Abstract
Background
Acute bronchitis is the most common respiratory disease. Mixture of Ivy Leaf Extract and Coptidis rhizome syrup has shown good treatment efficacy against chronic bronchitis and acute respiratory infections. This study aimed to evaluate the efficacy and safety of Mixture of Ivy Leaf Extract and Coptidis rhizome compared with those of Pelargonium sidoides extract, for the treatment of acute bronchitis.
Methods
We performed a multicenter, randomized, double-blind, active-controlled, parallel phase III study in 220 patients with acute bronchitis. The participants were offered either Mixture of Ivy Leaf Extract and Coptidis rhizome syrup (AGS) and placebo of P. sidoides tablet or placebo syrup and active tablet of P. sidoides (AGU) for 7 days. The primary endpoint was the change in the Bronchitis Severity Score (BSS) from the baseline visit (visit 2) to day 7 (visit 3).
Results
For the primary outcome, there was no significant difference in the change of total BSS between visits 2 and 3 (−4.10 ± 1.93 vs. −4.24 ± 1.85, p = 0.5125), and since the upper limit of the confidence interval (1.00) was smaller than the predetermined non-inferiority margin (1.17), it was confirmed that the AGS group was non-inferior to the AGU group. The changes in each symptom in the BSS between visits 2 and 3 also showed no significant differences. The overall improvement rate measured by the investigator (91.7 vs. 89.7%; p = 0.3506) and the satisfaction rate of the participants at visit 3 also showed no significant differences (97.2 vs. 94.4%; p = 0.4388). Regarding safety issues, adverse reactions were noted in both groups similarly, with no serious adverse events (4.55 vs. 3.64%, p > 0.999).
Conclusion
Mixture of Ivy Leaf Extract and Coptidis rhizome syrup is as effective and safe as P. sidoides in controlling symptoms of acute bronchitis.
Introduction
Acute bronchitis is among the most common diseases in primary care centers worldwide. Patients with acute bronchitis usually complain of acute cough with or without sputum that continues for <3 weeksCitation1–4. According to the United States National Health Interview Survey, acute bronchitis occurs in 5% of the general population in the United States and leads to 10 outpatient clinic visits per 1000 persons per yearCitation5. According to the 2016 National Health Insurance data, acute bronchitis is one of the most common diseases affecting over 15,000,000 patients per year in KoreaCitation6.
There are several concerns regarding the treatment of acute bronchitis. There have been issues with the prescription of antibiotics in patients with acute bronchitis, especially those with severe cough and purulent sputumCitation7,Citation8. Since the symptoms of acute bronchitis are usually relieved within 3 weeks, it is considered to have a self-limiting course, and routine treatment with antibiotics is not recommendedCitation1–3. In addition, β2-agonist bronchodilators may be useful only in selected patients with cough accompanied by wheezingCitation2. In the practice guideline for a chronic cough from acute bronchitis, antitussive agents are occasionally useful for short-term symptom relief but not mucokinetic agents in the aspect of coughCitation2. However, similar to other symptoms of acute bronchitis, sputum can be relieved by several mucokinetics or mucus expectorants from various sources, including herbs or natural extracts, without serious adverse reactions. Therefore, mucus expectorants and antitussive agents are frequently administered to patients with acute bronchitis.
There are several herbal medicines with antitussive and expectorant effects that originate from various native plants composed of a single material or mixtureCitation9–12. These include thyme, Pelargonium sidoides extracted from South African geranium, Hedera helix from ivy leaf extracts, and many other Chinese medicinal herbs. The remedies were made as pills and syrups to relieve acute cough or sputum. Mixture of Ivy Leaf Extract and Coptidis rhizome syrup was formulated using a dried 30% ethanolic extract of H. helix L. (ivy; Lamiaceae) leaves and a dried water-saturated butanolic extract of Coptis chinensis Franch (Ranunculaceae) rhizomes (3:1, w/w). It is an herbal product developed by a South Korean pharmaceutical company (Ahn-Gook Parmaceuticals Co., Ltd.), and it consists of Hederacoside C from ivy leaf extracts and berberine from Coptidis rhizome. Hedera helix and Coptidis rhizome extracts, the two main components of Mixture of Ivy Leaf Extract and Coptidis rhizome, have an expectorant and antitussive effect, respectively. The mixture of Hederacoside C and berberine exhibited anti-inflammatory effects by inhibiting NF-κB signaling and did not have any harmful effects in animal studiesCitation13,Citation14. Furthermore, it has been demonstrated to be effective in decreasing cough and sputum in patients with acute upper respiratory tract infection and chronic inflammatory bronchitis in a phase III clinical trial conducted in 2010 (NCT01151202). It was approved by the Korean Ministry of Food and Drug Safety in March 2011 for the treatment of cough and sputum production due to bronchitis.
