Abstract
Objective
To explore real-life use of the extemporaneous combination of nebivolol and valsartan (NV-EXC) in adult hypertensive patients in Europe.
Methods
Retrospective analysis of patients starting NV-EXC treatment conducted using prescription databases in Italy, Germany, Hungary, and Poland. The selection period during which study patients were identified covered a time span ranging from 3 to 9 years (until 30 June 2020) according to availability of the different data sources. Patient demographics, clinical information, and treatment adherence, measured by proportion of days covered, were evaluated. Additionally, the potential eligibility of Italian patients for the single pill combination (SPC) of nebivolol and valsartan over a one-year period was estimated.
Results
The study included 170,682 patients initiating NV-EXC across the databases. Most patients were females (from 51 to 60%) and primarily aged over 60 years. Few patients received prescriptions of both available dosages of valsartan (80 and 160 mg) during follow-up (from 3.2 to 8.5%). Common comorbidities included dyslipidemia (19.2%) and diabetes (19.1%). Around 59.5% of patients did not require cardiologic visits during the study period. Adherence to NV-EXC, as indicated by the Italian database, was low in 53.3% of patients, with only 16.1% showing high adherence. The Italian database revealed 680 prevalent NV-EXC users in 2019, estimating a potential 30,222 adult patients eligible for the nebivolol/valsartan SPC.
Conclusions
The combination of nebivolol and valsartan is frequently prescribed for hypertension, but adherence remains a challenge. A potential nebivolol/valsartan SPC holds promise in enhancing adherence and optimizing therapeutic outcomes for hypertension management.
Introduction
Hypertension is a significant issue for public health with over 1 billion people affected worldwideCitation1. In Italy, the prevalence of hypertension spans from 55 to 59% of the adult populationCitation2,Citation3. The substantial impact of high blood pressure on public health mainly depends on its high prevalence and unsatisfactory control in a large proportion of hypertensive patientsCitation4. Even with the notable advances in treatment of hypertension and the beneficial impact on cardiovascular results, the use of medications is frequently suboptimal, leading to inadequate blood pressure management in a significant portion of patientsCitation5. In addition, lack of adherence to antihypertensive treatment, which is very common in primary care, since it strongly influences control of blood pressure, has a relevant impact on the cardiovascular risk and on the financial burden sustained by healthcare systemsCitation5. This situation underscores a pressing need for innovative strategies that not only enhance therapeutic efficacy but also improve patient adherence. In order to achieve adequate control of blood pressure the large majority of patients with hypertension require 2 or 3 antihypertensive drugs. One of the measures introduced to maximize the benefits of these therapies was the development of single-pill combinations (SPCs) of drugs with complementary mechanisms of actionCitation6. Indeed, the use of SPCs allows for simplification of the treatment strategy for patients, with likely improvement of pharmacological adherenceCitation6. Among currently available antihypertensive treatments, β-blockers and angiotensin receptor blockers (ARBs) are supported by a substantial amount of evidence in terms of blood pressure lowering effects and cardiovascular protection.
β-Blockers are a heterogeneous drug class including agents with vasodilatory activity. Nebivolol is a third-generation, long-acting, and highly selective β1-adrenergic receptor antagonist, approved for the treatment of hypertension in the US and for hypertension and chronic heart failure in EuropeCitation7. Nebivolol has a unique pharmacological profile compared to other drugs in its class. In fact, while sharing cardioselectivity mediated via β1 receptor blockade, it also induces vasodilation via nitric oxide (NO) by activating endothelial NO synthase through β3 agonismCitation7. Research on nebivolol has demonstrated its beneficial impact on central blood pressure, aortic stiffness, and endothelial dysfunctionCitation7,Citation8.
Valsartan, an early member of the ARBs pharmacological class, has been used since 1996 in Europe and in the US since 1997. Valsartan is a nonpeptide angiotensin receptor antagonist that selectively blocks the binding of angiotensin II to the angiotensin II type 1 receptorCitation9,Citation10. Experimentally, valsartan inhibits the vasoconstriction induced by angiotensin II in a dose dependent manner and lowers blood pressure in renin-dependent models of hypertensionCitation9,Citation10. The efficacy, tolerability, and safety of valsartan have been shown in several dose-finding and large-scale comparative studies in patients with mild to moderate hypertensionCitation9,Citation10.
