Abstract
Objectives
To describe the clinical characteristics and treatment adherence in European adult hypertensive patients starting treatment with the extemporaneous combination of nebivolol and ramipril (NR-EXC).
Methods
Retrospective database analysis of patients receiving NR-EXC treatment across five European countries (Italy, Germany, France, Poland, Hungary) over a period ranging from 3 to 9 years (until 30 June 2020) according to data availability for the different data sources. Patient demographics, comorbidities, and treatment adherence were evaluated.
Results
We identified 592,472 patients starting NR-EXC. Most of them were over 60 years of age, with ramipril most commonly prescribed at 5 mg (from 30.0 to 57.2% of patients across the databases). Notable comorbidities included diabetes (19.2%) and dyslipidemia (18.2%). The study population was also highly subjected to polytherapy with antithrombotics, lipid-lowering agents, and other lowering blood pressure agents as the most co-prescribed medications, as resulted from Italian database. Up to 59% of the patients did not request a cardiologic visit during the study period. Adherence to therapy was low in 56.3% of the patients, and it was high only in 11.1% of them.
Conclusions
The combination of nebivolol and ramipril is frequently prescribed in Europe, but adherence to treatment is suboptimal. The transition to a single pill combination could enhance treatment adherence and streamline regimens, potentially leading to significant benefits. Improved adherence not only correlates with better blood pressure control but also reduces the risk of cardiovascular events, underscoring the importance of this development.
Introduction
Hypertension affects more than a billion people worldwideCitation1, with an estimated prevalence of more than 150 million people in central and eastern Europe aloneCitation1,Citation2. The reported hypertension prevalence in the adult Italian population ranges from 55 to 59%Citation3. Hypertension has therefore a significant impact on public health and blood pressure (BP) control is still not satisfactory in a large proportion of patients despite the availability of various BP-lowering drugsCitation2,Citation4. The use of these medications is frequently suboptimal and lack of adherence is very common, impacting both on patients’ cardiovascular risk and the finances of healthcare systemsCitation5.
Most hypertensive patients require two or three drugs to adequately control BP, and current guidelines recommend the combined use of drugs, ideally as single-pill combinations (SPCs) with complementary mechanisms of action to maximize the therapeutic effectiveness because of the synergistic effects on BPCitation2. SPCs also increase treatment adherence and promote treatment simplification, leading to better cardiovascular protectionCitation2. Considering the currently available treatments, the use of β-blockers and angiotensin-converting enzyme (ACE) inhibitors is supported by a significant amount of evidence highlighting their BP-lowering effects and cardiovascular protectionCitation2.
Among the β-blockers, the third-generation β1-adrenergic receptor antagonist nebivolol is currently approved for the treatment of hypertension and for both hypertension and chronic heart failure in the US and in Europe, respectivelyCitation6–8. Nebivolol is a long-acting and highly selective β-blocker, and it shows unique pharmacological characteristics compared to other drugs of the same class. In addition to the cardioselectivity mediated via the β1 receptor blockade, nebivolol also induces vasodilation by stimulating the endothelial nitric oxide (NO) synthase through β3 agonismCitation6,Citation9. Studies have demonstrated the beneficial impact of nebivolol on central BP, endothelial dysfunction, and aortic stiffnessCitation9,Citation10. Moreover, nebivolol has a favorable tolerability profile, which makes it appropriate for specific non-CV comorbidities (such as diabetes, respiratory obstructive diseases, or erectile dysfunction)Citation11.
Ramipril is an ACE inhibitor indicated to treat hypertension, to reduce the risk of cardiac attack, ictus, and renal disease, and to treat heart failure in patients who experienced myocardial infarctionCitation12. Ramipril inhibits the angiotensin-converting enzyme and decreases angiotensin II formation. This action leads to a reduction in sympathetic system activation, as well as a reduction in sodium and water reabsorption in the kidneys. Additionally, it causes the smooth muscles in the arterioles to relax. As a result, the BP decreasesCitation13,Citation14. Several clinical trials have demonstrated that oral administration of ramipril, ranging from 1.25 to 10 mg once daily, effectively lowers both systolic and diastolic BP in individuals with mild to moderate hypertension. This treatment results in adequate BP control for ∼50–70% of patients. Additionally, various studies have demonstrated the efficacy of ramipril in managing BP in patients with cardiovascular diseases, either as monotherapy or in combination with other antihypertensive drugsCitation15–17. Ramipril is usually well tolerated, with a low incidence of adverse events reported in clinical trials and during maintenance therapyCitation14.
Due to the complementary effects of β-blockers and ACE inhibitors on the interlinked pathways of the sympathetic nervous system and the renin–angiotensin–aldosterone systemCitation18, both of which significantly impact cardiovascular risk and disease outcomes, the combined use of these agents is supported by a robust pharmacological and clinical rationaleCitation19. This study aims to investigate the extent of the concomitant use of nebivolol and ramipril in hypertensive patients in common clinical practice in Europe.
For this purpose, we carried out a large retrospective observational cohort study to describe the demographics, clinical characteristics, and adherence to treatment of hypertensive patients initiating the extemporaneous combination of nebivolol and ramipril in Italy, Germany, France, Poland, and Hungary.
