Abstract
Objective
To evaluate the efficacy and safety of nivolumab in the second-line (2L) or later-line (LL) treatment of patients with locally advanced/metastatic non-small cell lung cancer (NSCLC) in real-life setting in Türkiye.
Methods
This study was designed as a national, multi-center, retrospective study. The study population was evaluated in two groups for the line of nivolumab therapy: those receiving nivolumab in the 2L (Group 2L) and third-line (3L) or LL (Group 3L/LL). Efficacy was evaluated based on one-year overall survival (OS) and progression-free survival (PFS). Safety was evaluated based on treatment-related adverse events (AEs) and nivolumab discontinuation rate.
Results
Of 244 patients, 52.9% were in Group 2L and 47.1% were in Group 3L/LL. Demographic and clinical characteristics did not differ between the groups. In Group 2L and Group 3L/LL, one-year OS and PFS rates were 60.8% and 61.4% (p = 0.592) and 31.2% and 21.3% (p = 0.078), respectively. The objective response rate (ORR) was 34.7% in Group 2L and 27.3% in Group 3L/LL (p = 0.262). The percentage of patients reporting at least one AE in Groups 2L and 3L/LL was 34.9% and 43.5%, respectively (p = 0.169). Fatigue was the most common (16.4%) treatment-related AE in each group. The groups were comparable regarding the AE frequency. Nivolumab was discontinued in 61 patients in Group 2L and 53 patients in Group 3L/LL, with the most common reason being disease progression (57.4% and 66.0%, respectively).
Conclusion
Nivolumab is safe and effective in the 2L or 3L/LL treatment of locally advanced/metastatic NSCLC and associated with acceptable AEs in real-life setting.
PLAIN LANGUAGE SUMMARY
Non-small cell lung cancer (NSCLC) is the most common type of lung cancer (around 85% of all lung cancers). Patients with NSCLC are usually diagnosed at advanced or metastatic stages. When cancer cells spread to other areas from where they first formed, it is called metastatic cancer. Surgery may not be a treatment option for such patients. Currently, immunotherapeutic agents are used in the treatment of NSCLC. Nivolumab is one of the approved immunotherapeutic agents in the treatment of patients with metastatic NSCLC, who have failed after receiving chemotherapy. Our study explored the efficacy and safety of nivolumab in real-life setting in Türkiye. Nivolumab effectiveness was evaluated by overall survival (OS) and progression-free survival (PFS) rates. OS indicates the proportion of patients who are still alive at a given time after diagnosis or treatment initiation. PFS refers to “the length of time during and after cancer treatment that a person lives with the disease but does not get worse.” In the present study, one-year OS for 244 patients who received nivolumab was 61.1% and one-year PFS was 26.4%. Nivolumab safety was evaluated based on the frequency of adverse events observed during nivolumab therapy. Of the patients 38.9% had at least one side effect, with fatigue being the most common (16.4%). Our results support the earlier studies and showed that nivolumab was a safe and effective agent and is associated with acceptable side effects.
Introduction
Lung cancer is a global public health problem and the leading cause of cancer deaths.Citation1 Histologically, non-small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancersCitation2,Citation3 and is usually diagnosed at advanced stages. More than half (60%) of patients with lung cancer have locally advanced or metastatic disease (stage III or IV) at diagnosis, and local resection is not an option for such patients.Citation4,Citation5
Currently, the use of immunotherapeutic agents alone or in combination with chemotherapy is the standard approach in the first-line treatment of advanced NSCLC.Citation6 However, their use in first-line (1L) treatment is still limited in some countries due to problems in reimbursement. In such patients, the use of second-line (2L) immunotherapy improves survival compared to the results of single-agent chemotherapy.Citation7
In recent years, the treatment of metastatic NSCLC has evolved into more biomarker-driven personalized care along with the inhibition of the programmed cell death protein-1 (PD-1)/programmed cell death protein ligand-1 (PD-L1) pathway, which is an immune checkpoint used by tumor cells to inhibit antitumor responses.Citation8,Citation9 Nivolumab is a human immunoglobulin G4 (IgG4) monoclonal antibody and specifically binds the PD-1 found on the activated immune cells. It inhibits the receptor to engage with its ligands (PD-L1: B7-H1/CD274 and PD-L2: B7-DC/CD273), and thereby enhances antitumor response of the host.Citation10,Citation11 Nivolumab was approved by the US Food and Drug Administration (FDA) in March 2015 to be used in the 2L and later-line (LL) treatments of patients with metastatic NSCLC, who showed progression while or after receiving platinum-based chemotherapy.Citation12 Since then, the efficacy and safety of nivolumab have been documented in various clinical trials. In Türkiye, Bristol-Myers Squibb Pharmaceuticals Inc. received the license for Nivolumab (Opdivo®) in April 2017;Citation13 however, data from real-life experience in Türkiye remains lacking. The present study aimed to evaluate the efficacy and safety of nivolumab in the 2L or LL treatment of patients with locally advanced/metastatic NSCLC in real-life setting in Türkiye.