One possible combination of a β-blocker and ARB is nebivolol with valsartan. Clinical evidence and pharmacological data suggest that nebivolol and valsartan combination offers clear benefit, bringing together β1-adrenoceptor and angiotensin II type 1 receptor (AT1) receptor blockade with β3 receptor activationCitation11,Citation12. The combination thus results in an increase in nitric oxide with subsequent vasodilation. An SPC of nebivolol and valsartan has been evaluated in an 8-week, phase 3, multicenter, randomized, double-blind, placebo-controlled, parallel-group study on adult patients with hypertensionCitation13. The study demonstrated the efficacy and tolerability of the SPC nebivolol and valsartan which is therefore a good option for patients with hypertension. However, an SPC of nebivolol and valsartan is not currently marketed in Europe. This gap in availability leads to the utilization of an extemporaneous combination of nebivolol and valsartan, administered as separate pills, a practice that has yet to be thoroughly explored in the context of real-world clinical settings across Europe. Existing literature predominantly focuses on the clinical trial data of SPCs or examines the use of single antihypertensive agents. However, there is a notable lack of comprehensive research assessing the real-world effectiveness, adherence, and overall impact of the extemporaneous combination of nebivolol and valsartan on hypertension management across diverse European populations.
To provide insights on the use of nebivolol and valsartan together as separate pills (i.e. extemporaneous combination), in common clinical practice in four European countries, we carried out a study based on real-world evidence. Patients initiating the extemporaneous combination of nebivolol and valsartan (NV-EXC) for the treatment of hypertension were evaluated in terms of demographics, clinical data, and adherence to treatment.
Materials and methods
Data source
The sources of data for this retrospective observational cohort study were the Italian IQVIA Longitudinal Patient Database (LPD), the Italian IQVIA LRx Database, the German IQVIA LRx Database, the Polish IQVIA LRx Database, and the Hungarian National Hospital Insurance Fund (NHIF) Database. The Italian IQVIA LPD gathers data from physician consultations (including diagnoses and prescriptions categorized by the International Classification of Diseases 9th revision–ICD-9, and the Anatomical Therapeutic and Chemical–ATC classification system). Additionally, it compiles medical and demographic details, creating an inclusive representation of the regular healthcare provided by general practitioners (GPs) in Italy. The database includes data routinely collected by around 900 GPs from about 1.2 million patients. The IQVIA Italian LPD is a reliable source of information for hypertension and other diseases, as previously shownCitation14.
IQVIA Lrx databases gather information on prescriptions at the patient level, obtained from retail pharmacy computers (excluding hospital-based pharmacies). These databases use an anonymous patient ID to monitor prescription activity over time. A key benefit of these prescription databases is their extensive coverage of specialty physicians and pharmacies. Drug utilization studies, based on this longitudinal prescription data, have been conducted worldwide in the past.
The financial database of the Hungarian NHIF contains detailed data from the entire Hungarian population and includes all patient data of state-funded services since 2000, information on pharmaceuticals, health spas, and suppliers for patient transport. This database is also used for scientific research purposes.
Cohorts definition
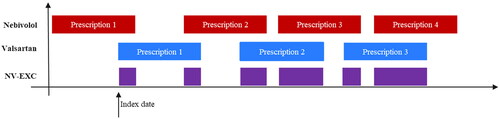
All patients who initiated treatment with NV-EXC of “5 mg nebivolol” (ATC code C07AB12) and “80 mg/160 mg valsartan” (ATC code C09CA03) within the study timeframe were included. For the Italian IQVIA LPD database, the selection period (i.e. the period of time during which study patients were identified) spanned from 1 July 2011, to 30 June 2020, while for other databases, it was from 1 July 2017, to 30 June 2020. The date of the first extemporaneous combination taken within this selection period was considered as the index date (). The index date is given by the initial prescription of the second component, provided it coincided with the duration of the first component’s prescription during the selection phaseCitation15. To identify prevalent users of NV-EXC, patients without a hypertension diagnosis (ICD-9 codes 401.xx and 402.xx) in the 12-month period before the index date (only for Italian IQVIA LPD) and underage patients (with a cut-off based on data source) were excluded. Furthermore, incident users of NV-EXC were defined by excluding patients with a prior NV-EXC prescription in the 6 months preceding the index date. The cohort of NV-EXC incident users identified was subsequently analyzed for demographic features, clinical profile, and adherence to treatment.
Figure 1. Visual representation of the extemporaneous combination of nebivolol and valsartan (NV-EXC) and of index date.