Methods
Data sources
Study data were retrieved from the following sources: the Italian IQVIA Longitudinal Patient Database (LPD), the IQVIA LifeLink Treatment Dynamics (LRx) databases from ItalyCitation20,Citation21, GermanyCitation22–24, FranceCitation25–27, and PolandCitation28,Citation29, and the National Hospital Insurance Fund (NHIF) database from HungaryCitation30,Citation31. The Italian IQVIA LPD consists of routinely collected data from physician consultations of about 1.2 million patients referring to around 900 general practitioners (GPs). It contains information on diagnoses, drug prescriptions, and other medical and demographic information. The IQVIA Italian LPD gives an accurate representation of the regular healthcare provided by GPs in Italy, and as such, it has already been used in studies for several conditions including hypertensionCitation32,Citation33. The IQVIA LRx databases contain information on individual patients’ prescriptions over time collected from retail (not hospital-based) pharmacies. The main advantage of the LRx databases is their broad coverage of specialty physicians and pharmacies. The Hungarian NHIF database includes detailed data on state-funded services (including pharmaceuticals) for all Hungarian patients since the year 2000. All data retrieved from the databases were anonymized.
Study cohorts’ definition
We included all patients starting treatment with “5 mg nebivolol“ (Anatomical Therapeutic and Chemical [ATC] code C07AB12) and “2.5/5/10 mg ramipril” (ATC code C09AA05) (NR-EXC) during the following selection periods: 1 July 2011 to 30 June 2020 for the Italian IQVIA LPD; 1 July 2017 to 30 June 2020 for the other databases. The starting date of the first extemporaneous combination was defined as the index date. We first define the NR-EXC prevalent users by excluding all the patients who did not have a diagnosis of hypertension (International Classification of Diseases-9th revision [ICD-9] codes 401.xx and 402.xx) in the 12 months before the index date (only for Italian IQVIA LPD) and all those underage (for all databases, using a cut-off depending on data source). We then defined the NR-EXC incident users by excluding all the patients who had a previous prescription of NR-EXC during the 6-month period before the index date. The NR-EXC incident users’ cohort was described in terms of demographics, clinical characteristics, and adherence to treatment. To estimate the number of adult hypertensive patients potentially eligible for a nebivolol/ramipril SPC in Italy over a 1-year period, we focused on the year 2019 to avoid possible bias in changing prescriptions due to the COVID-19 pandemicCitation34. Calculations were done following Volpe et al.Citation32,Citation33. Briefly, we included all the adult (≥18 years old) hypertensive patients with at least one NR-EXC prescription during 2019 and considered the total sample of adult hypertensive active patients within the Italian IQVIA LPD in the same year (around 360,000 patients) and the Italian adult hypertensive population (around 16 million patients) according to the Società Italiana dell’Ipertensione Arteriosa (SIIA)Citation35 and the Istituto Nazionale di Statistica (ISTAT)Citation36.
Sensitivity analysis
A sensitivity analysis was conducted by including patients receiving NR-EXC prescribed as either a single molecule (ATC codes C07AB12 for nebivolol and C09AA05 for ramipril) or as SPCs with hydrochlorothiazide (ATC codes C07BB12 for nebivolol and C09BA05 for ramipril) to investigate possible differences between cohorts.
Study variables and definitions
The following patients’ variables were retrieved: age, sex, ramipril dosage (from all databases), body mass index (BMI), comorbidities, concomitant pharmacological treatments, and cardiologic visit referrals (from the IQVIA Italian LPD only). For each patient, the pre-selection period was defined as the six-month period preceding the index date, while the follow-up period was defined as the six-month period starting on the index date. Comorbidities were searched for the pre-selection period using the ICD-9 codes, while co-prescriptions were searched for both the pre-selection and the follow-up periods using the ATC codes. Adherence to therapy, defined as the degree to which patients take the medication as prescribed by their GPs, was assessed as the proportion of days covered (PDC) (i.e. total days of medication supply over the length of the corresponding follow-up), as indicated by the Pharmacy Quality Alliance and previously definedCitation37. This approach provides a more conservative estimate of adherence compared to other indicators when multiple medications are used concomitantlyCitation38. The number of days supplied by each prescription was obtained by dividing the total amount of active drug in each prescription by the recommended defined daily dose for nebivolol (i.e. 5 mg) and the dose of the prescribed tablets for ramipril (i.e. if a patient was prescribed a pack of ramipril with tablets’ strength equal to 2.5 mg then the patient was assumed to take 2.5 mg per day). The days of supply contributed to the numerator in the calculations only when nebivolol and ramipril overlapped.
Statistical analysis
SAS software version 9.4 SAS Institute Inc. (2013), Cary, NC, USA, was used to perform the analyses. The demographic, clinical, and treatment adherence characteristics of the patients were described using the following descriptive statistics: frequencies and percentages for categorical variables, and mean values (with standard deviation, SD) or median values (with first and third quartiles, Q1 and Q3) for continuous variables. Treatment adherence was classified as low when PDC was lower than 40%, intermediate for PDC between 40 and 79%, and high for values of at least 80%. Additionally, we stratified the incident users of ramipril by the dose formulation (2.5, 5, or 10 mg) prescribed over the 6-month follow-up to quantify the number of patients who were prescribed the same dosage and how many switched to a different one.
