Abstract
Objective
Current cost-effectiveness analyses (CEA) emphasize drug costs as the differentiator between NICE recommended anti-VEGF treatments but may neglect real-world non-drug costs of running nAMD services in the UK. To address this, this study identified real-world non-drug service cost items relevant to UK NHS nAMD clinics, including costs arising from operational strain (demand exceeding capacity).
Methods
Cost items were identified by a structured literature review of peer-reviewed and grey literature, and an expert panel of 10 UK-based ophthalmologists with relevance to real-world practice. These items underwent meta-synthesis and were then determined in a consensus exercise.
Results
Of 237 cost items identified, 217 (91.6%) met the consensus threshold of >0.51 and were included in the nAMD Service Non-Drug Cost Instrument (nAS). Sensitivity of cost items taken from UK Health Technology Assessment (HTA) using the nAS as the reference standard was low (HTAmin: 1.84%, 95% CI 0.50–4.65%; HTAmax: 70.51%, 95% CI 63.96–76.49%). False negative rates showed variable likelihood of misclassifying a service by cost burden depending on prevalence. Scenario analysis using cost magnitudes estimated annual per-patient clinic cost at £845 (within capacity) to £13,960 (under strain) compared to an HTAmin estimate of £210. Accounting for cost of strain under an assumed 50% increase in health resource utilization influenced cost-effectiveness in a hypothetical genericisation scenario.
Conclusion
Findings suggested that HTA underestimates UK NHS nAMD clinic cost burden with cost of strain contributing substantial additional unmeasured expense with impact on CEA. Given potential undertreatment due to strain, durability is suggested as one of the relevant factors in CEA of nAMD anti-VEGF treatments due to robustness under limited capacity conditions affecting UK ophthalmology services.
PLAIN LANGUAGE SUMMARY
When considering how well treatments work versus how much they cost, the focus is usually only on the price of the medicine itself. However, other real-world costs exist. In the UK, when treating certain eye problems such as neovascular age-related macular degeneration (nAMD), there are additional expenses related to running clinics and managing treatments that often go unnoticed. To get a better understanding of these hidden costs, the study examined factors like clinic workload and the extra expenses that come with it. Ten eye doctors in the UK were consulted for their expert opinions and numerous research papers were reviewed to identify these additional costs. The study grouped different costs in a tool called the nAMD Service Non-Drug Cost Instrument (nAS). When the findings of the nAS tool were compared to the usual methods of calculating costs, it was found that the conventional approach overlooked many of the actual expenses. Busy clinics face unique challenges, such as higher operational costs associated with staffing for extended hours, emergency appointments, extended waiting times and the potential to miss optimal treatment windows. This can lead to disease progression and the onset of comorbidities, which require more complex and costly treatments. Recognizing these real costs is crucial when making decisions about treatments, especially when treatments require more frequent visits to eye clinics. This study emphasizes the importance of considering all expenses, not just the obvious ones like medication and doctor visits when determining the most effective way to manage eye conditions like nAMD in the UK.
Background
Neovascular age-related macular degeneration (nAMD), also known as wet macular degeneration is a late-stage age-related macular degeneration (AMD) occurring in an estimated 10–15% of AMD patients and is associated with high rates of vision loss if untreated. Cases of nAMD in the United Kingdom (UK) in 2015 were estimated at 411,000, projected to increase by 27% to 521,000 in 2025 and 64% to 672,000 in 2035Citation1. Increasing prevalence has implications for demand on nAMD healthcare services and associated service delivery costs, especially with increasing demand for National Health Service (NHS) ophthalmic clinics from continual quality and accessibility service improvementCitation2,Citation3. As a result, NHS clinic service capacity may fall short of the threshold needed to meet recommended follow-up intervals. Increasing demand for nAMD services exists in the context of a limited NHS budget. In 2008, estimated average societal cost for each bilateral nAMD patient treated in the UK was €5300/year, 23–63% of which comprised direct vision-related medical costsCitation4. Meeting recommended follow-up intervals may improve treatment outcomes, with potential cost-saving on the contribution of nAMD to the £3.0 billion direct healthcare system costs relating to sight loss and blindnessCitation5. Cost-effectiveness is a priority in nAMD service planning. Analyses have focused on nAMD treatment drug costs, which rank among the highest in the NHS medicines budget, neglecting non-drug-costs with apparent detriment to accuracy of cost-effectivenessCitation2,Citation6–9. Extent of this impact must be determined to support ophthalmology service planning in the UK.
Intravitreal anti-vascular endothelial growth factor (anti-VEGF) treatment was first licensed in the UK for ophthalmic indications in 2006 and is the gold standard of care for nAMDCitation10. The National Institute of Health and Care Excellence (NICE) has recommended four licensed anti-VEGF agents as options for nAMD treatment: aflibercept (Eylea®; Bayer Plc), brolucizumab (Beovu®; Novartis Pharmaceuticals UK Ltd), faricimab (Vabysmo®; Roche Products Ltd) and ranibizumab (Lucentis®; Novartis Pharmaceuticals UK Ltd), with mention but no explicit recommendation of bevacizumab (Avastin®; Roche Products Ltd), which is unlicensed for intravitreal useCitation11–15. National Institute for Health and Care Excellence (NICE) Technology Appraisals for ranibizumab cover three licensed ranibizumab biosimilars (Byooviz®, Ongavia® and Ximluci®), which are subject to NHS England commissioning recommendationsCitation16. Based on evidence from pivotal randomized controlled trials ANCHOR, MARINA, VIEW I & II, HAWK, HARRIER, TENAYA and LUCERNE, NICE recognizes no clinically significant differences in safety and efficacy between anti-VEGF treatments in nAMD but states that cost-effectiveness may be influenced by net acquisition, administration and monitoring costsCitation12,Citation17–21. Current cost-per-dose list prices are equivalent (aflibercept, £816; brolucizumab, £816; faricimab, £857), except for the reference ranibizumab product (Lucentis; £551), although the respective patient access schemes provide these treatments to the NHS at a confidential discountCitation22–25. Acquisition costs for biosimilar ranibizumab are lower. Costs in the company base case scenario for a cost-effectiveness analysis of faricimab included diagnosis costs of £130 for fundus fluorescein angiography (FFA); administration costs of £126 for optical coherence tomography (OCT) plus £54 intravitreal injection additional costs; and monitoring costs of £102 for a consultant-led outpatient visit, £126 for OCT and £82 for caregiver costsCitation15.
Most anti-VEGF treatments have been approved for treat and extend dose regimens, the aim of which is to maintain optimal clinical outcomes with reduced injection frequency compared to fixed and pro re nata regimensCitation22–26. Recommended follow-up times based on clinical trial efficacy vary () and have been attributed to variance in durability with expected benefit of high durability in reducing treatment and monitoring burden to patients and health systemsCitation27–29. Company base cases in recent health technology assessments (HTAs) have used costs per visit to value non-drug cost burden of nAMD anti-VEGF treatments according to expected per-patient annual administration and monitoring visit frequencyCitation11,Citation13–15. NICE recognizes that lower injection and monitoring visit frequency may reduce costs but has suggested that visit frequency is likely to be equivalent due to clinical practice minimum standardsCitation15. As such, technology appraisal guidance expects that non-drug costs are generally comparable across anti-VEGF treatments with resulting emphasis on acquisition cost in cost-effectiveness analysis and treatment decisionsCitation15,Citation16. However, routinely measured costs associated with monitoring visit and injection frequency omit costs incurred when per-patient monitoring visit and injection frequency produces overall demand that exceeds clinic capacity, including cost consequences of reduced clinical outcomes when services are unable to deliver optimal dose regimenCitation28,Citation30. Given the relation between undertreatment and suboptimal clinical response, high durability could be more important in clinical practice than initially thought, if commensurate clinical and health system impacts of missed monitoring and injection visits due to strain are reducedCitation6.