To provide an estimate of the number of adult hypertensive patients potentially eligible for the SPC of nebivolol and valsartan in overall adult population in Italy over a one-year period, we focused on the year 2019 since COVID-19 pandemic influenced diagnosis, prescription habits, and initiation of new therapies. Considering patients >18 years with at least one prescription of NV-EXC during 2019, and the total sample of >18 year active patients with a diagnosis of hypertension within the Italian IQVIA LPD database during 2019 (around 360,000 patients) and the Italian adult hypertensive population (around 16 million patients) according to the Società Italiana dell’Ipertensione Arteriosa (SIIA) and Istituto Nazionale di Statistica (ISTAT), we found the number of potentially eligible patients for the SPC of nebivolol and valsartan at the national level over a one-year periodCitation15,Citation16.
Study definitions
Information regarding NV-EXC incident users (age, sex, body mass index, comorbidities, concomitant pharmacological treatments, and cardiologic visits referrals) were extracted from the IQVIA Italian LPD while only age and sex were retrieved for the other databases. For each patient, the pre-selection period was defined as the six-month period preceding the Index Date, while the follow-up period started at the Index Date and lasted until six months from the Index Date. Comorbidities and co-prescriptions of interest were defined according to Volpe et al.Citation14. Comorbidities were searched during the pre-selection period using the recording of the corresponding ICD-9-CM code and co-prescriptions were searched during both the pre-selection period and the follow-up period separately using the recording of at least one prescription of the corresponding ATC code.
The degree to which patients take the medication following GP prescription is defined as adherence to therapy. Adherence was evaluated using the Proportion of Days Covered (PDC), which involves calculating the total days of medication supplied throughout the corresponding follow-up period. This measurement aligns with the criteria established by the Pharmacy Quality Alliance, as previously definedCitation15,Citation17. When multiple medications are used, PDC has been shown to be a more conservative assessment of adherence compared to other indicators. The number of days supplied by each prescription was obtained by dividing the total amount of active drug in each prescription by the recommended defined daily dose for nebivolol (i.e. 5 mg) and the dose of the tablets of the prescribed package for valsartan (i.e. if a patient was prescribed a pack of valsartan with tablets equal to 80 mg the patient was assumed to take 80 mg per day and, similarly, if a patient was prescribed a pack of valsartan with tablets equal to 160 mg the patient was assumed to take 160 mg per day). Days of supply contributed to the numerator only when nebivolol and valsartan overlapped.
Furthermore, categorizing incident NV-EXC users based on the prescribed dosage of valsartan (80 or 160 mg) during a 6-month follow-up allowed for the determination of the count of patients on each specific dosage and the identification of those who transitioned to an alternative dosage.
Statistical analysis
Analysis was performed as previously describedCitation14. Briefly, demographic and clinical features of the population and a summary of treatment adherence for incident users of NV-EXC are presented with descriptive statistics. Qualitative variables are described in terms of frequencies and percentages, whereas quantitative variables are reported as mean values, standard deviation (SD), median, and first and third quartiles (Q1 and Q3). Adherence to treatment was deemed low when PDC was <40%, intermediate if the PDC was between 40 and 79%, and high if the PDC was at least 80%. SAS software version 9.4 was used for statistical analyses on anonymized data.
Sensitivity analysis
In addition to the NV-EXC cohorts, patients receiving NV-EXC prescribed as either single molecule (ATC codes C07AB12 for nebivolol and C09CA03 for valsartan) or as SPC containing hydrochlorothiazide (ATC codes C07BB12 for nebivolol and C09DA03 for valsartan) were also analyzed to investigate possible differences. Furthermore, this additional information provides a broader picture of the well-established use of the extemporaneous combination in current practice, although patients receiving the SPC containing hydrochlorothiazide are not likely to be the target population for a potential nebivolol/valsartan SPC.
Results
Study populations and demographic characteristics (all data sources)
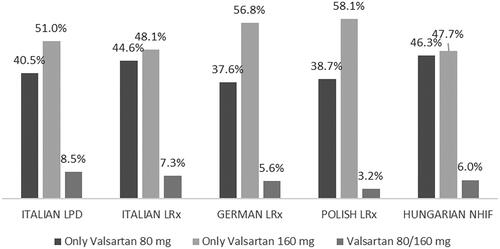
shows attritions for inclusion in the cohorts of prevalent and incident users of NV-EXC. Over the period investigated, a total of 2,390 patients in the Italian LPD, 40,188 in the Italian LRx, 32,326 in the German LRx, 63,863 in the Polish LRx, and 31,915 in the Hungarian NHIF started treatment with NV-EXC. Considering the incident users of NV-EXC, from 37.6% (German LRx) to 46.3% (Hungarian NHIF) of patients were prescribed only 80 mg valsartan, while from 47.7% (Hungarian NHIF) to 58.1% (Polish LRx) of the patients were prescribed only 160 mg valsartan over the 6-month follow-up period. A small proportion of patients (from 3.2% in Polish LRx to 8.5% in Italian LPD) switched product dose during the period investigated ().