Results
Incident users and demographic characteristics (all data sources)
A total of 9889 patients on Italian LPD, 169,097 on Italian LRx, 107,642 on German LRx, 45,119 on French LRx, 224,107 on Polish LRx, and 36,618 on Hungarian NHIF started treatment with NR-EXC over the investigated period (), and so were incident users. These accounted for 5.2 and 4.4% of the total number of patients with at least one prescription of “5mg nebivolol” and/or “2.5/5/10 mg ramipril” during the selection period from the Italian LPD and LRx databases, respectively. Lower percentages of incident users were registered from the German (1.4%), French (2.2%), and Hungarian (3.5%) databases, while they accounted for a higher proportion (7%) of the Polish database.
Table 1. Attrition table for the inclusion in the cohorts of prevalent and incident users of the extemporaneous combination of nebivolol/ramipril (NR-EXC).
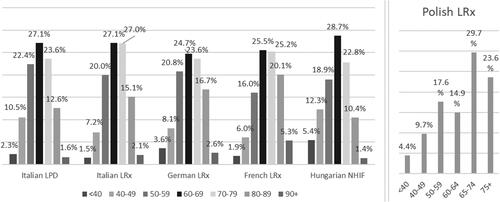
Male patients were slightly more represented in the databases from France and Poland (57.4 and 52.6% of the total, respectively), while the opposite was observed in Italy and Hungary. In Germany, male patients were 50.1% of the total. In the latter cohort, around 8% of data about sex distribution were missing (). The age distribution was comparable between the countries, with around half of the patients aged between 60 and 79 years ().
Figure 1. Distribution of incident users of the extemporaneous combination of nebivolol/ramipril (NR-EXC) by sex and country/database. Abbreviations. LPD, longitudinal patient database; LRx, longitudinal prescription database; NIHF, National Hospital Insurance Fund database.
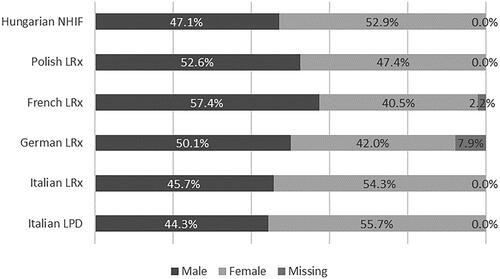
Figure 2. Distribution of incident users of the extemporaneous combination of nebivolol/ramipril (NR-EXC) by age group and country/database. Abbreviations. LPD, longitudinal patient database; LRx, longitudinal prescription database; NIHF, National Hospital Insurance Fund database.
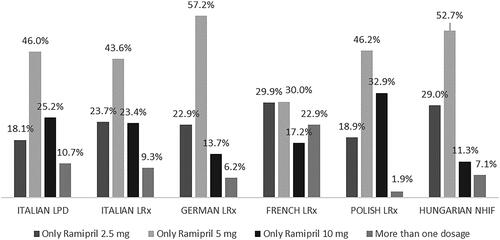
Considering the ramipril dosage in incident users, from 18.1% (Italian LPD) to 29.9% (French LRx) of the patients were prescribed only 2.5 mg, from 30.0% (French LRx) to 57.2% (German LRx) of the patients were prescribed only 5 mg, while from 11.3% (Hungarian NHIF) to 32.9% (Polish LRx) of the patients were prescribed only 10 mg ramipril over the 6-month follow-up period. A small proportion of patients (from 1.9% in Polish LRx to 10.7% in Italian LPD, and 22.9% in French LRx) switched the ramipril dose during the period investigated ().
Clinical characteristics and treatment adherence in Italian incident users (IQVIA Italian LPD)
The clinical characteristics of the incident users of the NR-EXC therapy retrieved from the Italian LPD are shown in . Among patients with a recorded value, the mean BMI value was 28.3 kg/m2 (±5.4 SD). Considering the BMI classes, overweight and obese subjects accounted for 40.0 and 32.5% of patients with available BMI values, respectively. Diabetes mellitus was the most common comorbidity (19.2%), followed by dyslipidemia (18.2%) and cardiac dysrhythmias (7.9%) (). Other conditions reported by at least 3% of the patients included other forms of chronic ischemic heart disease, acquired hypothyroidism, gout, asthma, chronic kidney disease, and occlusion and stenosis of precerebral arteries. The most frequently co-prescribed drugs during the pre-selection (six-month period before starting the NR-EXC therapy) were antithrombotics (31.8%), followed by lipid-lowering agents (29.7%), agents acting on the renin-angiotensin system other than ramipril (29.2%), calcium-channel blockers (24.2%), nonsteroidal anti-inflammatory drugs (NSAIDs) (24.0%), and diuretics (19.6%). Of note, around 60% of the patients had received at least two co-prescriptions during the pre-selection period. The co-prescribed drugs during the follow-up (six-month period after the index date) were very similar, with the most frequent being antithrombotics (38.9%) followed by lipid-lowering agents (35.9%), agents other than ramipril also acting on the renin-angiotensin system (28.4%), calcium-channel blockers (25.8%), diuretics (23.9%), and nonsteroidal anti-inflammatory drugs (22.8%). The proportion of patients with at least one cardiologic visit request only during the six-month period following initiation of treatment with NR-EXC was lower than the one observed only during baseline period (13.4 vs. 19.6%) (). Overall, almost 60% of the patients did not have any cardiologic visit request during the entire study period.