Table 1. Approved posology and administration for anti-VEGF treatments licensed for use in nAMD in the UK.
Specific costs associated with strain in nAMD service provision have not been defined or measured. Given the substantial burden of avoidable vision loss to patients, health systems and society that may arise due to strain, there is value in identifying and considering these costs in cost-effectiveness comparison of anti-VEGF treatmentsCitation31,Citation32. This study aimed to identify real-world cost items related to NHS nAMD treatment and monitoring service provision in the UK, including costs arising from strain, and understand the extent to which current HTAs value these costs accurately. Impact of cost of strain on anti-VEGF treatment cost-effectiveness comparison was considered using a hypothetical genericization scenario describing entry of a low-cost generic drug to a pharmaceutical market after brand-name patent expiration.
Methods
Study design
The study design was a mixed-methods approach. Real-world cost items associated with running an NHS nAMD treatment and monitoring service in the UK were identified using the Jandhyala method, an inductive approach incorporating a structured literature review, integrating findings from multiple studies (meta-synthesis) and experts’ structured agreement (consensus) exercise to develop highly accurate disease-specific construct measures and core datasets in contexts with paucity of data or where generic measures are inaccurateCitation33–38. Ecological validity of current HTA cost lists was determined by a Neutrality analysis of sensitivity and specificity using the list of identified real-world cost items as the reference standard. A case study of two hypothetical drugs in a genericization scenario showed the impact of accounting for cost of strain in nAMD anti-VEGF treatment, which may have a significant impact on cost-effective analysis (CEA).
Conceptual basis
Neutral theory is a conceptual framework that defines the accuracy of a construct measure relative to an absolute standard represented by theoretically ideal levels of sensitivity (S) and specificity (C), where sensitivity is defined as the inclusion of all relevant indicators and specificity is defined as the exclusion of all irrelevant indicators. The Jandhyala method aims to identify all evidence relevant to the target construct via an iterative structured literature review process and survey of expert opinion, viewing constructs as inherently situated and subjective. Meta-synthesis is informed by hermeneutic principles. Relevance of indicators to the construct is defined as operational implementation in a specific setting or population and is determined by a consensus panel of clinicians with experience in the real-world setting. Neutrality analysis uses the empirically defined construct as a reference standard with sensitivity and specificity showing ecological validity of existing measures within the limits of the defined study area and population, assuming its utmost importance in real-world utilityCitation38,Citation39. Within stated assumptions, the Jandhyala method has been used in this study to develop an accurate list of real-world cost items related to NHS nAMD treatment and monitoring service delivery in the UK for use in health economic analysis (HEA) and service planning.
Expert panel
The expert panel comprised 10 consultant ophthalmologists practicing in NHS nAMD services in the UK at the time of study. Expertise requisite to determining operational relevance of treatment and monitoring cost items to UK NHS clinical practice in nAMD was qualified as current experience in diagnosis and treatment of nAMD in an NHS setting in the UK; recent publications on anti-VEGF treatments for nAMD in high-impact journals and/or authorship of national and international guidelines for management of retinopathies. All members of the panel were given the opportunity to contribute as authors to this manuscript. Those listed as authors, who are not affiliated with Bayer PLC or Medialis Ltd., were members of the expert panel. Detailed affiliations and contributions of the expert panel members are acknowledged in the 'Acknowledgements’ section to ensure full transparency of their involvement and potential conflicts of interest.
Real-world cost items associated with NHS nAMD treatment and monitoring service delivery
Structured literature review cost item identification and meta-synthesis
Due to the limited and heterogeneous nature of existing evidence on nAMD treatment and monitoring service delivery, a structured literature review methodology was adopted. The approach was closely aligned with the systematic principles outlined in Higgins et al.Citation40. However, a formal quality assessment of the included evidence was not feasible, owing to the inclusion of non-peer-reviewed sources such as websites and conference abstracts. The objective of the structured literature review was to identify cost outcomes with potential relevance to the provision of an NHS nAMD treatment and monitoring service in a UK setting. The final review question was refined iteratively to reflect situated cost outcomes defined in the literature, “What cost burdens are incurred by individuals and caregivers as a result of nAMD and nAMD treatment and monitoring from patient, healthcare provider, and health system health economic perspectives?” Meta-synthesis was applied to narrow review findings to operational cost items from a healthcare clinic perspective.
A protocol was developed a priori and refined iteratively. Preliminary searching and a sample set of eight test papers was used to test and refine initial inclusion and exclusion criteria based on the population, intervention, comparator, outcome, setting, and timing (PICOST) framework with discrepancies settled by a senior systematic reviewerCitation41. Outstanding issues were resolved by testing and refining search strings, which were developed using keyword analysis and the established PICOST framework (Supplemental Table 1). The research context and exploratory nature of the review lent itself to ongoing refinement of the protocol and criteria based on principles of the Joanna Briggs Institute framework for systematic scoping reviews although some modifications were made to account for the specific, targeted nature of the search relating to cost concept identificationCitation42. Searching was conducted in four electronic databases: PubMed, the Cochrane Library, the University of York Database and Google Scholar and six sources of grey literature: websites of the Medicines and Healthcare Products Regulatory Agency (MHRA), NICE, the Royal College of Ophthalmologists, Macular Society, nAMD Barometer and Google. The grey literature included non-peer-reviewed reports, conference abstracts and website articles. Date of last access of all sources was 23 March 2023.
Initial title and abstract screening were conducted by two research analysts using Rayyan, a web-based tool designed to facilitate the systematic review process by streamlining the management and screening of citationsCitation43. Full-text articles were retrieved and submitted to eligibility assessment using refined criteria (). Reference lists of grey literature were hand-searched for eligible published studies. Reasons for exclusion were recorded. A standardized data extraction table was developed and refined iteratively. Research analysts extracted data on author, year, title, aim, method, population, outcome, study type and key findings relating to the review question. Targeted extraction of conceptual and operational definitions of cost burdens within the limits of the population, concept and context was employed. Analysts extracted data and completed quality checks independently. Senior reviewers (RW & RJ) with over 10 years of experience in systematic literature reviews arbitrated all discrepancies. Qualitative meta-synthesis to interpret key findings proceeded via an iterative process informed by thematic analysis methodology after an expert survey in Awareness Round (1) of the consensus exercise.
Table 2. Population, concept and context inclusion and exclusion criteria.
Expert panel cost item identification, meta-synthesis and consensus exercise
Further cost items were identified by an expert panel in Awareness Round (1) in January 2023. Awareness Round (1) comprised an anonymous, qualitative online survey with free-text responses to six open-ended questions. Experts were asked to provide at least three and up to 50 cost items relating to the pre-defined cost outcome categories per the real-world context. Health economic cost definitions were provided to standardize responses. Thematic analysis was used to code and standardize responses with those from the structured literature review with second and third order constructs developed to interpret findings as per-patient per-visit cost items from a health clinic health economic perspective. Meta-synthesis proceeded in a consensus exercise with the expert panel to determine relevance of identified cost items to the study context. In Consensus Round (2), completed in March 2023, aggregated cost items from the structured literature review and Awareness Round (1) were grouped by question and presented for expert rating of relevance on a 5-point Likert scale (strongly agree to strongly disagree). Awareness index was item frequency in respect of the frequency of the most commonly occurring item. Consensus index was the proportion of experts who agreed or strongly agreed with the inclusion of an item. Items with a consensus index of > 50% (CI > 0.5) represented real-world NHS nAMD treatment and monitoring service cost burden in the UK and formed the nAMD Service Non-Drug Cost Instrument (nAS). Consensus and awareness indexes were converted to scores denoting their overall relevance to the construct in question (lower scores indicated higher awareness and relevance).