There are studies indicating the effectiveness of Mixture of Ivy Leaf Extract and Coptidis rhizome syrup in various respiratory conditionsCitation15,Citation16. It showed significant improvement in the quality of life and reduced systemic inflammation in patients with chronic bronchitis type stable COPDCitation15. In addition, it significantly reduces overall bronchitis symptoms and respiratory quality of life in patients with chronic bronchitis and bronchiectasisCitation16. However, to date, there has been no study that investigated the effect of Mixture of Ivy Leaf Extract and Coptidis rhizome syrup in patients with acute bronchitis. Pelargonium sidoides is an herbaceous perennial plant in South Africa that has been proven to be effective and safe in the treatment of acute bronchitis, with its therapeutic effects validated in several prior randomized controlled trials and meta-analysesCitation11,Citation17,Citation18. In this study, we sought to determine the efficacy and safety of Mixture of Ivy Leaf Extract and Coptidis rhizome syrup compared with P. sidoides in the treatment of acute bronchitis.
Methods
Study design
This was a randomized, double-blinded, active-controlled, multicenter, parallel-designed, phase III clinical trial conducted at 13 centers in Korea. The day of the first patient screening and enrollment was 17 October 2019, and the day of the last patient’s closed-out visit was 29 July 2020.
Patients with symptoms of acute bronchitis were seen at the initial screening visit (visit 1) and randomly assigned to each treatment group at the baseline visit (visit 2). After 7 days of treatment, considering the inclusion of weekends when outpatient services are not available, the end of the treatment visit was performed from days 7 to 10 (visit 3). Eligible patients were randomized on the same day of the screening visit or within 3 days after the screening visit.
Written informed consent was obtained from all the participants. The protocol for this clinical trial was authorized by the Korean Food and Drug Administration, and all details of the clinical trial were approved by the institutional review board of each center. This trial was registered at ClinicalTrials.gov (NCT05344638).
Participants
The inclusion criteria were as follows: (1) patients who are adults based on the Korean Civil Code (19 years and older) and are under the age of 75; (2) symptoms of acute bronchitis with 5 points or more of BSS at the screening visit; (3) acute bronchitis with a duration of cough <2 weeks at the screening visit; and (4) those without fever with temperature <38.5 °C.
Patients diagnosed with severe lung diseases, such as pneumonia, active tuberculosis, bronchiectasis, asthma, COPD, interstitial lung disease, or lung cancer, were excluded. Patients with clinically significant comorbid conditions, lactose-intolerant, pregnant or lactating, and chronic smokers were also excluded.
Randomization
This clinical trial employed institution-stratified randomization. All enrolled patients underwent a screening test before the beginning of the clinical trial. Detailed history taking for symptoms, baseline characteristics with a medical history, laboratory examination, vital sign measurement, electrocardiogram, and pregnancy tests (in the case of women of childbearing age) were performed. After the screening test, the participants were randomly assigned to the Mixture of Ivy Leaf Extract and Coptidis rhizome syrup (AGS) or control (AGU) groups. Investigators used a randomization table to assign participants to their respective groups. In this clinical trial, the allocation ratio for each group was 1:1.
After randomization, concomitant administration of several medications that could influence patient symptoms was inhibited, including angiotensin-converting enzyme inhibitors, antibiotics, antiviral agents, glucocorticoids, antihistamines, mucolytics, antitussives, expectorants, β2-adrenergic agonists, antiplatelet or anticoagulative agents, and central nervous system stimulants. Concomitant medications that patients had been taking before enrollment that did not affect the trial were allowed.
Interventions
In the AGS group, Mixture of Ivy Leaf Extract and Coptidis rhizome syrup 15 mL and a placebo of P. sidoides 1 tablet for one dose were administered. In the control group, AGU (active tablet of P. sidoides) 1 tablet and a placebo of Mixture of Ivy Leaf Extract and Coptidis rhizome syrup 15 mL for one dose were administered. Each dose of medication was administered three times per day for 7 days, after which the patients underwent efficacy and safety assessments. In cases of symptom resolution of acute bronchitis within 7 days, a close-out visit of the clinical trial was performed.