Table 2. Clinical characteristics of the incident users of the extemporaneous combination of nebivolol and ramipril (NR-EXC) from the Italian longitudinal patient database (LPD).
The mean proportion of days effectively covered by the extemporaneous combination was 38.9 (±27.6 SD), meaning that patients on average were taking both drugs for 70 out of 180 days (i.e. the follow-up duration) (). During the 6-month follow-up period, more than half of the treated patients (56.3%) were found to have low adherence to therapy whereas 32.6 and 11.1% had an intermediate and high adherence, respectively.
Table 3. Treatment adherence of the incident users of the extemporaneous combination of nebivolol and ramipril (NR-EXC) from the Italian longitudinal patient database (LPD).
Prevalent users of NR-EXC in Italy (IQVIA Italian LPD)
A total of 3581 prevalent users of NR-EXC were identified in the Italian LPD in 2019 (). This number translated into an estimated 159,000 adult (≥18 years old) patients treated at the national level with the extemporaneous combination (data not shown) which were considered potentially eligible for the nebivolol/ramipril SPC.
Sensitivity analysis (IQVIA Italian LPD)
The analysis accounting for prescriptions of nebivolol and ramipril as FDCs with hydrochlorothiazide in addition to single-molecule prescriptions on Italian LPD found 12,907 patients meeting the criteria for inclusion in the cohort of incident users of NR-EXC (see Supplementary Material). Results from the analysis run on such a cohort were comparable to those of the incident users of NR-EXC, considering only nebivolol and ramipril as single molecules (data not shown, available upon request).
Discussion
The combined use of β-blockers and ACE inhibitors for better control of hypertension has been advocatedCitation19, mostly based on their synergistic and complementary effects on cardiovascular risk and disease outcomes. Nebivolol and ramipril are currently available in Europe either separately or in the form of SPC with other drugsCitation39–41.
Our study, based on the analysis of European databases, demonstrates that the extemporaneous prescription of these two drugs is consolidated in clinical practice in the countries examined. To the best of our knowledge, the present study is the first research reporting evidence on the use of these two agents in combination therapy for hypertension.
The detailed analysis of Italian data revealed that the combination was prescribed mainly to patients over 50 years of age, with comorbidities, such as diabetes and dyslipidemia. These findings are expected considering the known risk factors for hypertensionCitation42 and are comparable to the results of similar studies on hypertensive patientsCitation32,Citation33,Citation43,Citation44. Moreover, the analysis of different countries revealed that the predominant prescription dosage of ramipril was 5 mg.
Thanks to its peculiar vasodilating features, nebivolol is a cardio selective β-blocker associated with fewer side effects compared to other β-blockers. Indeed, nebivolol demonstrated superior efficacy over other β-blockers, such as metoprolol, in improving oxidative stress, insulin sensitivity, and inflammatory markers in hypertensive patients. These properties may contribute to a reduction in cardiovascular risk associated with hypertension, offering cardiovascular benefits beyond blood pressure controlCitation45. The benefits of nebivolol in improving pathophysiology of hypertension and its complications may be due to its unique properties that include endothelial-dependent vasodilation through the nitric oxide pathway and potential anti-inflammatory effects. The latter is confirmed by the decreased red cell distribution width and neutrophil/lymphocyte ratio, which are indicators of vascular inflammationCitation46.
The analysis suggested a low adherence to NR-EXC therapy in more than half of the patients. As the patients were supposedly taking other antihypertensive drugs, it cannot be ruled out that this might have affected overall treatment plans, influencing the adherence to the analyzed therapy. Treatment adherence is a central issue in the management of high BP since even modest changes in adherence can lead to clinically significant reduction in blood pressure values with consequent long-term reduction of cardiovascular eventsCitation47. However, the levels of adherence recorded in this analysis are in line with what has been shown in studies comparing treatment adherence of patients on extemporaneous combination of antihypertensive treatments vs. fixed doseCitation43,Citation44,Citation48,Citation49. Several studies demonstrated that SPC therapy increases compliance and persistence and also increases BP control rates in comparison to extemporaneous combination therapyCitation2.
Indeed, the results of a real-life study in hypertensive patients who were poorly adherent to individual drug combination showed that switching to a SPC significantly improved adherenceCitation50. This is especially important when considering people older than 60 years already prescribed a high number of pillsCitation51. The improved adherence has the potential to reduce healthcare utilization. By reducing the frequency of cardiovascular events and hospital visits, as pointed out by several studiesCitation52,Citation53, an SPC could decrease healthcare costs and improve resource allocation, which is particularly significant in the context of an aging population with multiple comorbiditiesCitation54,Citation55. In addition, the use of SPC results effective in reaching BP target levels and patients on SPC are usually not taking additional therapies for hypertension, thus adding to the overall cost-effectiveness of the therapyCitation52,Citation55. Furthermore, SPCs are often available in different doses of each component, thus offering convenience in reducing the need to adjust individual drug doses. This increases the ease of use of the medication, facilitating dose adjustment for the clinician, allowing to easily avoid the therapeutic inertiaCitation55.
