BMJ. 2000;320(7243):1197–1200. doi: 10.1136/bmj.320.7243.1197.
- Engebretsen I, Gilhus NE, Kristiansen IS, et al. The epidemiology and societal costs of myasthenia gravis in Norway: a non-interventional study using national registry data. Eur J Neurol. 2024;31(5):e16233.
- Farrugia ME, Goodfellow JA. A practical approach to managing patients with myasthenia gravis – opinions and a review of the literature. Front Neurol. 2020;11:604. doi: 10.3389/fneur.2020.00604.
- Misra UK, Kalita J, Singh VK, et al. A study of comorbidities in myasthenia gravis. Acta Neurol Belg. 2020;120(1):59–64.
- Laakso SM, Myllynen C, Strbian D, et al. Comorbidities worsen the prognosis of generalized myasthenia gravis post-thymectomy. J Neurol Sci. 2021;427:117549.
- Andersen JB, Owe JF, Engeland A, et al. Total drug treatment and comorbidity in myasthenia gravis: a population-based cohort study. Eur J Neurol. 2014;21(7):948–955.