Efficacy outcomes and general measurement
The primary endpoint of this study was the change in the total Bronchitis Severity Score (BSS) between the baseline visit (visit 2) and the end of the treatment visit (visit 3). The secondary endpoints were defined as follows: (1) change in each BSS component, (2) treatment response assessed by each physician, (3) overall improvement, and (4) degree of subjective satisfaction.
The main analysis of efficacy in this clinical trial was performed using the per-protocol set (PPS), and additional analysis was performed using the full analysis set (FAS). PPS was defined as the completion of all visit schedules without serious violations of the clinical trial protocol. FAS was defined as the administration of at least one dose of clinical trial medication after randomization and could obtain data on the primary efficacy evaluation.
Bronchitis Severity Score
Acute bronchitis is a self-limiting disease, and there is significant individual variability in the duration and course of the condition, making accurate assessment challengingCitation4. The BSS is a useful scale for assessing the severity and treatment outcomes of acute bronchitisCitation19. It is a scoring scale for the symptoms of acute bronchitis consisting of cough, sputum, rales or rhonchi, chest pain during coughing, and dyspnea. Each component was scored from 0 (absent) to 4 (very severe), and the total score was up to 20 points, which was the sum of each symptom score. The BSS was assessed for patients at each visit.
Treatment response was defined as the comparison of total BSS difference between before (visit 2) and after (visit 3) administration of the investigational drug, where meeting any of the following criteria was considered as a treatment response: (1) a total BSS score <3 points at visit 3; (2) a decrease in total BSS score more than 7 points at visit 3; and (3) a total BSS score <3 points and a decrease in total BSS score over 7 points.
Subjective measurement of treatment response
At the end of the investigational drug administration period, the investigators performed history taking on the subjects about their treatment outcomes and subjectively assessed the degree of change in treatment effects. The Integrative Medicine Outcomes Scale (IMOS), a 5-point scale, was applied for this evaluation, as follows: (1) deterioration; (2) no change; (3) slight to moderate improvement; (4) major improvement; and (5) complete recovery. At the same point, we assessed the patient’s degree of satisfaction on 5 points scale as follows: (1) very dissatisfied; (2) dissatisfied; (3) neutral; (4) satisfied; and (5) very satisfied.
Safety measurements
All patients were asked to report any adverse events they experienced during the clinical trial. At each visit, we investigated the presence of adverse symptoms, starting point and duration, severity, treatment response, and causal relationship with the study drugs. During this clinical trial, patients underwent physical examinations, laboratory examinations, vital signs, electrocardiograms, and pregnancy tests in women of childbearing age for safety evaluation, performed twice at visits 1 and 3.
Statistical analysis
Statistical analyses were performed using the SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA). In the case of a non-inferiority trial, a two-sided test at a 5% (α = 0.05) significance level and a 97.5% one-sided confidence interval (or upper limit of 95% two-sided confidence interval) were performed. In this clinical trial, we referred to the previous research on P. sidoides conducted in acute bronchitis patients to establish the non-inferiority margin for demonstrating the non-inferiority of the investigational drugCitation11,Citation17,Citation20,Citation21. In the previous study, after 7 days of administration of P. sidoides, the weighted mean change in the BSS was 6.47, with a pooled standard deviation of 2.65, while the mean change in the placebo group was 3.75, with a pooled standard deviation of 3.46. Based on this, the clinically acceptable margin was determined to be the difference between the weighted mean change in the P. sidoides group and the mean change in the placebo group, which is 2.72 points. The 95% confidence interval for this difference was estimated to be 2.34 to 3.10, and half of the lower limit of the confidence interval, 2.34, which is 1.17, was set as the non-inferiority clinical margin. If the upper margin of the confidence interval was <1.17, we considered the experimental group not inferior to the control group. For the comparison between treatment groups, if the continuous data satisfied the normality assumption based on normality tests, the two-sample t-test was employed. Alternatively, if the data did not meet the normality assumption, the Wilcoxon rank sum test was used to assess significance. For categorical data, the Chi-square test was applied, and in cases where the expected cell count was <5 in more than 20% of the total cells, Fisher’s exact test was utilized to examine significance. After assessing the normality of the primary efficacy evaluation variable, confidence intervals were estimated using Hodges-Lehmann for the non-normally distributed data. All statistical values were reported to two decimal places, and p-values were presented to four decimal places.