It is estimated that 25–35% of patients require more than two antihypertensive agents to achieve acceptable control of hypertensionCitation56, supporting the use of SPC to reduce the number of daily pills needed, as promoted by the latest guidelines by both the European and International Societies of Hypertension. Adoption of SPCs is also endorsed by the American College of Cardiology/American Heart Association Task ForceCitation2,Citation57,Citation58. The use of SPC might facilitate patient-centric care. The SPC simplifies the treatment regimen, which is likely to be particularly beneficial for elderly patients or those with cognitive impairments who might struggle with complex medication schedulesCitation55,Citation59,Citation60.
Overweight and obese patients represented a significant percentage of study population as highlighted form the IQVIA Italian LPD database. Although a similar detailed characterization is not available for the population of the other European countries, we believe this data are representative of the overall situation in Europe. Indeed, a study from Poland showed that uncontrolled hypertension was common in overweight and obese patients (39.4 and 48.5%, respectively), and similar data were reported in a study which analyzed German patientsCitation61,Citation62. Indeed, although an extensive clinical characterization of patient population from all the European countries included in the study is not present, it is likely to assume that the description of the Italian patients is representative of the overall cohort.
This study has the same limitations common to other real-world studies. First, it relies only on data coming from written prescriptions. Assumptions were made, presupposing the consumption of all prescribed medications. However, we acknowledge the potential divergence from reality, which may lead to an overestimation of adherence rates. Secondly, since visits done in private healthcare settings do not require a written prescription by a GP, the number of patients requesting cardiology appointments may have been underestimated. Third, we could not select patients based on the presence of a registered hypertension diagnosis from countries other than Italy because the European IQVIA LRx and Hungarian NHIF databases do not include information on patients’ diagnoses. However, since in the Italian IQVIA LPD, we found only a low proportion of patients treated with the NR-EXC who were not diagnosed with hypertension, it can be confidently assumed that the majority of patients included from the other European countries were hypertensive. In addition, the detailed clinical characteristics of the patients in Germany, France, Poland, and Hungary are not available as not supported by the analyzed databases, therefore they can only be assumed as similar to what was found from IQVIA Italian LPD database.
Notwithstanding the limitations, this study can be considered representative of the general European population due to the evaluation of a large number of patients in rigorous databases. This allowed a detailed clinical characterization of these patients in a real-world setting. Additionally, similar findings in different countries suggest the validity of our approach. To our knowledge, this is the first pan-European analysis of the current prescribing behaviors of nebivolol and ramipril for the management of hypertensive patients.
Recent data indicate that treatment with a BB and a RAS blocker is a well-tolerated and effective combination that may be helpful for many hypertensive individualsCitation63–65. In particular, nebivolol combined with a RAS blocker showed both an additional BP lowering effect and a good safety profileCitation63,Citation66. In light of their distinct pharmacological effects and their antihypertensive effectiveness, the development of an SPC including nebivolol and ramipril may offer a viable therapeutic option for managing hypertension. Further analysis of real-world data is required to better understand the potential therapeutic benefits of such SPC, particularly in terms of improved adherence and impact on BP control.
Conclusions
Our findings confirm that the co-prescription of nebivolol and ramipril is a common practice in different European countries. Patients prescribed the extemporaneous combination of nebivolol and ramipril often recorded multiple comorbidities and relevant concurrent treatments. In addition, the analysis of prescription adherence revealed a significant proportion of patients exhibiting low adherence levels. Thus, the development of an SPC of nebivolol and ramipril holds promise. Such a formulation could enhance treatment adherence and streamline regimens, potentially leading to significant benefits. Improved adherence not only correlates with better BP control but also reduces the risk of cardiovascular events, underscoring the importance of this development.
Transparency
Author contributions
All authors contributed to the study conception and design. Data extraction and analysis were performed by Riccardo Cipelli, Claudio Ripellino, and Valeria Pegoraro, who also wrote the first draft of the manuscript. All authors commented on previous versions of the manuscript. All authors take responsibility for the integrity of the work as a whole and have given final approval for the version to be submitted.
Ethical approval
All the analyses presented here did not involve any new study of human or animal subjects performed by any of the authors. The databases used for this study include only anonymized data in compliance with the provisions set forth in the applicable data protection laws, which allow the use of anonymous electronic medical records for research purposes under certain conditions. In accordance with this legislation, it is not necessary to obtain informed consent from patients or approval from a medical ethics committee for this type of observational study that contains no directly identifiable data. Since all patients were only queried as aggregates and no protected health information was available for queries, no institutional review board approval was required for the completion of this study in line with previous works.
Nebivolol_Ramipril_Supplementary.docx
Download MS Word (79.8 KB)Acknowledgements
Editorial support, funded by Menarini was provided by Corrado Minetti, PhD on behalf of Health, Publishing and Services s.r.l., according to Good Publication Practice.
Declaration of financial/other relationships
R.C. and V.P. are employees of IQVIA. C.R. worked for IQVIA as an external consultant. M.M., S.M., M.G., and P.F. have disclosed that he/she is currently an employee of Menarini Group and was employed at the time of study conduct. G.D. has nothing to declare. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary Materials.
Additional information
Funding
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398(10304):957–980. doi: 10.1016/S0140-6736(21)01330-1.
- Mancia G, Kreutz R, Brunström M, et al. 2023 ESH guidelines for the management of arterial hypertension the task force for the management of arterial hypertension of the European Society of Hypertension endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J Hypertens. 2023;41(12):1874–2071. doi: 10.1097/HJH.0000000000003480.