Methods
A national, multi-center, retrospective, non-interventional registry study was conducted between July 2015 and October 2021 in the patients diagnosed with locally advanced or metastatic NSCLC at eight centers across Türkiye. The study was conducted after the approval of the University of Health Sciences Gulhane Scientific Researches Ethics Committee (Date: 08.07.2021; Approval No: 2021/251) and in accordance with the Declaration of Helsinki. Male and female patients who were diagnosed with NSCLC in 2015 and later, at the age of ≥18 years at the time of initial diagnosis, received nivolumab therapy in the 2L or LL treatment of NSCLC after failure with previous systemic therapy, and had at least 6-month follow-up data prior to nivolumab therapy were considered eligible. Patients with insufficient epidemiological or clinical data were excluded.
The data were retrieved from the patients’ medical records and then recorded in the electronic case report forms (eCRFs). The data collected consisted of demographic and clinical characteristics, treatment pattern (duration of nivolumab therapy, reasons for discontinuation, treatment dose adjustment), treatment response by RECIST (response evaluation criteria in solid tumors) criteria, and survival status.
Study population was evaluated in two groups according to the line of nivolumab therapy: those receiving nivolumab in the 2L treatment of NSCLC (Group 2L) and those receiving nivolumab in the third-line (3L) or LL treatment of NSCLC (Group 3L/LL). These groups were then compared in terms of safety and efficacy of nivolumab.
Statistical analysis
Data analyses were performed using the Predictive Analytics Software (PASW) 18 (SPSS Inc., Chicago, IL, USA). Normality of data was tested using the visual (histogram and probability plots) and analytical (Kolmogorov-Smirnov/Shapiro-Wilk tests) methods. Demographic and clinical data were summarized using descriptive statistics. Descriptive statistics were expressed as numbers and percentages for categorical variables and as mean, standard deviation, median, and minimum-maximum for numerical variables. A p value <0.05 was considered the level of statistical significance.
The Kaplan-Meier method was used to assess overall survival (OS) and progression-free survival (PFS). The effects of selected categorical variables (sex, smoking and treatment line) on survival were investigated using the log-rank test. Mann-Whitney U test was used for non-normally distributed numerical variables. For categorical variables, the Chi-square test was used for two-group and multiple comparisons when Chi-square condition was met, whereas Fisher’s exact test was used for two-group and multiple comparisons when Chi-square condition was not met.
Results
Demographic and clinical characteristics
The medical records of 247 patients in eight centers across Türkiye were reviewed; among them, the data of 244 patients were analyzed (). Overall, the mean age was 60 ± 10 years, 80.3% were male (n = 196/244), 44.3% (n = 35/79) were former smokers, and 31.6% (n = 25/79) were current smokers (). The treatment-related information of the whole study population is presented in . Of the patients, 129 (52.9%) received nivolumab in the 2L and 115 (47.1%) in the 3L or LL treatment of NSCLC.