Ecological validity of HTA cost lists
Ecological validity of HTA cost lists (the extent to which the findings of the study can be generalized to actual NHS retina clinics, as opposed to idealized or theoretical settings often used in health economics models) was assessed using the nAS as the reference standard. HTA cost list was extracted from the base case scenario provided by Roche, detailed in TA800 (the most recent technology appraisal for nAMD anti-VEGF treatments by NICE at the time of writing) and included: monitoring visit cost items were OCT and consultant-led outpatient clinic; administration visit cost items were OCT, consultant-led outpatient clinic and additional intravitreal injection costs, defined as difference in cost between administration and monitoring visits as calculated by the evidence review group in the appraisal of aflibercept for diabetic macular edema (DMO)Citation15. It was necessary to standardize cost identification approach to provide for comparison between the more approximate HTA cost lists and itemized nAS. Consultant-led outpatient clinic cost items, not detailed in TA800, were itemized per a low estimate (HTAmin) with the minimum nAS direct cost items (n = 4) and high estimate (HTAmax) containing all nAS direct cost items (n = 153). Sensitivity and specificity showed inclusion and exclusion of indicators relevant and irrelevant to the study context. To ensure the robustness of the results, simulations were conducted 1000 times, each with a sample size of 30. Both the HTAmin and HTAmax scenario values were incorporated into these simulations. Sensitivity and specificity derived from contingency tables were utilized as foundational assumptions for these simulations. The probabilities of false positives and false negatives were calculated for three hypothetical prevalence rates of 20%, 50%, and 80%. Results from these simulations were visually represented using box plots to facilitate a clear presentation of the data’s distribution and variance. All analyses were conducted using RStudio v2023, a part of POSIT Software, ensuring high precision in statistical computations and graphical output.A threshold value of £102 was set, based on the TA800 reference cost for a consultant-led clinic in the UKCitation15.
Cost of strain in nAMD anti-VEGF cost-effectiveness analysis
Scenario analysis was conducted using HTAmin and HTAmax over a 1-year time horizon to assess clinic cost burden with the assumption that healthcare resource utilization would increase by 50% under strain. Healthcare resource utilization is affected by multiple factors, and there is little research on impact due to strain; structured literature review and UK clinical practice suggested an estimated increase of between 0–50% depending on clinic with 50% selected as an arbitrary value for the purposes of analysisCitation44. All costs were estimated in Great British Pound (GBP) from UK clinical practice in an NHS setting. A comparative cost-effectiveness decision-making scenario reflective of genericization, a common application of cost-effectiveness analysis, was applied to two hypothetical nAMD anti-VEGF treatments, a low-cost generic with higher recommended frequency of injection and monitoring visits (D1) and a higher cost standard with lower recommended frequency of injection and monitoring visits (D2). Heterogeneity of durability outcomes in clinical and real-world studies precludes direct comparison of durability as a characteristic of specific treatments; however extended treatment intervals at follow-up can be considered a secondary outcome relating to treatment durabilityCitation45. As such, higher recommended injection and monitoring visit frequency was an assumed indicator of lower treatment durability and associated higher risk of strain in the context of a limited capacity health system. Per-patient per-visit cost item magnitudes (GBP) were allocated from a HTA perspective, predominantly focusing on acquisition costs, and from a real-world NHS nAMD monitoring and treatment service perspective using acquisition costs and the nAS to chart direct and indirect non-drug clinic cost estimates and additional costs incurred by strain. Cost items of value less than £1 were excluded. Acquisition costs were based on BNF76Citation46. All other cost magnitudes were estimated based on UK clinical practice in an NHS setting.
Results
Real-world cost burdens related to NHS nAMD treatment and monitoring service provision
Structured literature review
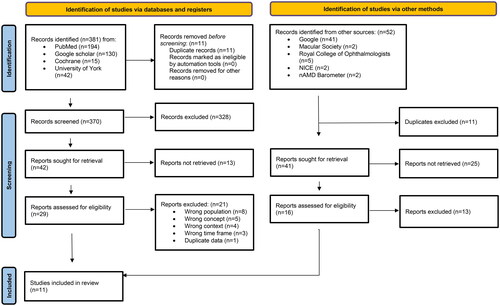
Searching identified 381 records from databases and 52 from sources of grey literature. Duplicates were removed from published studies (n = 11) and grey literature (n = 11) before initial screening (). Of 29 full-text and 16 grey literature records assessed for eligibility, eight published studies and three grey literature references meeting population, concept, context and time inclusion criteria were included. Of published studies, three cost-effectiveness analyses reported cost-effectiveness of diagnostic and monitoring tests and treatment, two cross-sectional surveys reported patient preferences and caregiver burden, one qualitative study reported patients’ views on ophthalmic care during the Coronavirus disease 2019 (COVID-19) pandemic and two expert opinion reviews reported recommendations for safe and effective intravitreal treatment service deliveryCitation44,Citation47–53. Systematic literature review and meta-analysis of diagnostic test accuracy were included in one health economic analysisCitation48. Health economic perspective and care setting were not reported in all studies. CEAs took a health and personal social services perspective; expert opinion recommendations for clinical practice covered primary, secondary and community settings. No studies reported cost values as real-world evidence findings from clinical practice centers within the study context; one expressed clinic burden data from NHS outpatient retina services and community optometry referral in the UK as clinical workload items in measurable unitsCitation44. Full-text eligibility screening excluded 21 published studies.
Figure 1. Modified preferred reporting items for systematic reviews and meta-analysis (PRISMA) flow chart adapted from Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi:10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/.
Three grey literature references were included; two reports from a healthcare provider perspective and one webpage containing patient education and recommendations for macular disease patients in the UKCitation6,Citation54,Citation55. The two reports included the Royal College of Ophthalmologists Commissioning Guidance for Age-related Macular Degeneration, which included systematic literature review and set out principles and minimum care standards for age-related macular degeneration services in England and WalesCitation6. The other was a national audit reporting visual acuity and safety outcomes and key care processes for providers of NHS-funded nAMD treatment at NHS Trusts or independent sector treatment sites in the UK using data collected from patients who started treatment for nAMD as part of routine care in NHS year 2020Citation54. Commissioning Guidance and the Macular Society webpage contained mixed populations but reported nAMD-specific data separately.
Cost burdens of nAMD and nAMD treatment and monitoring service delivery were reported from patient, caregiver, healthcare provider, health system perspectives. First order constructs generally referred to patient/caregiver cost burdens as disease impact on patient and caregiver activities and quality of life and treatment burden, for example number of injections, number of clinic appointments and total follow-up periodCitation50,Citation51. Cost burdens of nAMD treatment and monitoring from a health and personal social service use included health resource use, such as time to perform and interpret tests, grades of staff required, unit cost multipliers for hospital staff (inclusive of overheads), cost of test equipment, cost of equipment wear and tear, and health resource unit costs such as ophthalmology outpatient visitCitation47. Costs related to vision loss, such as unscheduled low vision appointments, use of antidepressants, general practitioner (GP) visits, visits from social services (mainly home caregivers), day center care, time in nursing homes, residential care, or sheltered housing were also reportedCitation49. Similar clinical workload costs were reported as considerations for impact of service innovation with additional costs and potential cost savings relating to mobile eye care clinics, non-consultant-led treatment and monitoring, and community optometry referralCitation44,Citation48,Citation53.