Results
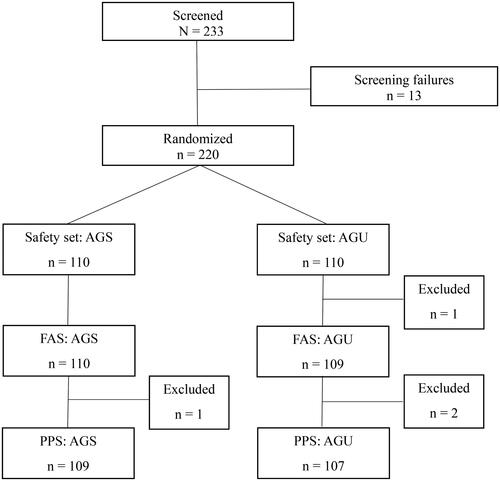
A total of 220 randomized patients who provided informed consent were enrolled. Four patients were excluded because of poor compliance or accidental duplicate assignments. As a result, 216 patients were analyzed as PPS, including 109 and 107 patients in the AGS and AGU groups, respectively ().
Figure 1. Enrolled patients with acute bronchitis. Abbreviations. AGS, investigational drug (Synatura syrup); AGU, comparative drug (Umckamin); FAS, full analysis set; PPS, per-protocol set.
Baseline characteristics
The baseline characteristics of the enrolled patients are presented in . Of all the enrolled patients, 63 (28.64%) were male. Their mean age [mean ± standard deviation (SD)] was 39.05 ± 11.58 years. The mean heights and weights of the patients were 164.59 ± 8.47 (cm) and 62.35 ± 13.73 (kg), respectively. Nineteen (8.64%) patients were current smokers. Duration of acute bronchitis (mean ± SD) was 6.01 ± 3.27 in the AGS group and 5.89 ± 3.10 in the AGU group. There were no significant differences in baseline characteristics between the AGS and AGU groups.
Table 1. Baseline characteristics of randomized patients.
Primary efficacy evaluation: total BSS change
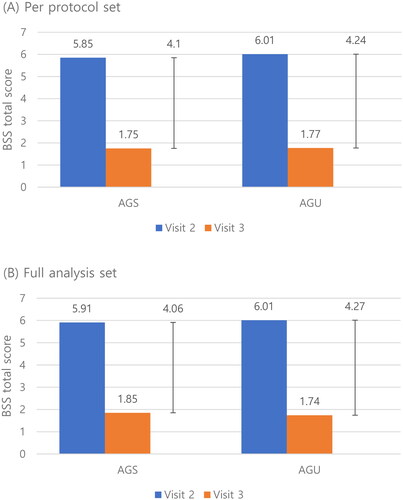
The total change of BSS between visits 2 and 3 of the AGS and AGU groups was not different in PPS (−4.10 ± 1.93 vs. −4.24 ± 1.85, p = 0.5125) and FAS (−4.06 ± 1.96 vs. −4.27 ± 1.84, p = 0.3793) (). Since the total BSS change was not normally distributed, Wilcoxon’s rank-sum test was performed. The difference between the AGS and AGU groups was 0.00, with no statistically significant difference in PPS and FAS. The Hodges–Lehmann estimator was used to measure the confidence intervals. The width of the confidence interval was 0.00–1.00, which did not exceed the non-inferiority margin (1.17) (). Therefore, we confirmed the non-inferiority of AGS to AGU.
Figure 2. Change of the total bronchitis severity score (BSS) of the AGS and AGU groups from the baseline visit (visit 2) to end of treatment visit (visit 3) in the per-protocol set (A) and full analysis set (B). Abbreviations. BSS, bronchitis severity score; AGS, investigational drug (Synatura syrup); AGU, comparative drug (Umckamin).