Figure 1. Study flow-chart. NSCLC: non-small cell lung cancer; eCRFs: electronic case report form; RECIST: Response evaluation criteria in solid tumors; OS: overall survival; PFS: progression-free survival; 2L: second-line, 3L/LL: third-line or later-line.
Table 1. Demographic and clinical characteristics of patients.
Table 2. Treatment-related information of the whole study population (N = 244).
No significant difference was found between the groups in terms of demographic and clinical characteristics except for lymph node metastasis. Demographic and clinical characteristics according to the line of treatment are summarized in . Additionally, the molecular characterization (driver mutations) of the population at baseline are summarized in ; no significant difference was found between the groups regarding the frequency of mutations.
Table 3. Comparison of demographic and clinical characteristics of the patients in the treatment line groups.
Table 4. Comparison of molecular characteristics (driver mutations) of the patients in the treatment line groups.
Efficacy
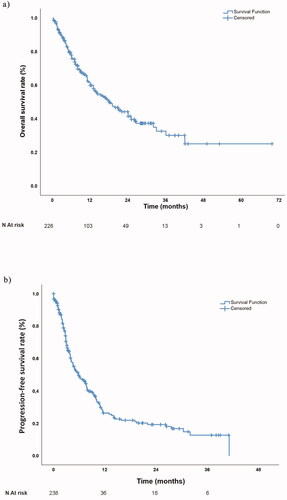
Regarding the whole study population, the median (minimum-maximum) OS was 18.6 (12.7–23.3) months, with a one-year OS rate of 61.1%. The OS for the whole study population is demonstrated in .
Figure 2. (a) Overall survival and (b) Progression-free survival curve for the whole study population.
One-year OS rates in Group 2L and Group 3L/LL were 60.8% and 61.4%, respectively. The median (minimum-maximum) OS was 22.0 (12.7–31.3) months for Group 2L and 17.8 (13.3–23.3) months for Group 3L/LL. OS showed no significant difference between the two groups (p = 0.592) (Figure S1). No difference was observed in OS according to smoking status or sex (Figures S2 and S3).
In the whole study population, the one-year PFS rate was 26.4%, with a median PFS of 5.85 (3.91–7.79) months ().
The one-year PFS rate was 31.2% for Group 2L and 21.3% for Group 3L/LL, with a median PFS of 7.75 (5.21–10.30) months and 4.63 (3.91–7.79) months, respectively. The PFS showed no significant difference between the treatment line groups (p = 0.078) (Figure S4). No difference was observed in PFS according to smoking status or sex (Figures S5 and S6).
In the whole study population, the response rates (RRs) per RECIST were available in 189 patients (101 patients in Group 2L and 88 patients in Group 3L/LL) (). In those patients, 59 had a response, resulting in an ORR of 31.2%; 3.7% showed complete response (CR), 27.5% showed partial response (PR), 13.8% had stable disease (SD), and 55.0% had progressive disease (PD). The RRs did not differ between the groups (p = 0.264). The ORR was 34.7% (n = 35) in Group 2L and 27.3% (n = 24) in Group 3L/LL, with no significant difference between the groups (p = 0.275).
Table 5. Treatment response rates by RECIST criteria according to the treatment line groups.
Safety
Among all patients, 38.9% (n = 95) reported at least one adverse event (AE) of any grade during nivolumab therapy, with fatigue being the most common AE (16.4%), followed by toxicity (14.6%), poor appetite (11.8%), and anemia (11.6%). In Group 2L and Group 3L/LL, the rate of patients reporting at least one AE of any grade was 34.9% and 43.5%, respectively, and the difference was not statistically significant (p = 0.169). The groups were comparable in terms of the frequency of AEs excluding skin rash, which was significantly higher in Group 3L/LL as compared to Group 2L (5.6% vs. 0%) (p = 0.009) ().
Table 6. Frequency of adverse events according to the treatment line groups.
Nivolumab therapy was discontinued in 114 patients (46.7%) (61 in Group 2L and 53 in Group 3L/LL). The most common reason for discontinuation of nivolumab therapy was disease progression in both Group 2L and Group 3L/LL (57.4% and 66.0%, respectively) ().