The context of UK ophthalmology services under strain was reported from a health system perspective in relation to increasing disease prevalence, aging population and a constrained workforceCitation44,Citation54. From a health provider perspective, strain was conceptualized as clinic workload exceeding capacity due to patient demand, limited capacity and treatment/monitoring burdenCitation44,Citation52,Citation54. Cost items incurred by strain were informed by clinical consequences of strain conditionsCitation31,Citation32,Citation52. Reduction of treatment interval and treatment re-initiation were recognized sequelae of undertreatment a likely impact of strainCitation6,Citation30. Therefore, it was expected that strain would incur direct and indirect medical and non-medical health system costs of re-treatment and management of increasing patient morbidity, deterioration of clinical condition and risk of harm as described by clinical workload items and allocation of clinical resource requirements. Costs of strain included additional patient and caregiver burden resulting from increased treatment visit burden, clinical deterioration, patient mental wellbeing, patient confidence in and access to eye clinic careCitation50–52.
Additional costs from a healthcare provider perspective related to mitigating the impact of strain on patient compliance, clinical deterioration, and patient wellbeing as well as potential additional direct medical costs of GP, eye clinic or emergency care for additional safety events and complicationsCitation55. Mitigation strategies included key care processes to facilitate early identification through monitoring and reduce hospital-initiated delay in treatment and monitoring appointments, including minimum standards for referral within and beyond NHS services and patient education and signpostingCitation6,Citation54,Citation55. Patient mitigation strategies included information seeking, self-monitoring and infection control activities at homeCitation55. Some costs of strain, such as costs associated with did-not-attend appointments and baseline visual acuity, were beyond health provider controlCitation6,Citation54.
Expert panel cost item identification, meta-synthesis and consensus exercise
Ten consultant ophthalmologists completed Awareness Round (1) and Consensus Round (2) with no attrition. Data saturation was reached at six experts (Supplemental Figure 1). From free-text responses in Awareness Round (1), 234 cost items were captured and coded. These were combined with unique cost items identified by the structured literature review (n = 3), to provide 237 cost items for assessment by the panel in Consensus Round (2). Of these, 217 (91.56%) met the consensus threshold and were included in the nAS (Supplemental Table 6).
Ecological validity of HTA cost lists
Sensitivity (1.84%, 95% CI 0.50–4.65%) and Neutrality (1.02; 50.1%) of HTAmin using the nAS as the reference standard were low. Including real-world direct costs of consultant-led outpatient visit increased sensitivity and Neutrality (HTAmax: S = 70.51%, 95% CI 63.96–76.49%; N = 1.71, 85.5%) with remaining error in comparison to the reference standard suggested due to underestimation of costs relating to strain (, Supplemental Table 5). Specificity for both was ideal, that is, they did not contain items irrelevant to the assessment of real-world NHS nAMD service costs. Median false negative rates for HTAmin at 20%, 50% and 80% prevalence (0.24, 0.57, 0.84, respectively) were higher than those for HTAmax (0.13, 0.38, 0.71, respectively), reflecting higher risk of misclassifying a ‘costly’ clinic as a ‘non-costly’ clinic due to underestimating clinic cost burden (). False positive rate for both HTA cost list estimates was 0, in line with specificity results.
Figure 2. Clinic cost misclassification plot showing false negative and false positive rates for HTAmin and HTAmax at 20%, 50% and 80% prevalence. Minimum: HTAmin; Maximum: HTAmax. False positive rate = 0, following specificity results.
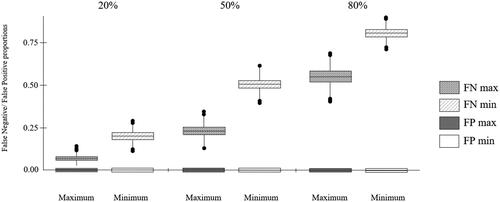
Table 3. Neutrality analysis for HTAmin and HTAmax.
Cost of strain in nAMD anti-VEGF cost-effectiveness analysis
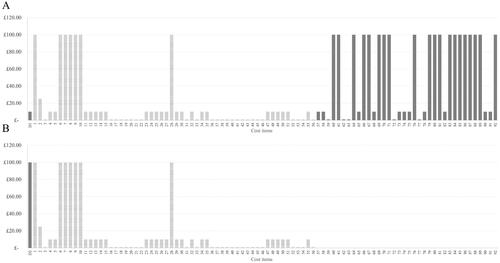
Scenario analysis of estimated cost magnitudes suggested annual per-patient real-world NHS nAMD non-drug clinic cost burden at a range of £845 (demand within clinic capacity) to £13,960 (clinic under strain conditions) compared to an HTAmin estimate of £210. The hypothetical CEA decision-making scenario considered per-patient per-visit drug and non-drug treatment costs. A distribution of high cost magnitudes was observed for drug cost (D2), additional expenditure due to strain (D1) and selected direct medical costs, mainly relating to diagnosis and monitoring (D1 and D2). Given equivalence of estimated per-patient per-visit diagnosis and monitoring costs among treatments, treatments were differentiated on the basis of cost-effectiveness by drug cost when using the HTA perspective () and additional expenditure due to strain when using the nAS () in decision-making. For a full list of cost items considered in the scenario, see Supplemental Table 7.
Figure 3. Comparative cost-effectiveness decision-making from HTA perspective showing estimated drug acquisition cost magnitudes for hypothetical drug 1 (A) and drug 2 (B): D1, D2: anti-VEGF drug acquisition cost; Items 1–3: non-anti-VEGF drug acquisition costs. Shaded bars show differentiating cost items.
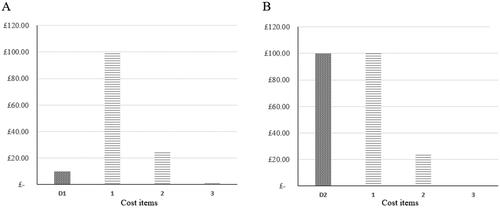
Figure 4. Comparative cost-effectiveness decision-making using the nAS, illustrating the impact of strain costs on two hypothetical nAMD anti-VEGF treatments. This figure shows that the lower cost drug (D1) incurs higher costs of strain, despite its lower acquisition cost. This is attributed to the higher frequency of treatments and monitoring required to maintain its efficacy, which increases operational costs during periods of clinic strain. The higher cost drug (D2), while more expensive upfront, demands fewer clinic visits, thereby incurring lower strain-related costs. This visualization underscores the importance of considering visit frequency and operational strain in total treatment cost assessments. The list of cost items is provided in Appendix 7: Supplemental Table 7.
Discussion
This study suggested that the real-world costs of running an nAMD clinic are drastically underestimated by current health economic analyses, especially under strain conditions. Company base cases in previous HTAs have used costs per visit to value non-drug cost burden of nAMD anti-VEGF treatments according to expected per-patient annual administration and monitoring visit frequencyCitation11–15. Real-world service cost burden of nAMD clinic provision cannot be accurately expressed solely in terms of expected per-patient annual administration and monitoring visit frequency due to the limit imposed by clinic capacity ceiling on clinic ability to meet recommended follow-up times, with strain likely to impact health economic costs at patient, system and societal levelsCitation30. According to approved UK posology, recommended injection and monitoring visit frequency required for optimal clinical outcome varies among nAMD anti-VEGF treatments; with potential undertreatment due to strain, durability is suggested as a relevant factor in robustness under limited capacity conditions currently affecting UK ophthalmology services.
To date, cost-effectiveness analysis and decision-making has distinguished between nAMD anti-VEGF treatments based on acquisition cost due to generally equivalent safety and efficacy findingsCitation2,Citation7,Citation8,Citation12,Citation16,Citation56. This study suggests that such decision-making may be somewhat of a false economy due to failure to account for non-drug clinic cost burden arising from strain. In other words, a treatment may appear better value only because its true cost exists in dimensions that have not been measuredCitation57. Findings were generally supported by previous research showing that HEAs frequently underestimate costs, and lack of standardization and transparency in methods for health technology cost estimation are common sources of inaccuracy in HTA and cost-effectiveness decision-makingCitation58–66. Systematic review has shown low quality and validity of published CEAs of anti-VEGF treatments for nAMD with conflicting findings attributable to cost assumptions used and omission of relevant costsCitation67. There is sparse evidence showing the impact of accurate cost estimation in CEA compared to use of less applicable assumptions. However, more ecologically valid estimation of HEA parameters significantly impacted cost-effectiveness of anti-VEGF treatments for nAMD in a study of patient versus surrogate utilitiesCitation68.