Table 1. Patient attrition table for inclusion in the cohort of prevalent and incident users of the extemporaneous combination (NV-EXC).
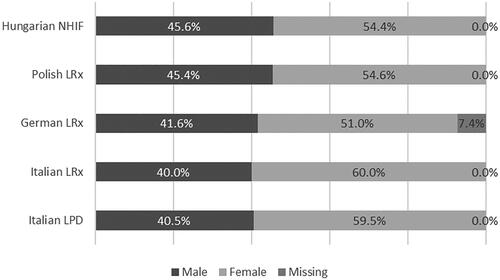
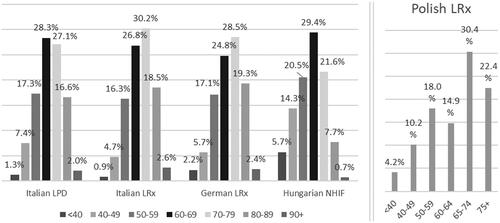
Women represented between 51.0% (German LRx) to 60.0% (Italian LRx) of the population (). Moreover, around three-quarters of patients aged more than 60 years, with a slightly younger population found in Polish LRx and Hungarian NHIF ().
National-level estimate of patients eligible for the SPC (IQVIA Italian LPD)
In the Italian LPD, there were 680 prevalent users of NV-EXC identified in the database during 2019. The number of prevalent users allowed to estimate 30,222 adult patients (≥18 years old) treated in Italy with NV-EXC in 2019. These are the patients potentially eligible for the SPC of nebivolol and valsartan.
Clinical characteristics (IQVIA Italian LPD)
Mean BMI value was 28.6 kg/m2 (±5.3 SD) considering patients reporting this information. Focusing on BMI classes, overweight and obese accounted for 38.3 and 35.1% of patients, respectively (). The most common comorbidity was dyslipidemia (19.2%), followed by diabetes mellitus (19.1%), and cardiac dysrhythmias (9.5%) (). Other comorbidities reported in at least 3% of the cohort included other forms of chronic ischemic heart disease, gout, chronic kidney disease, acquired hypothyroidism, occlusion and stenosis of precerebral arteries, nontoxic nodular goiter, and asthma ().
Table 2. Clinical characteristics of incident users of the extemporaneous combination of nebivolol and valsartan (NV-EXC) – Italian LPD.
The most frequently co-prescribed drugs recorded during the 6-month period before starting the extemporaneous combination were agents acting on the renin-angiotensin system other than valsartan (48%), followed by antithrombotics (37.2%), lipid-lowering agents (33.0%), calcium-channel blockers (26.6%), diuretics (23.2%), and nonsteroidal anti-inflammatory drugs (NSAIDs) (23.2%). Of note, around 70% of patients had received at least 2 co-prescriptions during pre-selection period ().
The most frequently co-prescribed drugs recorded during the 6-month period after the index date were antithrombotics (42.9%), followed by lipid-lowering agents (38.1%), agents acting on the renin-angiotensin system other than valsartan (29.8%), diuretics (29.1%), calcium-channel blockers (24.3%) and nonsteroidal anti-inflammatory drugs (23.9%). Nearly 70% of the patients had received at least 2 co-prescriptions during follow-up period ().
Stratification of patients by the presence/absence of cardiologic visits requests during pre-selection and follow-up period revealed that almost 60% of patients did not have any requests during the study period. During the 6-month period following the initiation of treatment with NV-EXC, the percentage of patients who requested at least one cardiologic visit was lower than the one observed during the baseline period (13.3 versus 18.8%) ().
Adherence to treatment (IQVIA Italian LPD)
Mean PDC, which is the proportion of days effectively covered by the extemporaneous combination, was 42.6% (±29.0 SD) from the Italian LPD, meaning that patients on average were taking both two agents for 77 days of 180 days (i.e. follow-up duration) (). During the 6-month period after the index date, more than half of patients treated with NV-EXC (53.3%) were low adherent (PDC <40%) to therapy, whereas 30.6% had a 40% ≤ PDC < 80%, and 16.1% a PDC >80% ().