Table 7. Reasons for discontinuation of nivolumab therapy.
Discussion
In the present study, we evaluated the safety and efficacy of nivolumab used in the 2L and 3L/LL treatment of patients with locally advanced and metastatic NSCLC on the basis of real-life data. The treatment line groups were comparable in terms of demographic and clinical characteristics. We found that nivolumab therapy is associated with improved OS and PFS rates and low AE rates regardless of treatment line.
According to the 2021 cancer statistics, the lung cancer survival rate is low with a median 5-year survival rate being 6% for metastatic disease and 59% for localized disease in the US.Citation14 The development of immune checkpoint inhibitors completely changed the treatment paradigm. Improved survival is reported in considerably more patients with metastatic NSCLC treated with immune checkpoint inhibitors than with chemotherapy alone.Citation7
Nivolumab (BMS-936558/ONO-4538) was the first PD-1- specific monoclonal antibody and initially approved in 2015 for the 2L treatment of advanced/metastatic NSCLC after failure with 1L chemotherapy.Citation15 The approved indications have been moved to earlier lines and extended to the other types of cancer in a relatively short period of time. In two phase III studies, nivolumab monotherapy was associated with statistically superior survival benefit compared to docetaxel, which was the standard of care at the time of trials in 2L treatment of patients with advanced NSCLC with failure to previous treatment.Citation16,Citation17
Efficacy
Approval of nivolumab for the treatment of patients with advanced NSCLC was based on two Phase 3 trials (CheckMate 057 and CheckMate 017), which showed survival benefit over docetaxel across histological types.Citation16,Citation17 So far, a number of clinical trials on nivolumab have demonstrated satisfactory efficacy with improved OS and PFS rates in the treatment of patients with NSCLC as described below. Both three-year outcomes from CheckMate 057 and CheckMate 017 studiesCitation18 and the results of a pooled analysis of ONO-4538-05 and ONO-4538-06 studiesCitation19 revealed durable responses in some of the patients with NSCLC.
A real world study from Israel, where the majority (64%) of patients received nivolumab in the 2L treatment, reported a median OS of 5.9 months (95% CI 4.7–7.4) with Eastern Cooperative Oncology Group Performance Status (ECOG PS) being the only variable significantly associated with OS. The response, which was evaluated in 49 patients, revealed an ORR of 35% and a median PFS of 2.8 months (95% CI, 1.8–7.7).Citation20 A systematic review of 11 randomized controlled clinical trials (RCTs) on 7,581 patients comparing the efficacy and safety of licensed 2L treatments including the checkpoint inhibitors nivolumab, atezolizumab and pembrolizumab in patients with advanced-stage NSCLC reported superior OS benefit over docetaxel and determined that nivolumab was associated with the highest survival benefit compared to atezolizumab and pembrolizumab (based on a meta-analysis and not on head-to-head trial comparison).Citation15 PFS benefit was also the highest with nivolumab therapy. Moreover, nivolumab was found significantly safer as compared to atezolizumab and pembrolizumab. Nivolumab, pembrolizumab and atezolizumab exhibited superior benefit/risk balance compared to other licensed drugs used in advanced-stage NSCLC.Citation15 In another study investigating the long-term efficacy and safety of nivolumab in 129 patients with advanced NSCLC pretreated with ≥3 prior therapies, nivolumab showed durable clinical activity with a median OS of 9.9 months and with one-year and two-year OS rates of 42% and 24%, respectively.Citation21 A study from Japan investigated 901 patients with NSCLC, the majority of whom received nivolumab as 2L treatment.Citation22 In that study, the median OS (14.6 months), one-year survival rate (54.3%) and the median PFS (2.1 months) were lower than those found in our study, which found a median OS of 18.6 months; one-year OS of 61.1%; and a median PFS of 5.85 months.