- Tocci G, Nati G, Cricelli C, et al. Prevalence and control of hypertension in the general practice in Italy: updated analysis of a large database. J Hum Hypertens. 2017;31(4):258–262. doi: 10.1038/jhh.2016.71.
- Carey RM, Muntner P, Bosworth HB, et al. Prevention and control of hypertension: JACC health promotion series. J Am Coll Cardiol. 2018;72(11):1278–1293. doi: 10.1016/j.jacc.2018.07.008.
- Mennini FS, Marcellusi A, von der Schulenburg JMG, et al. Cost of poor adherence to anti-hypertensive therapy in five European countries. Eur J Health Econ. 2015;16(1):65–72. doi: 10.1007/s10198-013-0554-4.
- Münzel T, Gori T. Nebivolol: the somewhat-different beta-adrenergic receptor blocker. J Am Coll Cardiol. 2009;54(16):1491–1499. doi: 10.1016/j.jacc.2009.05.066.
- FDA. Nebivolol drug approval package; 2007 [cited 2023 Oct 10]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/021742s000TOC.cfm#:∼:text=Approval%20Date%3A%2012/17/2007
- EMA. Nebivolol; 2015 [cited 2023 Oct 10]. Available from: https://www.ema.europa.eu/en/medicines/human/paediatric-investigation-plans/emea-001761-pip01-15
- Fongemie J, Felix-Getzik E. A review of nebivolol pharmacology and clinical evidence. Drugs. 2015;75(12):1349–1371. doi: 10.1007/s40265-015-0435-5.
- Hayek SS, Poole JC, Neuman R, et al. Differential effects of nebivolol and metoprolol on arterial stiffness, circulating progenitor cells, and oxidative stress. J Am Soc Hypertens. 2015;9(3):206–213. doi: 10.1016/j.jash.2014.12.013.
- Ferri C. The role of nebivolol in the management of hypertensive patients: from pharmacological profile to treatment guidelines. Future Cardiol. 2021;17(8):1421–1433. doi: 10.2217/fca-2021-0048.
- Chauhan M, Patel JB, Ramipril AF. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537119/
- Mancia G, Parati G, Bilo G, et al. Ambulatory blood pressure values in the ongoing telmisartan alone and in combination with ramipril global endpoint trial (ONTARGET). Hypertension. 2012;60(6):1400–1406. doi: 10.1161/HYPERTENSIONAHA.112.199562.
- Todd PA, Benfield P. Ramipril. A review of its pharmacological properties and therapeutic efficacy in cardiovascular disorders. Drugs. 1990;39(1):110–135. doi: 10.2165/00003495-199039010-00009.
- Miranda RD, Mion D, Rocha JC, et al. An 18-week, prospective, randomized, double-blind, multicenter study of amlodipine/ramipril combination versus amlodipine monotherapy in the treatment of hypertension: the assessment of combination therapy of amlodipine/ramipril (ATAR) study. Clin Ther. 2008;30(9):1618–1628.
- Bull S, Loudon M, Francis JM, et al. A prospective, double-blind, randomized controlled trial of the angiotensin-converting enzyme inhibitor ramipril in aortic stenosis (RIAS trial). Eur Heart J Cardiovasc Imaging. 2015;16(8):834–841.
- Lüders S, Schrader J, Berger J, et al. The PHARAO study: prevention of hypertension with the angiotensin-converting enzyme inhibitor ramipril in patients with high-normal blood pressure: a prospective, randomized, controlled prevention trial of the German hypertension league. J Hypertens. 2008;26(7):1487–1496. doi: 10.1097/HJH.0b013e3282ff8864.
- Mancia G, Dell’Oro R, Quarti-Trevano F, et al. Angiotensin-sympathetic system interactions in cardiovascular and metabolic disease. J Hypertens Suppl. 2006;24(1):S51–S56. doi: 10.1097/01.hjh.0000220407.84363.fb.
- Strauss MH, Hall AS, Narkiewicz K. The combination of beta-blockers and ACE inhibitors across the spectrum of cardiovascular diseases. Cardiovasc Drugs Ther. 2023;37(4):757–770. doi: 10.1007/s10557-021-07248-1.
- Putignano D, Bruzzese D, Orlando V, et al. Differences in drug use between men and women: an Italian cross sectional study. BMC Womens Health. 2017;17(1):73.
- Federici MO, McQuillan J, Biricolti G, et al. Utilization patterns of glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes mellitus in Italy: a retrospective cohort study. Diabetes Ther. 2018;9(2):789–801. doi: 10.1007/s13300-018-0396-2.
- Helwig U, Kostev K, Schmidt C. Comparative analysis of 3-year persistence with vedolizumab compared with antibodies against tumor necrosis factor-alpha in patients with inflammatory bowel disease in Germany: retrospective analysis of a large prescription database. J Clin Gastroenterol. 2021;55(1):e1–e7. doi: 10.1097/MCG.0000000000001323.
- Richter H, Dombrowski S, Hamer H, et al. Use of a German longitudinal prescription database (LRx) in pharmacoepidemiology. Ger Med Sci. 2015;13:doc14.
- Kaplan S, Ehlken B, Hamann X. Drug utilization patterns of flupirtine following implementation of risk minimization measures in Germany. Curr Med Res Opin. 2019;35(8):1397–1403. doi: 10.1080/03007995.2019.1594743.