Table 3. Treatment adherence of incident users of the extemporaneous combination (NV-EXC) – Italian LPD.
Sensitivity analysis
After evaluation of prescriptions of nebivolol and valsartan as SPCs with hydrochlorothiazide, in addition to single-molecule prescriptions, a total of 4,359 patients meeting the inclusion criteria for the cohort of incident users of NV-EXC on Italian LPD were identified (Supplementary material). Results obtained from analyzing this cohort were similar to those obtained from incident users of NV-EXC considering only nebivolol and valsartan single molecules (data not displayed but obtainable on request).
Discussion
Current evidence strongly suggests that the combination of nebivolol and valsartan provides definite clinical benefitCitation11. Nebivolol is dissimilar to other β-blockers since it activates the β3-adrenoceptor to increase endothelial NO and produce vasodilation; at the same time, it strongly blocks the β1-adrenoceptor. In addition, due to its anti-inflammatory properties nebivolol has positive effects on endothelial dysfunction and vascular remodelingCitation11. By selectively blocking the angiotensin AT1-receptor, valsartan reduces angiotensin II-induced vasoconstriction and the production of aldosterone. Additionally, valsartan can decrease the production of reactive oxygen speciesCitation11. The distinct mechanisms of action through which nebivolol and valsartan exert their action, impacting endothelial function, β3-adrenoreceptor activation, inhibiting the renin-angiotensin-aldosterone system and oxidative stress highlighted by the results of clinical trials, provided a strong foundation for the development of this SPC by the US FDA in 2007Citation18. Other studies are available outside US analyzing the effectiveness of a SPC of nebivolol/valsartan in the effective treatment lowering peripheral and central blood pressure in patients with hypertensionCitation19. Thus, there is strong rationale for the use of the combination of nebivolol and valsartan given their complementary actions on hypertension. Despite this evidence, few studies report evidence from real-life, and no data are available across the European area.
The present study based on real-world data from prescribing databases in four European countries provides evidence that co-prescription of NV-EXC is well consolidated in daily practice. As can be expected, the combination was prescribed predominately in patients over 50 years of age. On average, a slightly higher percentage of female patients among those initiating the extemporaneous combination was found in Italy than in the other countries.
Across countries, the prescribed doses of valsartan were rather similar with slightly more patients being prescribed 160 mg. Moreover, a large proportion of patients were frequently co-prescribed other drugs. While the high percentage of patients receiving polypharmacy can be expected given the frequent comorbidities in an ageing population as seen in the cohort studied herein, it also indicates that prescribers feel confident in administering a wide range of agents to patients with hypertension, with the combination of nebivolol and valsartan being a common choice. Nebivolol/valsartan has been demonstrated to be partially additive, with comparable results with respect to other non-β-blocker/ARB inhibitor SPCs. This challenges the previous assumption that β-blocker/ARB inhibitor combinations are less effective due to overlapping mechanisms, and supports the clinical utility of nebivolol/valsartan SPCsCitation20.
Nebivolol/valsartan cold be the appropriate choice for a wide range of patient population, including both obese and nonobese patientsCitation21, justifying its use in a cohort composed by a high percentage of overweight and obese patients, as reported for the Italian cohort.
Unfortunately, but in line with previous observationsCitation5, overall adherence to therapy was low. In fact, more than half of patients treated with NV-EXC were low adherent to therapy, around one-third were intermediate adherent while only 16% were high adherent. Despite not providing a direct proof of increased adherence with the use of a SPC, this evidence emphasizes that an SPC of nebivolol and valsartan may have the potential to improve adherence to therapy by simplifying treatment and reducing the pill burden, especially in light of the high polypharmacy characterizing this populationCitation22. Better adherence has the potential to reduce cardiovascular riskCitation5, a relevant observation also in consideration that dyslipidemia and diabetes mellitus were present in about 20% of patients. The latter diseases, together with hypertension, places them at higher risk for cardiovascular events as previously observedCitation23.