Secondary outcome
At the baseline visit, there was no significant difference in the severity of each component of BSS (cough, 2.62 ± 0.68 vs. 2.58 ± 0.66; sputum, 2.22 ± 0.58 vs. 2.26 ± 0.66; rales/rhonchi, 0.38 ± 0.70 vs. 0.38 ± 0.70; chest pain during coughing, 0.35 ± 0.55 vs. 0.47 ± 0.55; dyspnea, 0.28 ± 0.55 vs. 0.32 ± 0.58; all of p > 0.05). As described in , the differences in each component of the BSS between the AGS and AGU groups were not statistically significant between visits 2 and 3 (all of p > 0.05). The treatment response rates of the clinical trials between visits 2 and 3 are shown in . In PPS and FAS, none of the treatment response rate categories showed a statistically significant difference between the AGS and AGU groups (p > 0.05). shows the distribution of the degree of improvement in the AGS and AGU groups. We defined “overall improvement” as a score of 4 (complete recovery) or 5 (major improvement) on the IMOS. The rate of overall improvement between the AGS and AGU groups did not show the difference in PPS (69.72 vs. 74.77%, p = 0.4082) or FAS (69.09 vs. 75.23%, p = 0.311). shows the patients’ treatment satisfaction. Patients in both groups did not show differences in treatment satisfaction in PPS (p = 0.4388) or FAS (p = 0.5957).
Table 2. Difference in each BSS component of the AGS and AGU groups with treatment.
Table 3. Treatment response rate between visits 2 and 3.
Table 4. Treatment response rate between visits 2 and 3.
Table 5. Subjective satisfaction of patients.
Adverse events
The rate of adverse effects in this clinical trial was 4.55% (five patients, five cases) in the AGS group and 3.64% (four patients, seven cases) in the AGU group (). Among these cases, the rate of adverse effects suspected to have a causal relationship with the medication used in the clinical trial was 4.55% (five patients, five cases) in the AGS group and 1.82% (two patients, five cases) in the AGU group. However, the differences were not statistically significant. All these adverse effects were mild; therefore, no additional treatment was needed. Diarrhea was the most common adverse event in the AGS and AGU groups [three patients (2.73%) and three patients, respectively]. In the AGS group, mild adverse events occurred in 4.55% (five patients, five cases) of the patients, whereas in the AGU group, mild adverse events occurred in 3.64% (four patients, seven cases). A critical adverse event that could lead to withdrawal from the clinical trial was not unknown. On laboratory examination, one patient in the AGS group showed clinically significant alanine aminotransferase elevation, and one patient in the AGU group had significantly elevated aspartate aminotransferase levels. However, this is unlikely to be generalized statistically because of its low incidence. Other safety evaluation tests did not reveal any adverse outcomes.
Table 6. Adverse events during the clinical trial.
Discussion
This multicenter, randomized, double-blinded, active-controlled, parallel-designed phase III study showed that Mixture of Ivy Leaf Extract and Coptidis rhizome syrup was as effective as P. sidoides in the treatment of acute bronchitis. This study is significant in that it is the first to investigate the potential of the Mixture of Ivy Leaf Extract and Coptidis rhizome syrup as a therapeutic agent for symptom relief, not only in respiratory diseases with recognized indications but also in acute bronchitis, demonstrating its effectiveness and safety.
The difference in the primary outcome, total BSS, between the AGS and AGU groups was 4.10 and 4.24, respectively, with no statistically significant difference observed between the groups. Compared to previous studies involving P. sidoides and placebo, a lower difference in total BSS was observedCitation20. However, the weighted mean difference in BSS change calculated from previous studies using P. sidoides was 2.72, with a 95% confidence interval of (2.34, 3.10)Citation11,Citation17,Citation20,Citation21. In our study, we adopted half of the lower limit of the confidence interval, 1.17, as the non-inferiority clinical margin to facilitate a clearer evaluation of non-inferiority comparison. Thus, it can be concluded that the AGS group exhibits non-inferiority compared to the AGU group in terms of the difference in total BSS.