- Vilcu AM, Blanchon T, Sabatte L, et al. Cross-validation of an algorithm detecting acute gastroenteritis episodes from prescribed drug dispensing data in France: comparison with clinical data reported in a primary care surveillance system, winter seasons 2014/15 to 2016/17. BMC Med Res Methodol. 2019;19(1):110. doi: 10.1186/s12874-019-0745-5.
- Etude de l’observance médicamenteuse des patients diabétiques de type II en Île-de-France. Agence Régionale de Santé Ile-de-France; 2016 [cited 2023 Oct 24]. Available from: https://www.iledefrance.ars.sante.fr/sites/default/files/2017-01/Observance-Diabete-type-II-ARSIDF-IMS-2016.pdf
- Joumaa H, Sigogne R, Maravic M, et al. Artificial intelligence to differentiate asthma from COPD in medico-administrative databases. BMC Pulm Med. 2022;22(1):357. doi: 10.1186/s12890-022-02144-2.
- Rathmann W, Czech M, Franek E, et al. Regional differences in insulin therapy regimens in five European countries. Int J Clin Pharmacol Ther. 2017;55(5):403–408.
- Rathmann W, Czech M, Franek E, et al. Treatment persistence in the use of basal insulins in Poland and Germany. Int J Clin Pharmacol Ther. 2017;55(2):119–125.
- Bekele BB, Harsha N, Kőrösi L, et al. Is prescription nonredemption a source of poor health among the Roma? Cross-sectional analysis of drug consumption data from the national health insurance fund of Hungary. Front Pharmacol. 2021;12:616092. doi: 10.3389/fphar.2021.616092.
- Kurti Z, Vegh Z, Golovics PA, et al. Nationwide prevalence and drug treatment practices of inflammatory bowel diseases in Hungary: a population-based study based on the National Health Insurance Fund database. Dig Liver Dis. 2016;48(11):1302–1307.
- Volpe M, Pegoraro V, Heiman F, et al. Extemporaneous combination therapy with amlodipine/zofenopril in hypertensive patients: a real-world data analysis in Italy. Curr Med Res Opin. 2023;39(12):1593–1601. doi: 10.1080/03007995.2023.2192607.
- Volpe M, Pegoraro V, Peduto I, et al. Extemporaneous combination therapy with nebivolol/zofenopril in hypertensive patients: usage in Italy. Curr Med Res Opin. 2022;38(10):1673–1681. doi: 10.1080/03007995.2022.2096352.
- Iqvia. Osservatorio sull’impatto della pandemia COVID-19 sull’accesso alle cure. Periodo dati: 2019–2020; 2021.
- SIIA. Ipertensione: i numeri in Italia [cited 2023 Oct 24]. Available from: https://siia.it/per-il-pubblico/ipertensione/ipertensione-i-numeri-in-italia/
- ISTAT. Popolazione residente al 1° gennaio; 2023. Available from: http://dati.istat.it/Index.aspx?QueryId=42869
- Prieto-Merino D, Mulick A, Armstrong C, et al. Estimating proportion of days covered (PDC) using real-world online medicine suppliers’ datasets. J Pharm Policy Pract. 2021;14(1):113. doi: 10.1186/s40545-021-00385-w.
- Asamoah-Boaheng M, Osei Bonsu K, Farrell J, et al. Measuring medication adherence in a population-based asthma administrative pharmacy database: a systematic review and meta-analysis. Clin Epidemiol. 2021;13:981–1010.
- Paton DM. Nebivolol/valsartan: fixed-dose combination for treatment of hypertension. Drugs Today. 2017;53(1):19–26. doi: 10.1358/dot.2017.53.1.2560078.
- Oigman W, Gomes MA, Pereira-Barretto AC, et al. Efficacy and safety of two ramipril and hydrochlorothiazide fixed-dose combination formulations in adults with stage 1 or stage 2 arterial hypertension evaluated by using ABPM. Clin Ther. 2013;35(5):702–710. doi: 10.1016/j.clinthera.2013.03.015.
- Jain SD, Biradar S, Periyandavar I, et al. Effects of oral fixed-dose combinations of telmisartan plus ramipril and losartan plus ramipril in hypertension: a multicenter, prospective, randomized, double-blind, phase III trial in adult Indian patients. Curr Ther Res Clin Exp. 2005;66(6):630–642.
- Mancia G, Volpe R, Boros S, et al. Cardiovascular risk profile and blood pressure control in Italian hypertensive patients under specialist care. J Hypertens. 2004;22(1):51–57.
- Yang W, Chang J, Kahler KH, et al. Evaluation of compliance and health care utilization in patients treated with single pill vs. free combination antihypertensives. Curr Med Res Opin. 2010;26(9):2065–2076. doi: 10.1185/03007995.2010.494462.
- Mazzaglia G, Ambrosioni E, Alacqua M, et al. Adherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patients. Circulation. 2009;120(16):1598–1605. doi: 10.1161/CIRCULATIONAHA.108.830299.
- Celik T, Iyisoy A, Kursaklioglu H, et al. Comparative effects of nebivolol and metoprolol on oxidative stress, insulin resistance, plasma adiponectin and soluble P-selectin levels in hypertensive patients. J Hypertens. 2006;24(3):591–596. doi: 10.1097/01.hjh.0000209993.26057.de.