In a real-life study in patients with hypertension who were poorly adherent to individual drug combination, the switch to an SPC substantially improved adherence to antihypertensive treatmentCitation24. This is a central issue since even relatively modest changes in adherence may lead to clinically significant reductions in hypertensionCitation25. To achieve long-lasting control of hypertension the use of SPCs is among the suggested actionsCitation26. The guidelines from 2013 by ESH/ESC outlined the rationale for the use of SPCs, aiming to reduce the number of daily pills while enhancing blood pressure managementCitation27. The ESC/ESH guidelines from 2018 emphasized and endorsed the integration of SPCs into routine clinical approachesCitation6. The International Society of Hypertension global practice guidelines also promote the use of SPCs with the aim to improve adherence to antihypertensive therapyCitation28. Other recommendations such as those from Hypertension Canada organization and the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA consensus guidelines also favor the adoption of SPCsCitation29,Citation30. Thus, there appears to be broad consensus that SPCs should be routinely prescribed to manage patients with hypertension in routine practice, especially in light of the fact that 25–35% of patients will require more than two antihypertensive agents to achieve control of hypertension, with one-third of patients needing three or moreCitation31.
Limitations
A general limitation, which is intrinsic of the use of electronic medical records like IQVIA Italian LPD is that findings rely on the accuracy of the information recorded, that is not ad-hoc collected for the study objectives, and is subject to potential for recording/coding errors or biasCitation32. Another limitation, characteristic of real-world studies, is that only data on written/dispensed prescriptions were available. Our evaluation assumed that any written or dispensed prescription was actually taken by patients. However, given that this is not likely to be the case for all prescriptions, our analysis may have overestimated the rates of true adherence.
Moreover, Italian IQVIA LPD does not account for drug prescriptions when prescribed by physicians working in the private sector. However, it should be noted that antihypertensive drugs are completely reimbursed by the National Healthcare System when prescribed by GPs. Thus, our estimates should be considered accurate in this regardCitation4.
Regarding the number of patients requesting cardiology appointments, it should be noted that those might also be lower than actual numbers, as visits performed in the private healthcare settings do not require a written prescription by the GP. Finally, European IQVIA LRx and Hungarian NHIF does not include information on patients’ diagnoses, thus it was not possible to select patients based on the presence of a registered hypertension diagnosis. However, the low proportion (11%) of patients treated with the NV-EXC without a diagnosis of hypertension found in the Italian IQVIA LPD, allowed the authors to assume that the majority of patients included in the analysis performed on European countries were hypertensive.
In addition, the study does not address therapy-resistant hypertension which is “the failure to achieve target blood pressure despite being treated with maximal doses of three antihypertensive drugs, including a diuretic”Citation33. This is a different condition from uncontrolled hypertension because patients with therapy-resistant hypertension might still achieve target blood pressure levels upon therapy with more medications. In patients experiencing therapy-resistant hypertension a more comprehensive patient-centered approach from healthcare professionals is warranted, to actively involve patients in the decision-making process regarding their hypertension management. This includes educating patients about their condition, discussing treatment options, and addressing any concerns or barriers to adherenceCitation34.
The large number of patients included in the databases and the fact that similar findings were seen in different countries are indeed a strength that suggest the validity of our approach. To our current knowledge, this is the first real-world pan-European analysis on current prescribing behaviors for management of patients with hypertension and treated with nebivolol and valsartan. The databases used provide information on a large number of patients, who can be considered as representative of the general population. This allowed for detailed clinical characterization of these patients in a real-world setting. The findings of this study provide some practical implication in the clinical management of hypertension. Indeed, the possible development of a SPC of nebivolol/valsartan would be beneficial for: (i) patients already on the treatment, with potential improvement in their adherence; (ii) healthcare providers, who could have a wider array of efficacious therapeutic options to prescribe; (iii) policymakers, as the consideration of the number of patients under nebivolol/valsartan therapy and the potential improvement in adherence could be the base for cost effectiveness evaluation of the long-term therapy.
Conclusions
Our analysis suggests that the administration of the extemporaneous combination of nebivolol and valsartan is a common practice in several European countries including Italy, Germany, Hungary, and Poland. Patients receiving this combination likely had multiple comorbidities and received concomitant medications. However, regrettably, large proportions of patients are not adherent to antihypertensive therapy. SPCs may represent a valid option in clinical practice to simplify treatment regimens, as it is expected to minimize low adherence, and likely enhance the overall therapeutic goals of antihypertensive therapy. Considering those potential advantages, our results emphasize that a possible development of SPC of nebivolol and valsartan might have clinical utility for both prescribers and patients.
Transparency
Declaration of funding
This study was funded by Menarini.