In both the AGS and AGU groups, the most severe symptom among the components of the BSS at the randomization point (visit 2) was cough, and at the end of treatment (visit 3), the most significant improvement in symptoms was also observed, with no statistically significant difference between the two groups. This is presumed to be attributed to the pharmacological actions of α-Hederin and berberine, the main components of the Mixture of Ivy Leaf Extract and Coptidis rhizome syrup, as revealed in previous studies. A meta-analysis of two randomized, double-blind, placebo-controlled trials showed that Ivy Leaf Extract reduces the intensity of cough associated with acute respiratory tract infection and accelerates the time to recoveryCitation22. α-Hederin, the chief ingredient of H. helix, increases the activity of the β2-adrenergic receptor and cyclic AMP (cAMP) levels. cAMP increases the number of alveolar type II cells, which elevates their secretolytic effect and bronchospasmolytic activityCitation23,Citation24. Berberine is the main ingredient of Coptidis rhizome and has been shown to suppress inflammation by inhibiting lipopolysaccharide (LPS)-stimulated pro-inflammatory cytokines, such as interleukin-6 and LPS-mediated nuclear factor kappa B activationCitation25. In addition, it was reported that berberine suppresses the expression of the MUC5AC gene, the key factor involved in mucin production in inflammatory airway disease, and other studies have shown that berberine exhibits antimicrobial and bronchodilator functionsCitation26,Citation27. Therefore, the combination drug Mixture of Ivy Leaf Extract and Coptidis rhizome syrup may have potent anti-inflammatory, mucus-suppressive, or mucokinetic effects. The effect of Mixture of Ivy Leaf Extract and Coptidis rhizome syrup on airway inflammation has been done mainly for the mechanism in animal studiesCitation15,Citation16,Citation28. Based on the mechanism, in previous studies, Mixture of Ivy Leaf Extract and Coptidis rhizome syrup demonstrated its efficacy in the treatment of chronic bronchitis type stable COPD and chronic bronchitis with bronchiectasisCitation15,Citation16.
In this study, both the AGS and AGU groups demonstrated symptom improvement in patients with acute bronchitis. Considering the results of previous studies comparing Mixture of Ivy Leaf Extract and Coptidis rhizome syrup with P. sidoides and placebo groups, it can be anticipated that Mixture of Ivy Leaf Extract and Coptidis rhizome syrup will improve patients’ BSS in acute bronchitis compared to the placebo groupCitation10–12. In addition to its efficacy and its superiority in the treatment of acute bronchitis compared with a placebo, we conducted a non-inferiority trial to determine whether these courses of anti-inflammatory action have a symptom-relieving effect in patients with acute bronchitis. In this study, we used P. sidoides as a control medication for acute bronchitis. Compared with P. sidoides, Mixture of Ivy Leaf Extract and Coptidis rhizome syrup was not inferior in efficacy or safety in this trial.
In terms of safety, previous studies utilizing P. sidoides and placebo revealed a slightly higher risk of adverse events in the P. sidoides group. However, no cases of serious adverse events were reported. Additionally, the frequency of drug-related adverse effects is much lower compared to the use of antibiotics in acute bronchitis. In this study, adverse events occurring in both the AGS and AGU groups were all mild in severity, and no clinical interventions or corrective treatments related to manifested adverse reactions were required. The frequencies of all types of adverse events and adverse drug reactions were <5%, indicating a low occurrence rate. Furthermore, there were no significant differences observed compared to the safety-proven P. sidoides in previous studies.
This clinical trial had some limitations. First, although 13 centers participated in this trial, it was a single-nation study; therefore, the possible effects of racial or international differences could not be reflected in the results. Second, our study included only adult patients; however, acute bronchitis is a common disease in all age groups, including children and adolescents. Further investigations were needed to generalize the efficacy and safety of Mixture of Ivy Leaf Extract and Coptidis rhizome syrup in the treatment of acute bronchitis in all age groups. Third, this study is a non-inferiority trial, and unlike other placebo-controlled studies, we did not establish a placebo groupCitation10,Citation12. Since acute bronchitis is a self-limiting disease, both the AGS group and AGU group showed improvements in BSS. As a result, the efficacy of each medication was not evaluated in comparison to a placebo group.
Despite these limitations, this study suggests a novel treatment option for acute bronchitis. In future studies, a wider range of participants is necessary to confirm the results of this study.
Conclusion
Mixture of Ivy Leaf Extract and Coptidis rhizome is proven to improve the symptoms of acute bronchitis as effectively and safely as P. sidoides.
Transparency
Declaration of funding
This research was funded by the Ahn-Gook Pharmaceutical Co., LTD.