In a study investigating the real-world experience with nivolumab in 58 patients with NSCLC of any histological subtype, who have received ≥2 prior therapies, the median OS was lower than that found in the present study (11.7 months vs. 18.6 months). Of the patients, 46.6% were alive at the time of data cut-off. No difference was determined between 1 vs. >1 prior lines of therapy in terms of OS.Citation23
In two studies, despite shorter PFS, favorable OS benefit was reported with nivolumab therapy during median 17 to18-month follow-up period.Citation7,Citation24 No statistically significant differences were detected between sexes, according to age groups, histological subtypes or number of prior treatment lines.Citation7
Smoking is the major risk factor and related to 80–90% of deaths from lung cancer.Citation25 In Türkiye, smoking is responsible for 90% of lung cancer cases.Citation26 Nishio et al.Citation27 reported better treatment response with 3 mg/kg nivolumab therapy after failure with platinum-containing chemotherapy in current/former smokers vs. nonsmokers with advanced or recurrent non-squamous NSCLC. Similarly, Morita et al.Citation22 reported that smoking is associated with better PFS. However, the present study found no significant correlation of OS or PFS with smoking status although OS seemed to be improved, which was consistent with that reported by Areses Manrique et al.Citation7 In two studies, each of PR, SD and PD were reported in around one-third of the patients with NSCLC, with only 1.4%Citation24 and 1.6%Citation7 showing CR. In another study, 14.5% of the patients with advanced, refractory, squamous NSCLC showed objective response (assessed by an independent radiology review committee) and 26% had SD, supporting the efficacy of nivolumab reported in randomized, controlled, phase 3 studies of 1L and 2L treatment.Citation28 In the present study, however, ORR was higher by half (31.2%), but the rate of SD was lower by half (13.8%). These differences might be due to the differences in samples sizes, nivolumab doses and/or study designs.
Safety
In the literature, the frequency of observing at least one AE during nivolumab therapy were reported at a rate between 58% and 84%.Citation7,Citation17,Citation24,Citation27,Citation28 Nevertheless, most of the AEs were low-grade AEs with discontinuation of the nivolumab due to treatment-related AEs being as low as <5%.Citation7,Citation17 In our study, the frequency of at least one AE during nivolumab therapy was found to be lower (38.9%) probably due to its retrospective nature. Fatigue has been reported as the most common AE during the nivolumab therapy ranging from 16% to 33%.Citation7,Citation17,Citation24,Citation28 Similarly, we also found fatigue as the most common AE (16.4%) in our study population.
As in many retrospective studies, this study also has some limitations. First, there were missing data in the patients’ medical records, which may limit the interpretation of the analyses. Second, duration of follow-ups showed significant variability among patients, leading to a difficulty in interpreting the outcomes of interest. In addition, the safety and efficacy of nivolumab were not compared between the histological subtypes. Therefore, it is difficult to draw a definite and generalizable conclusion from our results. Nevertheless, this is the first study demonstrating safety and efficacy of nivolumab in patients with NSCLC in a real-life setting in Türkiye, contributing to the world-wide limited data from real-life.
Conclusion
Our results are consistent with earlier studies and showed that nivolumab was a safe and effective agent to be used in the 2L or LL treatment of patients with locally advanced or metastatic NSCLC after failure with chemotherapy or systemic therapy regardless of the number of prior therapies, and that it is associated with acceptable AEs. Further prospective studies investigating all checkpoint inhibitors, as well as switching between two different checkpoint inhibitors, in larger cohorts are required.