Declaration of financial/other relationships
R.C., V.P. have disclosed that they are employees of IQVIA. C.R. worked for IQVIA as an external consultant. G.D. reported personal fees from Menarini Corporate, Bayer, Servier and AlfaSigma. M.M., S.M., M.G., P.F. declare to be employees of Menarini. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors contributed to the study conception and design. Data extraction and analysis were performed by Riccardo Cipelli, Claudio Ripellino, and Valeria Pegoraro, who also wrote the first draft of the manuscript. All authors commented on previous versions of the manuscript. All authors take responsibility for the integrity of the work as a whole and have given final approval for the version to be submitted.
Acknowledgements
Editorial support, funded by Menarini, was provided by Patrick Moore on behalf of Health, Publishing and Services s.r.l., according to Good Publication Practice.
Ethics statement
All the analyses presented here did not involve any new study of human or animal subjects performed by any of the authors. The databases used for this study includes only anonymized data in compliance with the provisions set forth in the applicable data protection laws. Italian, German, Polish, and Hungarian laws allow the use of anonymous electronic medical records for research purposes under certain conditions. In accordance with this legislation, it is not necessary to obtain informed consent from patients or approval from a medical ethics committee for this type of observational study that contains no directly identifiable data. Since all patients were only queried as aggregates and no protected health information was available for queries, no institutional review board approval was required for the use of these databases or the completion of this study in line with previous works.
Manuscript Nebivolol_Valsartan_supplementary material.docx
Download MS Word (76.4 KB)References
- World Health Organization. High blood pressure: a public health problem [cited 2022 Jun 1]. Available from: http://www.emro.who.int/media/world-health-day/public-health-problem-factsheet-2013.html.
- Tocci G, Muiesan ML, Parati G, et al. Trends in prevalence, awareness, treatment, and control of blood pressure recorded from 2004 to 2014 during world hypertension day in Italy. J Clin Hypertens. 2016;18(6):551–556.
- Tocci G, Nati G, Cricelli C, et al. Prevalence and control of hypertension in the general practice in Italy: updated analysis of a large database. J Hum Hypertens. 2017;31(4):258–262. doi: 10.1038/jhh.2016.71.
- Levi M, Pasqua A, Cricelli I, et al. Patient adherence to olmesartan/amlodipine combinations: fixed versus extemporaneous combinations. J Manag Care Spec Pharm. 2016;22(3):255–262.
- Mennini FS, Marcellusi A, von der Schulenburg JMG, et al. Cost of poor adherence to anti-hypertensive therapy in five european countries. Eur J Health Econ. 2015;16(1):65–72. doi: 10.1007/s10198-013-0554-4.
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104. doi: 10.1093/eurheartj/ehy339.
- Münzel T, Gori T. Nebivolol: the somewhat-different beta-adrenergic receptor blocker. J Am Coll Cardiol. 2009;54(16):1491–1499. doi: 10.1016/j.jacc.2009.05.066.
- Hayek SS, Poole JC, Neuman R, et al. Differential effects of nebivolol and metoprolol on arterial stiffness, circulating progenitor cells, and oxidative stress. J Am Soc Hypertens. 2015;9(3):206–213.
- Black HR, Bailey J, Zappe D, et al. Valsartan: more than a decade of experience. Drugs. 2009;69(17):2393–2414. doi: 10.2165/11319460-000000000-00000.
- Mistry NB, Westheim AS, Kjeldsen SE. The angiotensin receptor antagonist valsartan: a review of the literature with a focus on clinical trials. Expert Opin Pharmacother. 2006;7(5):575–581. doi: 10.1517/14656566.7.5.575.
- Sander GE, Giles TD. Nebivolol and valsartan as a fixed-dose combination for the treatment of hypertension. Expert Opin Pharmacother. 2015;16(5):763–770. doi: 10.1517/14656566.2015.1020790.
- Varagic J, Punzi H, Ferrario CM. Clinical utility of fixed-dose combinations in hypertension: evidence for the potential of nebivolol/valsartan. Integr Blood Press Control. 2014;7:61–70. doi: 10.2147/IBPC.S50954.
- Giles TD, Weber MA, Basile J, et al. Efficacy and safety of nebivolol and valsartan as fixed-dose combination in hypertension: a randomised, multicentre study. Lancet. 2014;383(9932):1889–1898.
- Volpe M, Pegoraro V, Heiman F, et al. Extemporaneous combination therapy with amlodipine/zofenopril in hypertensive patients: a real-world data analysis in Italy. Curr Med Res Opin. 2023;39(12):1593–1601.