Declaration of financial/other relationships
The authors declare that they have no relevant conflicts of interest. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Kyung-il Han: writing-original draft, writing-review and editing; Tae-Hyung Kim: conceptualization, data collection, investigation, supervision, writing-original draft, writing-review and editing; Seung Won Ra, Hyoung-Kyu Yoon, Deog Kyeom Kim, Chin Kook Rhee, Jung Woong Park, Yong-il Hwang, Hye Yun Park, Yee Hyung Kim, Yong Bum Park, Kyeong-Cheol Shin, and Seong Yong Lim: data collection, formal analysis, investigation, writing-review and editing; Kwang Ha Yoo: conceptualization, data collection, formal analysis, investigation, supervision, writing-review and editing.
Acknowledgements
Not applicable.
Ethical approval
Hanyang University Guri Hospital: GURI 2019-08-011; Ulsan University Hospital: UUH 2019-08-006.
Yeouido St. Mary’s Hospital: 2019-2743-0013; Seoul National University Seoul Metropolitan Government Boramae Medical Center: 10-2019-66; Seoul St. Mary’s Hospital: 2019-2762-0017.
Gachon University Gil Medical Center: GAIRB 2019-353; Hallym University Sacred Heart Hospital: HALLYM 2019-08-011; Samsung Medical Center: SMC 2019-08-068; Kyung Hee University Hospital at Gangdong: KHNMC 2019-08-011; Hallym University Kangdong Sacred Heart Hospital: KANGDONG 2019-08-011; Yeungnam University Medical Center: YUMC 2019-08-006; Kangbuk Samsung Hospital: KBSMC 2019-08-017; Konkuk University School of Medicine: 2019-09-007.
References
- Gonzales R, Sande MA. Uncomplicated acute bronchitis. Ann Intern Med. 2000;133(12):981–991. doi: 10.7326/0003-4819-133-12-200012190-00014.
- Braman SS. Chronic cough due to acute bronchitis: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 Suppl):95s–103s. doi: 10.1378/chest.129.1_suppl.95S.
- Albert RH. Diagnosis and treatment of acute bronchitis. Am Fam Physician. 2010;82(11):1345–1350.
- Kinkade S, Long NA. Acute bronchitis. Am Fam Physician. 2016;94(7):560–565.
- Adams PF, Hendershot GE, Marano MA. Current estimates from the national health interview survey, 1996. Vital Health Stat. 1999;10(200):1–203.
- Lyu Y, Park S, Lee EJ, et al. A systemic review of clinical trials using medication for acute bronchitis: a pre-study on the development of traditional Korean medicine clinical practice guideline. J Korean Med. 2017;38(1):93–111. doi: 10.13048/jkm.17009.
- Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302(7):758–766. doi: 10.1001/jama.2009.1163.
- Park S, Oh KC, Kim K-S, et al. Role of atypical pathogens and the antibiotic prescription pattern in acute bronchitis: a multicenter study in Korea. J Korean Med Sci. 2015;30(10):1446–1452. doi: 10.3346/jkms.2015.30.10.1446.
- Kim W-Y, Park MJ, Rhee CK, et al. HL301 versus Umckamin in the treatment of acute bronchitis: a phase III, randomized, controlled, double-blind, multicenter study. Curr Med Res Opin. 2020;36(3):503–508. doi: 10.1080/03007995.2019.1706044.
- Ra SW, Kim SY, Lim YY, et al. The safety and efficacy of CKD-497 in patients with acute upper respiratory tract infection and bronchitis symptoms: a multicenter, double-blind, double-dummy, randomized, controlled, phase II clinical trial. J Thorac Dis. 2021;13(1):1–9. doi: 10.21037/jtd-20-1567.
- Matthys H, Eisebitt R, Seith B, et al. Efficacy and safety of an extract of Pelargonium sidoides (EPs 7630) in adults with acute bronchitis. A randomised, double-blind, placebo-controlled trial. Phytomedicine. 2003;10(Suppl 4):7–17. doi: 10.1078/1433-187x-00308.
- Lee SW, Lyu YR, Kim SY, et al. Efficacy and safety of GHX02 in the treatment of acute bronchitis and acute exacerbation of chronic bronchitis: a phase II, randomized, double-blind, placebo-controlled, multicenter trial. Front Pharmacol. 2021;12:761575. doi: 10.3389/fphar.2021.761575.
- Yoon YY, Choi WS, Gil KC, et al. Mixture of ivy leaves and coptidis rhizoma suppresses pulmonary inflammation induced by air particles- and lipopolysaccharide-induced chronic obstructive pulmonary disease in mice. Yakhak Hoeji. 2019;63(2):95–102. doi: 10.17480/psk.2019.63.2.95.