Our findings suggest that existing approaches of accounting for parameter uncertainty in HEA using statistical methods are insufficient and that definition and accounting of all relevant costs of service delivery are neededCitation15,Citation69. HTAs for nAMD anti-VEGF treatments do not appear to take the kind of systematic approach necessary for this. One issue highlighted here is lack of high-quality healthcare cost data in the literature. Previous studies have reported methodological issues affecting the validity of health resource utilization data estimated by non-clinicians and suggest that the problem of omission of relevant cost items extends to databases routinely collecting cost data and development of standard costing measuresCitation70–71. Additionally, lack of standardized guidelines on cost estimation for CEA has been identified as a potential source of bias in CEA and related decision-makingCitation72. Guidelines on health economic reporting quality, critical appraisal of reviews with cost and CEA outcomes, and assessment of model relevance omit detailed guidance on methods for estimating costsCitation73–75. The need to interpret health economic cost data for appropriate real-world application has also been noted, but there are no gold standards for how to achieve thisCitation66. Parallels can be drawn between the present study’s cost identification approach and recommendations set out in the Professional Society for Health Economics and Outcomes Research (ISPOR) Good Practices for Outcomes Research Task Force Report for identification, review and use of health state utilities in cost-effectiveness modelsCitation76. Similarly, application of hermeneutic principles has been suggested to reduce error in HTA, and the NICE Decision Support Unit has suggested implementation of broad selection criteria, conceptual modeling and an iterative approach to identify all relevant evidenceCitation65,Citation77,Citation78. Previous application of a similar approach in palliative care identified cost items often omitted from HEACitation63,Citation64. As such, development of guidelines for cost estimation in HTA similar to those developed for estimating utilities and patient reported outcomes in HEA is suggested.
This study confirmed previous findings that strain is a key issue in current UK NHS ophthalmology practice in terms of providing a service that meets patient needs despite increasing demand within a limited capacity health systemCitation10,Citation30,Citation54. Our findings have important implications given that treatment decisions based on estimates of cost-effectiveness that omit cost of strain could contribute further to issues of strain if low durability and high frequency of monitoring requirement reflect poor robustness under real-world conditionsCitation6,Citation29. In this case, even if discounted price after Sale (PAS) cost-per-dose list prices are used, cost of strain is still expected to differentiate between anti-VEGF cost-effectiveness. This study estimated the annual per-patient non-drug cost burden of running an NHS nAMD clinic under strain at £13960, compared to £845 when demand is within capacity. This represents a more than 16-fold increase in non-drug costs, attributed to the expenses associated with additional clinic resources, increased clinic administration events, emergency care, and delays in care leading to disease progression. The most likely cause of non-response and subsequent treatment re-initiation is undertreatment due to protocol deviation, suggesting significant direct medical costs arising from administration visits missed due to strain. While patient adherence and other factors beyond health provider control, such as mobility and other social determinants of health, influence patient compliance with treatment protocols, a substantial proportion of avoidable vision loss and patient harm has been attributed to hospital-initiated delay and loss of patients to follow-up. Inclusion of costs of undertreatment from societal and patient perspectives, intangible cost consequences of strain and overheads excluded here would likely increase differential in cost-effectiveness of anti-VEGF treatmentsCitation79–81. Distributional CEA is further likely to demonstrate disproportionate disadvantage to patients with lower socioeconomic status, who present more frequently with lower baseline visual acuity, one of the strongest predictors of anti-VEGF treatment outcomeCitation82,Citation83.
Expected increase in the prevalence of nAMD placing increasing demand on workforce-constrained UK ophthalmology services with training shortfalls has made mitigation of ophthalmology service strain a strategic priority for health systemsCitation84,Citation85. Service innovation has aimed to manage key capacity issuesCitation44,Citation53. Innovations aiming to reduce clinical workload through decision-making supported by artificial intelligence and reduce the need for patients to attend clinic through digitally-enabled remote services and home monitoring may free up available resources to meet higher levels of demandCitation84,Citation86. High-dose anti-VEGF treatments for nAMD have the potential to reduce treatment and monitoring visit burden by increasing durability; given the projected escalation in per-patient burden of nAMD service delivery, translation of these benefits to clinical practice could be key in reducing demand to manageable levels alongside effective workforce planningCitation10,Citation27–29.
Limitations
This study was a conceptual examination of impact of cost of strain on cost-effectiveness analysis of nAMD anti-VEGF treatments. While it was possible to define current real-world costs associated with running an NHS nAMD treatment and monitoring clinic, lack of cost value data in the literature has limited its application to predicted impact of cost of strain on cost-effectiveness considerations based on estimated cost magnitudes. Further research to determine real-world cost values could extend this work toward development of a model to estimate contribution of cost of strain to anti-VEGF treatment cost-effectiveness. By taking into account variability in HEA findings arising from health resource unit costs (Sculpher et al. 2004) such work could provide comment on the magnitude of cost of strain in different locationsCitation87. This study only considered cost of strain from a healthcare clinic perspective; consideration of cost magnitude of strain to patients, caregivers and society would likely demonstrate further cost savings arising from treatment durability. Finally, the hypothetical scenario depicting strain was selected based on anecdotal evidence and may differ across retina clinics. The 50% increase served as a reference point, enabling readers to extrapolate resource utilization across varying levels of increased consumption.
Conclusion
This study showed that current assumptions of monitoring and treatment service cost equivalence among anti-VEGF treatments for nAMD are likely to result in inaccurate CEA due to omission of non-drug cost items associated with running an NHS nAMD clinic, especially those arising from strain. Cost of strain under an assumed 50% increase in health resource utilization differentiated between treatments in our analysis and has been estimated here in the region of £13115 per-patient annual costs to nAMD treatment and monitoring service providers.
Transparency
Declaration of financial/other relationships
Sivaprasad, S has received financial support from AbbVie, Amgen, Apellis, Bayer, Biogen, Boehringer Ingelheim, Eyebiotech, Eyepoint Phamaceuticals, Janssen Pharmaceuticals, Nova Nordisk, Optos, Ocular Therapeutix, Kriya Therapeutics, OcuTerra, Roche, Stealth Biotherapeutics and Sanofi. Bailey, C has received honoraria for advisory board work, lectures, and travel support from Alimera Sciences, Apellis, Bayer, Boehringer-Ingelheim, Janssen, Novartis, and Roche. Downey, L has received advisory board and presentation honoraria from Allergan, Alimera, Bayer, Biogen, Novartis and Roche; travel grants from Allergan, Bayer, Novartis, and Roche and research funding from Allergan, Alimera, Bayer, Novartis, and Roche. Gale, R has received financial support from Apellis, Bayer, Biogen, Boehringer Ingelheim, and Roche. Kotagiri, A has received educational funding from Bayer, Novartis, and Roche and also been on their advisory boards. Mahmood, S has received funding for research, travel grants and honoraria for lectures from Bayer, Novartis and Roche. Narendran, N has received honoraria and travel grants from Bayer, Novartis and Roche. Pearce, I has received compensation for consultancy and honoraria for lectures, as well as travel grants from Apellis, Bayer, and Roche, and has received compensation for consultancy from Alimera, Boehringer Ingelheim, and Novartis. Rennie, C has received compensation as a consultant for AbbVie and Alimera Sciences, for advisory board attendances for AbbVie, Alimera Sciences, Bayer, Novartis and Roche and travel grants from AbbVie, Bayer and Roche. Talks, J has received compensation for advisory boards and travel/meeting expenses from Bayer and Roche, and participated in research for Alexion, Bayer, Boehringer Ingelheim and Roche. Gilbert R., Morgan-Warren P. and Napier J. are employees of Bayer Plc. Wojcik, R is an employee of Medialis Ltd. Jandhyala, R is the CEO and founder of Medialis Ltd as well as the developer of the Jandhyala Method which is free of commercial licensing restrictions and while used as part of proprietary methodology, is not a direct means of commercial gain for the author.