Transparency
Declaration of financial/other relationships
Huseyin Abali has received lecture and meeting support from BMS and participated in Advisory Board for BMS. Perran Fulden Yumuk has participated in Advisory Board for Amgen, GSK, BMS, Roche, and Pfizer and has received support for attending meetings and/or travel from Astra Zeneca, BMS, and Roche and payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Amgen. Mustafa Ozguroglu has received consulting fees from Astellas, Regeneron, Sanofi; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Regeneron; payment for expert testimony from Astra Zeneca, support for attending meetings and/or travel from Regeneron; has participated in a Data Safety Monitoring Board or Advisory Board for MSD; have taken part in Steering Board for Astra Zeneca and Bayer. Saadettin Kilickap has received support for travel from Regeneron. All other authors have declared no conflicts of interest. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Nuri Karadurmus: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Roles/Writing - original draft, Writing - review & editing; Muhammet Ali Kaplan: Investigation, Writing - review & editing; Mehmet Ali Nahit Sendur: Investigation, Writing - review & editing; Yuksel Urun: Investigation, Writing - review & editing; Umut Demirci: Investigation, Writing - review & editing; Saziye Burcak Karaca: Investigation, Writing - review & editing; Sabin Goktas Aydin: Investigation, Writing - review & editing; Baris Aykan: Investigation, Writing - review & editing; Ahmet Bilici: Investigation, Writing - review & editing; Ahmet Sezer: Investigation, Writing - review & editing; Ulku Yilmaz: Investigation, Writing - review & editing; Huseyin Abali: Investigation, Writing - review & editing; Perran Fulden Yumuk: Investigation, Writing - review & editing; Serkan Degirmencioglu: Investigation, Writing - review & editing; Ahmet Demirkazik: Investigation, Writing - review & editing; Semra Paydas: Investigation, Writing - review & editing; Cem Mirili: Investigation, Writing - review & editing; Hande Turna: Investigation, Writing - review & editing; Aysegul Kargi: Investigation, Writing - review & editing; Mustafa Ozdogan: Investigation, Writing - review & editing; Deniz Can Guven: Investigation, Writing - review & editing; Mustafa Ozguroglu: Investigation, Writing - review & editing; Saadettin Kilickap: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Roles/Writing - original draft, Writing - review & editing.
Supplemental Material
Download MS Word (23 KB)Supplemental Material
Download PDF (670.4 KB)Supplemental Material
Download PDF (707.3 KB)Supplemental Material
Download PDF (652.7 KB)Supplemental Material
Download PDF (750.6 KB)Supplemental Material
Download PDF (683.5 KB)Supplemental Material
Download PDF (660.4 KB)Acknowledgements
None.
Additional information
Funding
References
- World Health Organization: Cancer [Internet]. WHO. c2023 [cited 2022 Sep 13]. Available from: https://www.who.int/news-room/fact-sheets/detail/cancer
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 world health organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243–1260. doi:10.1097/JTO.0000000000000630.
- Travis WD. Pathology of lung cancer. Clin Chest Med. 2011;32(4):669–692. doi:10.1016/j.ccm.2011.08.005.
- Cersosimo RJ. Lung cancer: a review. Am J Health Syst Pharm. 2002;59(7):611–642. doi:10.1093/ajhp/59.7.611.
- Goksel T, Eser S, Guclu S, et al. Prognostic factors affecting survival in cases with lung cancer [a lung cancer mapping project in Turkey (LCMPT)]. Eur Respir J. 2013;42(Suppl 57):2920.
- Gridelli C, Peters S, Mok T, et al. First-line immunotherapy in advanced non-small-cell lung cancer patients with ECOG performance status 2: results of an international expert panel meeting by the Italian association of thoracic oncology. ESMO Open. 2022;7(1):100355. doi:10.1016/j.esmoop.2021.100355.
- Areses Manrique MC, Mosquera Martínez J, García González J, et al. Real world data of nivolumab for previously treated non-small cell lung cancer patients: a Galician lung cancer group clinical experience. Transl Lung Cancer Res. 2018;7(3):404–415. doi:10.21037/tlcr.2018.04.03.
- Ezeife DA, Leighl NB. Personalized medicine for non-small cell lung cancer: where are we now and where can we go? Expert Rev Respir Med. 2018;12(2):81–82. doi:10.1080/17476348.2018.1411805.
- Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33(17):1974–1982. doi:10.1200/JCO.2014.59.4358.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi:10.1038/nrc3239.
- Raju S, Joseph R, Sehgal S. Review of checkpoint immunotherapy for the management of non-small cell lung cancer. Immunotargets Ther. 2018;7:63–75. doi:10.2147/ITT.S125070.