- Volpe M, Pegoraro V, Peduto I, et al. Extemporaneous combination therapy with nebivolol/zofenopril in hypertensive patients: usage in Italy. Curr Med Res Opin. 2022;38(10):1673–1681. doi: 10.1080/03007995.2022.2096352.
- IQVIA. Osservatorio sull’impatto della pandemia COVID-19 sull’accesso alle cure. Periodo dati: 2019–2020; 2021 Available from: https://www.farmindustria.it/app/uploads/2021/03/Osservatorio-IQVIA-sullimpatto-pandemia-sullaccesso-alle-cure_Marzo-2021.pdf.
- Prieto-Merino D, Mulick A, Armstrong C, et al. Estimating proportion of days covered (PDC) using real-world online medicine suppliers’ datasets. J Pharm Policy Pract. 2021;14(1):113.
- Wang SJ, Sander GE. Nebivolol/valsartan combination for the treatment of hypertension: a review. Future Cardiol. 2021;17(4):573–583. doi: 10.2217/fca-2020-0079.
- Farag SM, Rabea HM, Mahmoud HB. Effect of amlodipine/valsartan versus nebivolol/valsartan fixed dose combinations on peripheral and central blood pressure. High Blood Press Cardiovasc Prev. 2018;25(4):407–413. doi: 10.1007/s40292-018-0286-8.
- Ishak J, Rael M, Punzi H, et al. Additivity of nebivolol/valsartan single-pill combinations versus other single-pill combinations for hypertension. J Clin Hypertens. 2018;20(1):143–149.
- Mende CW, Giles TD, Bharucha DB, et al. Efficacy of nebivolol-valsartan single-pill combination in obese and nonobese patients with hypertension. J Clin Hypertens. 2017;19(6):632–639. doi: 10.1111/jch.12965.
- Schmieder RE, Wassmann S, Predel H-G, et al. Improved persistence to medication, decreased cardiovascular events and reduced all-cause mortality in hypertensive patients with use of single-pill combinations: results from the START-study. Hypertension. 2023;80(5):1127–1135. doi: 10.1161/HYPERTENSIONAHA.122.20810.
- Mancia G, Volpe R, Boros S, et al. Cardiovascular risk profile and blood pressure control in Italian hypertensive patients under specialist care. J Hypertens. 2004;22(1):51–57.
- Rea F, Savaré L, Franchi M, et al. Adherence to treatment by initial antihypertensive Mono and combination therapies. Am J Hypertens. 2021;34(10):1083–1091. doi: 10.1093/ajh/hpab083.
- Conn VS, Ruppar TM, Chase JA, et al. Interventions to improve medication adherence in hypertensive patients: systematic review and meta-analysis. Curr Hypertens Rep. 2015;17(12):94. doi: 10.1007/s11906-015-0606-5.
- Peacock E, Krousel-Wood M. Adherence to antihypertensive therapy. Med Clin North Am. 2017;101(1):229–245. doi: 10.1016/j.mcna.2016.08.005.
- Hypertension EETFftMoA. 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC task force for the management of arterial hypertension. J Hypertens. 2013;31(10):1925–1938.
- Unger T, Borghi C, Charchar F, et al. 2020 International society of hypertension global hypertension practice guidelines. J Hypertens. 2020;38(6):982–1004. doi: 10.1097/HJH.0000000000002453.
- Hypertension Canada. Guidelines-Hypertension Canada [cited 2022 Jun 7]. Available from: https://guidelines.hypertension.ca/chep-resources/.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2018;71(19):e127–e248. doi: 10.1016/j.jacc.2017.11.006.
- Düsing R, Waeber B, Destro M, et al. Triple-combination therapy in the treatment of hypertension: a review of the evidence. J Hum Hypertens. 2017;31(8):501–510. doi: 10.1038/jhh.2017.5.
- Camm AJ, Fox KAA. Strengths and weaknesses of 'real-world’ studies involving non-vitamin K antagonist oral anticoagulants. Open Heart. 2018;5(1):e000788. doi: 10.1136/openhrt-2018-000788.
- Forzano I, Mone P, Varzideh F, et al. The selective aldosterone synthase inhibitor baxdrostat significantly lowers blood pressure in patients with resistant hypertension. Front Endocrinol. 2022;13:1097968. doi: 10.3389/fendo.2022.1097968.
- Trimarco V, Izzo R, Mone P, et al. Therapeutic concordance improves blood pressure control in patients with resistant hypertension. Pharmacol Res. 2023;187:106557. doi: 10.1016/j.phrs.2022.106557.