Peer reviewers on this manuscript have received an honorarium from CMRO for their review work but have no other relevant financial relationships to disclose.
Author contributions
Jandhyala, R and Wojcik, R conducted the study and prepared, authored and approved the manuscript. Sivaprasad, S., Bailey, C., Downey, L., Gale, R., Kotagiri, A., Mahmood, S., Narendran, N., Pearce, I., Rennie, C and Talks, J prepared, authored and approved the manuscript. The authors affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Ethical approval
Written informed consent was obtained from all experts before the study commenced. Responses were anonymized, and consensus round list items were not identifiable. The study was conducted in accordance with the Declaration of Helsinki, and favorable ethical opinion was granted by the King’s College London Ethics Committee (ref MRA-22/23-33972).
Appendices v2.docx
Download MS Word (132 KB)Acknowledgements
The authors would like to thank biostatistician Akshay Patil, research analyst and medical writer Ziyaad Rahman, medical writer Lauri Naylor and medical writer and project manager Obuchinezia Anyanwu, who contributed to this work as part of their roles as paid employees of Medialis Ltd.
Additional information
Funding
References
- Buchan J, Norman P, Shickle D, et al. Failing to plan and planning to fail. Can we predict the future growth of demand on UK eye care services. Eye. 2019;33(7):1029–1031. doi: 10.1038/s41433-019-0383-5.
- Hollingworth W, Jones T, Reeves BC, et al. A longitudinal study to assess the frequency and cost of antivascular endothelial therapy, and inequalities in access, in England between 2005 and 2015. BMJ Open. 2017;7(10):e018289. doi: 10.1136/bmjopen-2017-018289.
- Laali S. 1205 Clinical audit demonstrating an increase in referrals to the ophthalmology AMD clinic at University Hospital Southampton. Br J Surg. 2021;108(Supplement_6):znab259.133. doi: 10.1093/bjs/znab259.133.
- Cruess AF, Zlateva G, Xu X, et al. Economic burden of bilateral neovascular age-related macular degeneration: multi-country observational study. PharmacoEconomics. 2008;26(1):57–73. doi: 10.2165/00019053-200826010-00006.
- Pezzullo L, Streatfeild J, Simkiss P, et al. The economic impact of sight loss and blindness in the UK adult population. BMC Health Serv Res. 2018;18(1):63. doi: 10.1186/s12913-018-2836-0.
- The Royal College of Ophthalmologists. Commissioning Guidance: age Related Macular Degeneration Services. 2021.
- van Asten F, Michels CTJ, Hoyng CB, et al. The cost-effectiveness of bevacizumab, ranibizumab and aflibercept for the treatment of age-related macular degeneration—a cost-effectiveness analysis from a societal perspective. PLOS One. 2018;13(5):e0197670. doi: 10.1371/journal.pone.0197670.
- Patel JJ, Mendes MAS, Bounthavong M, et al. Cost-utility analysis of bevacizumab versus ranibizumab in neovascular age-related macular degeneration using a Markov model. J Eval Clin Pract. 2012;18(2):247–255. doi: 10.1111/j.1365-2753.2010.01546.x.
- Elshout M, van der Reis MI, Webers CAB, et al. The cost-utility of aflibercept for the treatment of age-related macular degeneration compared to bevacizumab and ranibizumab and the influence of model parameters. Graefes Arch Clin Exp Ophthalmol. 2014;252(12):1911–1920. doi: 10.1007/s00417-014-2641-3.
- Chopra R, Preston G, Keenan TDL, et al. Intravitreal injections: past trends and future projections within a UK tertiary hospital. Eye. 2022;36(7):1373–1378. doi: 10.1038/s41433-021-01646-3.
- The National Institute for Health and Care Excellence (NICE). Ranibizumab and pegaptanib for the treatment of age-related macular degeneration. [TA155]. Published August 27, 2008. Accessed August 25, 2023. https://www.nice.org.uk/guidance/ta155.
- The National Institute for Health and Care Excellence (NICE). Age-related macular degeneration. NICE guideline [NG82]. Published January 23, 2018. Accessed August 25, 2023. https://www.nice.org.uk/guidance/ng82.
- The National Institute for Health and Care Excellence (NICE). Aflibercept solution for injection for treating wet age‑related macular degeneration. [TA294]. Published July 24, 2013. Accessed August 25, 2023. https://www.nice.org.uk/guidance/ta294/chapter/4-Consideration-of-the-evidence.
- The National Institute for Health and Care Excellence (NICE). Brolucizumab for treating wet age-related macular degeneration. [TA672]. Published February 3, 2021. Accessed August 25, 2023. https://www.nice.org.uk/guidance/ta672.
- The National Institute for Health and Care Excellence (NICE). Faricimab for treating wet age-related macular degeneration. Technology appraisal guidance [TA800]. Published June 29, 2022. Committee papers. Accessed August 25, 2023. https://www.nice.org.uk/guidance/ta800/evidence/committee-papers-pdf-11127512989.
- National Health Service England. Operational note: Updated commissioning recommendations for medical retinal vascular medicines following the national procurement for ranibizumab biosimilars. Accessed August 25, 2023. https://www.england.nhs.uk/long-read/operational-note-updated-commissioning-recommendations-for-medical-retinal-vascular-medicines-following-the-national-procurement-for-ranibizumab-biosimilars/.
- Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481.
- Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. doi: 10.1056/NEJMoa062655.
- Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–2548. doi: 10.1016/j.ophtha.2012.09.006.
- Dugel PU, Koh A, Ogura Y, et al. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127(1):72–84. doi: 10.1016/j.ophtha.2019.04.017.
- Heier JS, Khanani AM, Quezada Ruiz C, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and Lucerne): two randomised, double-masked, phase 3, non-inferiority trials. Lancet. 2022;399(10326):729–740. doi: 10.1016/S0140-6736(22)00010-1.
- Medicines and Healthcare products Regulatory Agency. 2021. Summary of product characteristics [Eyelea]. Accessed August 25, 2023. Available from: https://mhraproducts4853.blob.core.windows.net/docs/d24a783ac377311dea10a8fc983667447044d61a.
- Medicines and Healthcare products Regulatory Agency. 2021. Summary of product characteristics [Beovu]. Accessed August 25, 2023. Available from: https://mhraproducts4853.blob.core.windows.net/docs/856fe193cab742573bd558275ed4a05dfa42c0aa.
- Medicines and Healthcare products Regulatory Agency. 2022. Summary of product characteristics [Vabysmo]. Accessed August 25, 2023. Available from: https://mhraproducts4853.blob.core.windows.net/docs/cbe8dd3b2ac286047361d5ca94f2eba02db79878.
- Medicines and Healthcare products Regulatory Agency. 2021. Summary of product characteristics [Lucentis]. Accessed August 25, 2023. Available from: https://mhraproducts4853.blob.core.windows.net/docs/8f9f82d6ec696188fc316bdef8ef2f7243a279f4.