- Kazandjian D, Suzman DL, Blumenthal G, et al. FDA approval summary: nivolumab for the treatment of metastatic non-small cell lung cancer with progression on or after platinum-based chemotherapy. Oncologist. 2016;21(5):634–642. doi:10.1634/theoncologist.2015-0507.
- TC. Sağlık Bakanlığı Türkiye İlaç ve Tıbbi Cihaz Kurumu: Kısa Ürün Bilgisi [Internet]. Ankara: TİTCK [cited 2023 Jul 13]. Available from: https://titck.gov.tr/storage/Archive/2022/kubKtAttachments/TTCKOnaylKBOPDIVO40MG_d3b2004d-15f5-4052-a983-9c83bf3f0ce2.pdf.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. Erratum in: CA Cancer J Clin. 2021;71(4):359. doi:10.3322/caac.21654.
- Armoiry X, Tsertsvadze A, Connock M, et al. Comparative efficacy and safety of licensed treatments for previously treated non-small cell lung cancer: a systematic review and network meta-analysis. PLoS One. 2018;13(7):e0199575. doi:10.1371/journal.pone.0199575.
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi:10.1056/NEJMoa1507643.
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi:10.1056/NEJMoa1504627.
- Vokes EE, Ready N, Felip E, et al. Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol. 2018;29(4):959–965. doi:10.1093/annonc/mdy041.
- Horinouchi H, Nishio M, Hida T, et al. Three-year follow-up results from phase II studies of nivolumab in Japanese patients with previously treated advanced non-small cell lung cancer: pooled analysis of ONO-4538-05 and ONO-4538-06 studies. Cancer Med. 2019;8(11):5183–5193. doi:10.1002/cam4.2411.
- Dudnik E, Moskovitz M, Daher S, et al. Effectiveness and safety of nivolumab in advanced non-small cell lung cancer: the real-life data. Lung Cancer. 2018;126:217–223. doi:10.1016/j.lungcan.2017.11.015.
- Gettinger SN, Horn L, Gandhi L, et al. Long-term survival, clinical activity, and safety of nivolumab (anti-PD-1; BMS-936558, ONO-4538) in patients (pts) with advanced non-small cell lung cancer (NSCLC) metastatic non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2014;90(5):S34. doi:10.1016/j.ijrobp.2014.08.209.
- Morita R, Okishio K, Shimizu J, et al. Real-world effectiveness and safety of nivolumab in patients with non-small cell lung cancer: a multicenter retrospective observational study in Japan. Lung Cancer. 2020;140:8–18. doi:10.1016/j.lungcan.2019.11.014.
- Brustugun OT, Sprauten M, Helland Å. Real-world data on nivolumab treatment of non-small cell lung cancer. Acta Oncol. 2017;56(3):438–440. doi:10.1080/0284186X.2016.1253865.
- Figueiredo A, Almeida MA, Almodovar MT, et al. Real-world data from the Portuguese nivolumab expanded access program (EAP) in previously treated non small cell lung cancer (NSCLC). Pulmonology. 2020;26(1):10–17. doi:10.1016/j.pulmoe.2019.06.001.
- Centers for Disease Control and Prevention. What are the risk factors for lung cancer? [Internet] Atlanta (GA): CDC [cited 2022 Sep10]. Available from: https://www.cdc.gov/cancer/lung/basic_info/risk_factors.htm.
- T.C. Sağlık Bakanlığı Türkiye Halk Sağlığı Kurumu: Ulusal Kanser Kontrol Planı 2013 – 2018 [Internet]. Ankara: Halk Sağlığı Genel Müdürlüğü; c2023 [cited 2022 Sep 10]. Available from: https://www.iccp-portal.org/system/files/plans/Ulusal_Kanser_Kontrol_Plani_2013_2018.pdf
- Nishio M, Hida T, Atagi S, et al. Multicentre phase II study of nivolumab in japanese patients with advanced or recurrent non-squamous non-small cell lung cancer. ESMO Open. 2016;1(4):e000108. doi:10.1136/esmoopen-2016-000108.
- Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257–265. doi:10.1016/S1470-2045(15)70054-9.