- Fici F, Celik T, Balta S, et al. Comparative effects of nebivolol and metoprolol on red cell distribution width and neutrophil/lymphocyte ratio in patients with newly diagnosed essential hypertension. J Cardiovasc Pharmacol. 2013;62(4):388–393.
- Conn VS, Ruppar TM, Chase J-AD, et al. Interventions to improve medication adherence in hypertensive patients: systematic review and meta-analysis. Curr Hypertens Rep. 2015;17(12):94. doi: 10.1007/s11906-015-0606-5.
- Tocci G, Presta V, Ferri C, et al. Blood pressure targets achievement according to 2018 ESC/ESH guidelines in three European excellence centers for hypertension. High Blood Press Cardiovasc Prev. 2020;27(1):51–59. doi: 10.1007/s40292-020-00359-0.
- Del Pinto R, Desideri G, Ferri C, et al. Real-world antihypertensive treatment patterns, treatment adherence, and blood pressure control in the elderly: an Italian awareness-raising campaign on hypertension by senior Italia FederAnziani, the Italian Society of Hypertension and the Italian Federation of General Practitioners. High Blood Press Cardiovasc Prev. 2021;28(5):457–466. doi: 10.1007/s40292-021-00465-7.
- Rea F, Savaré L, Franchi M, et al. Adherence to treatment by initial antihypertensive mono and combination therapies. Am J Hypertens. 2021;34(10):1083–1091. doi: 10.1093/ajh/hpab083.
- OSMED. L'uso dei farmaci in Italia. Rapporto nazionale anno 2022; 2023 [cited 2023 Oct 10]. Available from: https://www.aifa.gov.it/documents/20142/1967301/Rapporto-OsMed-2022.pdf
- Bramlage P, Schmidt S, Sims H. Fixed-dose vs free-dose combinations for the management of hypertension–an analysis of 81 958 patients. J Clin Hypertens. 2018;20(4):705–715.
- Hilleman DE. Adherence and health care costs with single-pill fixed-dose combinations in hypertension management. J Manag Care Pharm. 2014;20(1):93–100. doi: 10.18553/jmcp.2014.20.1.93.
- Jones CH, Dolsten M. Healthcare on the brink: navigating the challenges of an aging society in the United States. NPJ Aging. 2024;10(1):22. doi: 10.1038/s41514-024-00148-2.
- Mancia G, Cappuccio FP, Burnier M, et al. Perspectives on improving blood pressure control to reduce the clinical and economic burden of hypertension. J Intern Med. 2023;294(3):251–268. doi: 10.1111/joim.13678.
- Düsing R, Waeber B, Destro M, et al. Triple-combination therapy in the treatment of hypertension: a review of the evidence. J Hum Hypertens. 2017;31(8):501–510. doi: 10.1038/jhh.2017.5.
- Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75(6):1334–1357. doi: 10.1161/HYPERTENSIONAHA.120.15026.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice guidelines. Hypertension. 2018;71(6):e13–e115.
- Sarzani R, Laureti G, Gezzi A, et al. Single-pill fixed-dose drug combinations to reduce blood pressure: the right pill for the right patient. Ther Adv Chronic Dis. 2022;13:20406223221102754. doi: 10.1177/20406223221102754.
- Munger MA. Polypharmacy and combination therapy in the management of hypertension in elderly patients with co-morbid diabetes mellitus. Drugs Aging. 2010;27(11):871–883. doi: 10.2165/11538650-000000000-00000.
- Chudek A, Owczarek AJ, Ficek J, et al. A stronger effect of body mass index and waist circumference on the prevalence of uncontrolled hypertension among Caucasian men than women. Kidney Blood Press Res. 2021;46(3):298–309. doi: 10.1159/000514346.
- DiBonaventura M, Nicolucci A, Meincke H, et al. Obesity in Germany and Italy: prevalence, comorbidities, and associations with patient outcomes. Clinicoecon Outcomes Res. 2018;10:457–475. doi: 10.2147/CEOR.S157673.
- Weber MA, Basile J, Stapff M, et al. Blood pressure effects of combined beta-blocker and angiotensin-converting enzyme inhibitor therapy compared with the individual agents: a placebo-controlled study with nebivolol and lisinopril. J Clin Hypertens. 2012;14(9):588–592.
- Boytsov SA, Burtsev YP, Khomitskaya YV, et al. Effectiveness and tolerability of the single-pill combination of bisoprolol and perindopril in patients with arterial hypertension and stable coronary artery disease in daily clinical practice: the STYLE study. Adv Ther. 2021;38(6):3299–3313. doi: 10.1007/s12325-021-01754-2.
- Abeel M, Gupta A, Constance C. Concomitant treatment of hypertensive patients with bisoprolol and perindopril in routine clinical practice: a post hoc analysis of the CONFIDENCE II, PROTECT I, and PROTECT III observational studies. Adv Ther. 2022;39(1):391–404. doi: 10.1007/s12325-021-01958-6.
- Neutel JM, Smith DH, Gradman AH. Adding nebivolol to ongoing antihypertensive therapy improves blood pressure and response rates in patients with uncontrolled stage I–II hypertension. J Hum Hypertens. 2010;24(1):64–73. doi: 10.1038/jhh.2009.33.