- Lanzetta P, Loewenstein A,. Fundamental principles of an anti-VEGF treatment regimen: optimal application of intravitreal anti–vascular endothelial growth factor therapy of macular diseases. Graefes Arch Clin Exp Ophthalmol. 2017;255(7):1259–1273. doi: 10.1007/s00417-017-3647-4.
- Adrean S. High-dose aflibercept with increased dosing intervals as a new standard of care for DMO and nAMD. Published online: may 11th 2023. touchREVIEWS in Ophthalmology. 2023;17(1):8–9. doi: 10.17925/USOR.2023.17.1.8.
- Chakravarthy U, Armendariz BG, Fauser S. 15 Years of anti-VEGF treatment for nAMD: success or failure or something in between? Eye . 2022;36(12):2232–2233. doi: 10.1038/s41433-022-02153-9.
- Mehta H, Kim LN, Mathis T, et al. Trends in real-world neovascular AMD treatment outcomes in the UK. Clin Ophthalmol. 2020;14:3331–3342. doi: 10.2147/OPTH.S275977.
- Gale R, Cox O, Keenan C, et al. Health technology assessment of new retinal treatments; the need to capture healthcare capacity issues. Eye (Lond). 2022;36(12):2236–2238. doi: 10.1038/s41433-022-02149-5.
- Davis A, Baldwin A, Hingorani M, et al. A review of 145 234 ophthalmic patient episodes lost to follow-up. Eye. 2017;31(3):422–429. doi: 10.1038/eye.2016.225.
- Foot B, MacEwen C. Surveillance of sight loss due to delay in ophthalmic treatment or review: frequency, cause and outcome. Eye . 2017;31(5):771–775. doi: 10.1038/eye.2017.1.
- Jandhyala R. Concordance between the schedule for the evaluation of individual quality of life – direct weighting (SEIQoL-DW) and the EuroQoL-5D (EQ-5D) measures of quality of life outcomes in adults with x-linked hypophosphatemia. Orphanet J Rare Dis. 2022;17(1):81. doi: 10.1186/s13023-022-02250-8.
- Damy T, Conceição I, García-Pavía P, et al. A simple core dataset and disease severity score for hereditary transthyretin (ATTRv) amyloidosis. Amyloid. 2021;28(3):189–198. doi: 10.1080/13506129.2021.1931099.
- Freedman S, de-Madaria E, Singh VK, et al. A simple core dataset for triglyceride-induced acute pancreatitis. Curr Med Res Opin. 2022;39(1):37–46. doi: 10.1080/03007995.2022.2144054.
- Jandhyala R. A novel method for observing proportional group awareness and consensus of items arising from list-generating questioning. Curr Med Res Opin. 2020;36(5):883–893. doi: 10.1080/03007995.2020.1734920.
- Jandhyala R. Delphi, non-RAND modified delphi, RAND/UCLA appropriateness method and a novel group awareness and consensus methodology for consensus measurement: a systematic literature review. Curr Med Res Opin. 2020;36(11):1873–1887. doi: 10.1080/03007995.2020.1816946.
- Jandhyala R. PAC-19QoL: design, validation and implementation of the post-acute (long) COVID-19 quality of life (PAC-19QoL) instrument. Health Qual Life Outcomes. 2021;19(1):229. doi: 10.1186/s12955-021-01862-1.
- Jandhyala R. Neutral theory: applicability and neutrality of clinical study endpoints where a disease-specific instrument is available. BMC Med Res Methodol. 2023;23(1):121. doi: 10.1186/s12874-023-01947-z.
- Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. 2nd Edition. Chichester (UK): John Wiley & Sons, 2019.
- Hartmann KE, Matchar DB, Chang S. Chapter 6: assessing applicability of medical test studies in systematic reviews. J Gen Intern Med. 2012;27(Suppl 1):S39–S46. doi: 10.1007/s11606-011-1961-9.
- Peters MDJ, Godfrey CM, Khalil H, et al. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13(3):141–146. doi: 10.1097/XEB.0000000000000050.
- Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan—a web and mobile app for. Syst Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4.
- Amoaku W, Blakeney S, Freeman M, et al. Action on AMD. Optimising patient management: act now to ensure current and continual delivery of best possible patient care. Eye 2012;26(Suppl 1):S2–S21. doi: 10.1038/eye.2011.343.
- Patel PJ, Villavicencio P, Hanumunthadu D. Systematic review of neovascular age-related macular degeneration disease activity criteria use to shorten, maintain or extend treatment intervals with anti-VEGF in clinical trials: implications for clinical practice. Ophthalmol Ther. 2023;12(5):2323–2346. Joint Formulary Committee. doi: 10.1007/s40123-023-00768-z.
- British National Formulary (online). London: BMJ Group and Pharmaceutical Press. Updated September 2018. Available from: https://vnras.com/wp-content/uploads/pdf/BNF-76.pdf.
- Hernandez R, Kennedy C, Banister K, et al. Early detection of neovascular age-related macular degeneration: an economic evaluation based on data from the EDNA study. Br J Ophthalmol. 2022;106(12):1754–1761. doi: 10.1136/bjophthalmol-2021-319506.
- Mowatt G, Hernández R, Castillo M, et al. Optical coherence tomography for the diagnosis, monitoring, and guiding of treatment for neovascular age-related macular degeneration: a systematic review and economic evaluation. Health Technol Assess. 2014;18(69):1–254. doi: 10.3310/hta18690.
- Grieve R, Guerriero C, Walker J, et al. Verteporfin photodynamic therapy cohort study: report 3: cost effectiveness and lessons for future evaluations. Ophthalmology. 2009;116(12):2471–2477.e2. doi: 10.1016/j.ophtha.2009.10.023.
- Skelly A, Taylor N, Fasser C, et al. Patient preferences in the management of wet age-related macular degeneration: a conjoint analysis. Adv Ther. 2022;39(10):4808–4820. doi: 10.1007/s12325-022-02248-5.
- Gohil R, Crosby-Nwaobi R, Forbes A, et al. Caregiver burden in patients receiving ranibizumab therapy for neovascular age related macular degeneration. PLOS One. 2015;10(6):e0129361. doi: 10.1371/journal.pone.0129361.
- O'Connor, Seán R, Treanor, Charlene, Ward, Elizabeth, et al. The COVID-19 pandemic and ophthalmic care: a qualitative study of patients with neovascular age-related macular degeneration (nAMD). Int J Environ Res Public Health 2022;19(15):9488. doi: 10.3390/ijerph19159488.
- Amoaku W, Bailey C, Downey L, et al. Providing a safe and effective intravitreal treatment service: strategies for service delivery. Clin Ophthalmol. 2020;14:1315–1328. doi: 10.2147/OPTH.S233061.
- The Royal College of Ophthalmologists. National Ophthalmology Database Audit: The First Report of Age-related Macular Degeneration Audit (AMD). 2023. Available from: https://nodaudit.org.uk/sites/default/files/2023-02/NOD%20AMD%20Audit%20Full%20Annual%20Report%202023_0.pdf.
- Macular Society. Treatments. 2022. Accessed September 1, 2023. Available from: https://www.macularsociety.org/diagnosis-treatment/treatments/.
- Yanagi Y, Takahashi K, Iida T, et al. Cost-effectiveness analysis of ranibizumab biosimilar for neovascular age-related macular degeneration in Japan. Ophthalmol Ther. 2023;12(4):2005–2021. doi: 10.1007/s40123-023-00715-y.
- Weisbrod BA, Test MA, Stein LI. Alternative to mental hospital treatment: II. Economic benefit-cost analysis. Arch Gen Psychiatry. 1980;37(4):400–405., cited in Beecham and Knapp. Costing psychiatric interventions. In Thornicroft G. (Ed). Measuring Mental Health Needs (2nd edition). 2001. Gaskell, Royal College of Psychiatrists, London. doi: 10.1001/archpsyc.1980.01780170042004.