- Chang SI, Ryu DY. Assessment of subchronic toxicity and toxicokinetics of AG NPP709 in Sprague-Dawley rats. J Ethnopharmacol. 2023;317:116801. doi: 10.1016/j.jep.2023.116801.
- Lee EG, Rhee CK. The clinical efficacy of AG NPP709 (Synatura(®)) in patients with chronic bronchitis type stable chronic obstructive pulmonary disease. J Thorac Dis. 2020;12(5):2435–2442. doi: 10.21037/jtd.2020.03.61.
- Hong G, Kim Y-I, Park SJ, et al. Effects of a mixture of ivy leaf extract and coptidis rhizome on patients with chronic bronchitis and bronchiectasis. Int J Environ Res Public Health. 2021;18(8):4024. doi: 10.3390/ijerph18084024.
- Chuchalin AG, Berman B, Lehmacher W. Treatment of acute bronchitis in adults with a Pelargonium sidoides preparation (EPs 7630): a randomized, double-blind, placebo-controlled trial. Explore. 2005;1(6):437–445. doi: 10.1016/j.explore.2005.08.009.
- Agbabiaka TB, Guo R, Ernst E. Pelargonium sidoides for acute bronchitis: a systematic review and meta-analysis. Phytomedicine. 2008;15(5):378–385. doi: 10.1016/j.phymed.2007.11.023.
- Kardos P, Lehrl S, Kamin W, et al. Assessment of the effect of pharmacotherapy in common cold/acute bronchitis – the Bronchitis Severity Scale (BSS). Pneumologie. 2014;68(8):542–546. doi: 10.1055/s-0034-1377332.
- Matthys H, Heger M. Treatment of acute bronchitis with a liquid herbal drug preparation from Pelargonium sidoides (EPs 7630): a randomised, double-blind, placebo-controlled, multicentre study. Curr Med Res Opin. 2007;23(2):323–331. doi: 10.1185/030079906X167318.
- Matthys H, Lizogub VG, Malek FA, et al. Efficacy and tolerability of EPs 7630 tablets in patients with acute bronchitis: a randomised, double-blind, placebo-controlled dose-finding study with a herbal drug preparation from Pelargonium sidoides. Curr Med Res Opin. 2010;26(6):1413–1422. doi: 10.1185/03007991003798463.
- Völp A, Schmitz J, Bulitta M, et al. Ivy leaves extract EA 575 in the treatment of cough during acute respiratory tract infections: meta-analysis of double-blind, randomized, placebo-controlled trials. Sci Rep. 2022;12(1):20041. doi: 10.1038/s41598-022-24393-1.
- Greunke C, Hage-Hulsmann A, Sorkalla T, et al. A systematic study on the influence of the main ingredients of an ivy leaves dry extract on the beta2-adrenergic responsiveness of human airway smooth muscle cells. Pulm Pharmacol Ther. 2015;31:92–98.
- Song KJ, Shin YJ, Lee KR, et al. Expectorant and antitussive effect of Hedera helix and rhizoma coptidis extracts mixture. Yonsei Med J. 2015;56(3):819–824. doi: 10.3349/ymj.2015.56.3.819.
- Lee C-D, Lee J-K, Jeong JH, et al. Inhibitory effects of an extract mixture of ivy (Hedera helix) leaves and coptidis rhizoma on ovalbumin-induced allergic lung inflammation by co-exposure to Asian sand dust in mice. Yakhak Hoeji. 2018;62(1):21–29. doi: 10.17480/psk.2018.62.1.21.
- Holzinger F, Chenot JF. Systematic review of clinical trials assessing the effectiveness of ivy leaf (Hedera helix) for acute upper respiratory tract infections. Evid Based Complement Alternat Med. 2011;2011:382789–382789. doi: 10.1155/2011/382789.
- Baharara H, Moghadam AT, Sahebkar A, et al. The effects of ivy (Hedera helix) on respiratory problems and cough in humans: a review. Adv Exp Med Biol. 2021;1328:361–376. doi: 10.1007/978-3-030-73234-9_23.
- Matthys H, Kamin W. Positioning of the Bronchitis Severity Score (BSS) for standardised use in clinical studies. Curr Med Res Opin. 2013;29(10):1383–1390. doi: 10.1185/03007995.2013.832183.