- Stone PW, Chapman RH, Sandberg EA, et al. Measuring costs in cost-utility analyses: variations in the literature. Int J Technol Assess Health Care. 2000;16(1):111–124. doi: 10.1017/S0266462300161100.
- Hughes DA, Tilson L, Drummond M. Estimating drug costs in economic evaluations in Ireland and the UK. Pharmacoeconomics. 2009;27(8):635–643. doi: 10.2165/10899570-000000000-00000.
- Kim DD, Silver MC, Kunst N, et al. Perspective and costing in cost-effectiveness analysis, 1974–2018. Pharmacoeconomics. 2020;38(10):1135–1145. doi: 10.1007/s40273-020-00942-2.
- Drummond MF, Iglesias CP, Cooper NJ. Systematic reviews and economic evaluations conducted for the National Institute for Health and Clinical Excellence in the United Kingdom: a game of two halves? Int J Technol Assess Health Care. 2008;24(2):146–150. doi: 10.1017/S0266462308080203.
- García-Mochón L, Špacírová Z, Espín J. Costing methodologies in european economic evaluation guidelines: commonalities and divergences. Eur J Health Econ. 2022;23(6):979–991. doi: 10.1007/s10198-021-01414-w.
- Gardiner C, Ingleton C, Ryan T, et al. What cost components are relevant for economic evaluations of palliative care, and what approaches are used to measure these costs? A systematic review. Palliat Med. 2017;31(4):323–337. doi: 10.1177/0269216316670287.
- Gardiner C, Ryan T, Gott M. What is the cost of palliative care in the UK? A systematic review. BMJ Support Palliat Care. 2018;8(3):250–257. doi: 10.1136/bmjspcare-2018-001519.
- Kaltenthaler E, Tappenden P, Paisley S, et al. NICE DSU Technical Support Document 13: Identifying and reviewing evidence to inform the conceptualisation and population of cost-effectiveness models: report by the Decision Support Unit. 2011. Available at: https://www.sheffield.ac.uk/media/34222/download?attachment.
- Urbich M, Globe G, Pantiri K, et al. A systematic review of medical costs associated with heart failure in the USA (2014–2020). Pharmacoeconomics. 2020;38(11):1219–1236. doi: 10.1007/s40273-020-00952-0.
- Elshout M, Webers CAB, van der Reis MI, et al. A systematic review on the quality, validity and usefulness of current cost-effectiveness studies for treatments of neovascular age-related macular degeneration. Acta Ophthalmol. 2018;96(8):770–778. doi: 10.1111/aos.13824.
- Brown GC, Brown MM, Chaudhry I, et al. Opportunities to reduce potential bias in ophthalmic cost-utility analysis. JAMA Ophthalmol. 2021;139(4):389–397. doi: 10.1001/jamaophthalmol.2020.6591.
- Briggs AH, Weinstein MC, Fenwick EAL, et al. Model parameter estimation and uncertainty: a report of the ISPOR-SMDM modeling good research practices task force-6. Value Health. 2012;15(6):835–842. doi: 10.1016/j.jval.2012.04.014.
- Franklin M, Lomas J, Walker S, et al. An educational review about using cost data for the purpose of cost-effectiveness analysis. Pharmacoeconomics. 2019;37(5):631–643. doi: 10.1007/s40273-019-00771-y.
- Thorn JC, Brookes ST, Ridyard C, et al. Core items for a standardized resource use measure: expert delphi consensus survey. Value Health. 2018;21(6):640–649. doi: 10.1016/j.jval.2017.06.011.
- Langley P. Facilitating bias in cost-effectiveness analysis: CHEERS 2022 and the creation of assumption-driven imaginary value claims in health technology assessment. F1000Res. 2022;11:993. doi: 10.12688/f1000research.123709.1.
- Mandrik O (, Severens JL(, Bardach A, et al. Critical appraisal of systematic reviews with costs and cost-effectiveness outcomes: an ISPOR good practices task force report. Value Health. 2021;24(4):463–472. doi: 10.1016/j.jval.2021.01.002.
- Caro J, Eddy DM, Kan H, et al. Questionnaire to assess relevance and credibility of modeling studies for informing health care decision making: an ISPOR-AMCP-NPC good practice task force report. Value Health. 2014;19(8):1039–1054. doi: 10.1016/j.jval.2014.01.003.
- Husereau D, Drummond M, Augustovski F, et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Int J Technol Assess Health Care. 2022;38(1):e13. doi: 10.1017/S0266462321001732.
- Brazier J, Ara R, Azzabi I, et al. Identification, review, and use of health state utilities in cost-effectiveness models: an ISPOR good practices for outcomes research task force report. Value Health. 2019;22(3):267–275. doi: 10.1016/j.jval.2019.01.004.
- Chilcott J, Tappenden P, Rawdin A, et al. Avoiding and identifying errors in health technology assessment models: qualitative study and methodological review. Health Technol Assess. 2010;14(25):iii. iii-iv, ix-xii, doi: 10.3310/hta14250.
- Kaltenthaler E, Tappenden P, Paisley S. Reviewing the evidence to inform the population of cost-effectiveness models within health technology assessments. Value Health. 2013;16(5):830–836. doi: 10.1016/j.jval.2013.04.009.
- Cheung R, Yu B, Iordanous Y, et al. The prevalence of occupational burnout among ophthalmologists: a systematic review and meta-analysis. Psychol Rep. 2021;124(5):2139–2154. doi: 10.1177/0033294120954135.
- Dewa CS, Loong D, Bonato S, et al. How does burnout affect physician productivity? A systematic literature review. BMC Health Serv Res. 2014;14:325. doi: 10.1186/1472-6963-14-325.
- Dewa CS, Loong D, Bonato S, et al. The relationship between physician burnout and quality of healthcare in terms of safety and acceptability: a systematic review. BMJ Open. 2017;7(6):e015141. doi: 10.1136/bmjopen-2016-015141.
- More P, Almuhtaseb H, Smith D, et al. Socio-economic status and outcomes for patients with age-related macular degeneration. Eye (Lond). 2019;33(8):1224–1231. doi: 10.1038/s41433-019-0393-3.
- Finger RP, Wickremasinghe SS, Baird PN, et al. Predictors of anti-VEGF treatment response in neovascular age-related macular degeneration. Surv Ophthalmol. 2014;59(1):1–18. doi: 10.1016/j.survophthal.2013.03.009.
- The Royal College of Ophthalmologists. Response from The Royal College of Ophthalmologists (RCOphth) to the HEE Strategic Framework Call for Evidence. 2021b. Available at: https://www.rcophth.ac.uk/wp-content/uploads/2021/09/RCOphth-response-to-HEE-Strategic-Framework-Call-for-Evidence-6-Sept-final-1.pdf.
- The Royal College of Ophthalmologists. Facing workforce shortages and backlogs in the aftermath or COVID-19: The 2022 census of the ophthalmology consultant, trainee and SAS workforce. Census Report. Available at: https://www.rcophth.ac.uk/wp-content/uploads/2023/03/2022-Ophthalmology-census-Facing-workforce-shortages-and-backlogs-in-the-aftermath-of-COVID-19.pdf.
- Wickham L, Hay G, Hamilton R, et al. The impact of COVID policies on acute ophthalmology services—experiences from Moorfields eye hospital NHS foundation trust. Eye. 2020;34(7):1189–1192. doi: 10.1038/s41433-020-0957-2.
- Claxton K, Ginnelly L, Sculpher M, et al. Generalisability in economic evaluation studies in healthcare: a review and case studies. Health Technol Assess. 2004;8(31):1–103, iii. iii-iv, doi: 10.3310/hta8490.
