Abstract
Objective
To evaluate the overall survival (OS) of patients with chronic lymphocytic leukemia (CLL) receiving either ibrutinib monotherapy as a first-line (1L) treatment or chemotherapy/chemoimmunotherapy-based (CT/CIT) regimens in 1L followed by ibrutinib in the second line (1L CT/CIT-2L ibrutinib) after disease progression by emulating a randomized trial comparing both treatment sequences.
Methods
Patient-level data from the RESONATE-2 trial (NCT01722487) and real-world PHEDRA databases were analyzed. Three scenarios were considered using the following data sources: (1) RESONATE-2, (2) combined RESONATE-2/PHEDRA, (3) combined RESONATE-2/PHEDRA for 1L ibrutinib and PHEDRA for 1L CT/CIT-2L ibrutinib. Propensity score-based weights and inverse probability of censoring weighting were used to adjust for baseline (Scenarios 2 and 3) and time-dependent confounding (all scenarios), and to address potential biases. A weighted Cox proportional hazards model was used to estimate the OS hazard ratio (HR) and 95% confidence interval (CI) for 1L ibrutinib versus 1L CT/CIT-2L ibrutinib.
Results
Results from Scenario 1 showed a significantly lower risk of death with 1L ibrutinib compared with 1L chlorambucil followed by 2L ibrutinib (HR 0.35 [95% CI 0.20–0.62]). Results from Scenarios 2 and 3 demonstrated a reduced risk of death with 1L ibrutinib compared with 1L CT/CIT-2L ibrutinib (HR 0.35 [0.21–0.61] and 0.64 [0.39–1.04], respectively).
Conclusion
The analyses consistently showed a reduced risk of death when ibrutinib was used as a 1L treatment in CLL compared with delaying its use until 2L after CT/CIT regimens, which suggests that initiating ibrutinib in 1L is advantageous for improving survival outcomes.
Introduction
Ibrutinib is a first-in-class oral covalent inhibitor of Bruton’s tyrosine kinase (BTK), approved in the United States (US) and the European Union (EU) for the treatment of patients with previously untreated or relapsed/refractory (R/R) chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and several other indications, either as monotherapy or in combination with other agentsCitation1,Citation2.
According to international guidelines, recommended treatment regimens for previously untreated CLL include targeted agents and chemotherapy (CT), either alone (such as cyclophosphamide, fludarabine, bendamustine, or chlorambucil) or as chemoimmunotherapy (CIT) in combination with the anti-CD20 antibodies rituximab or obinutuzumabCitation3,Citation4. The European Society for Medical Oncology (ESMO) guidelines recommend ibrutinib as a first-line (1L) and R/R treatment option for CLLCitation3. Despite the regulatory approval and clinical recommendations of targeted agents, some patients may still receive CT/CIT as their 1L treatment for CLL.
Although newer BTK inhibitors such as acalabrutinib and zanubrutinib are now available, neither has yet demonstrated a significant overall survival (OS) benefit as monotherapy over CT or CIT in randomized clinical trials (RCTs)Citation5–7. In contrast, ibrutinib monotherapy administered in RCTs to previously untreated patients with CLL has significantly improved OS compared with CT (chlorambucil)Citation8.
The objective of this study was to compare OS between patients with CLL receiving continuous ibrutinib monotherapy (referred to as “ibrutinib” hereafter) in 1L versus second line (2L) after 1L CT/CIT. As there are no RCTs available to address the optimal sequencing of ibrutinib in a treatment pathway, a randomized trial was emulated where patients either received 1L ibrutinib or 1L CT/CIT and subsequently, after disease progression, received ibrutinib treatment in the 2L. Trial emulation has been used previously when a new study drug or regimen that was evaluated in a single-arm trial needed to be compared with the standard of careCitation9–11.
Data from two sources were used: the phase 3 RESONATE-2 RCT and the PHEDRA (Platform for Hematology in Europe, Middle East, Africa) real-world project. The RESONATE-2 trial compared the efficacy and safety of 1L ibrutinib versus chlorambucil in patients aged ≥65 years with previously untreated CLL/SLL without the deletion of the short arm of chromosome 17 (del17p)Citation8,Citation12. Chlorambucil was chosen as a comparator to ibrutinib in the RESONATE-2 trial due to its established status as a standard of care for older patients at the time of the study initiation. In the current treatment landscape, if patients with CLL receive CT/CIT in the 1L, it is more likely, that they are treated with CIT regimens containing rituximab or obinutuzumabCitation3. The PHEDRA dataset provides information on a variety of commonly used treatments to conduct the analyses. PHEDRA is a Janssen-sponsored, noninterventional project that collected secondary real-world (RW) patient-level data. It was developed to assess treatment practice and outcomes in hematological malignancies (CLL, mantle cell lymphoma, and Waldenström macroglobulinemia), and to compare outcomes between patients treated with ibrutinib in RCTs and those treated with physician’s choice treatments in clinical practiceCitation13,Citation14. PHEDRA methodology has been published previouslyCitation13. Patient-level data for CLL within PHEDRA from the CLLEAR registry in the Czech Republic and the Lyon-Sud University Hospital database in France were analyzed retrospectively for this trial emulation. Therefore, RESONATE-2 provided data on ibrutinib and CT (use of chlorambucil) in 1L and on ibrutinib in 2L following CT (chlorambucil), whereas the two country databases within PHEDRA provided data on CT/CIT (commonly used regimens) in 1L, and on ibrutinib use in 1L and 2L, for the treatment of CLL.
Here, an emulated randomized trial investigating outcomes of treatment sequencing of ibrutinib in 1L versus 2L treatment following 1L CT/CIT is presented.
Methods
Eligibility criteria
The analysis included patients with previously untreated CLL or SLL who met all eligibility criteria for each of the datasets employed in this study. The entire list of RESONATE-2 eligibility criteria has been published previouslyCitation12. RESONATE-2 patients were eligible for inclusion in this analysis if they received at least one treatment of either ibrutinib or chlorambucil. For the PHEDRA dataset, to account for the availability of effective treatments for CLL, data from 2009 onward were included in the analysis. Patients from the PHEDRA databases were eligible for inclusion if they were diagnosed with CLL prior to initiation of 1L treatment, received at least one treatment of either ibrutinib or CT/CIT in 1L, and had recorded treatment start and end dates for their treatment lines. Patients from the PHEDRA databases who received any ibrutinib combinations or who were enrolled in any clinical trial for 1L or 2L CLL treatment were excluded.
No additional patient or ethics review committee approval was required for this clinical trial emulation. The RESONATE-2 trial was approved by the institutional review board or independent ethics committee at each institution and was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelinesCitation12. PHEDRA dataset includes only deidentified patient-level data extracted by the database owners at their respective sites prior to data transferCitation13 and permissions for use in PHEDRA were obtained according to local regulations by database owners where applicableCitation14.
Outcomes
The primary endpoint for this analysis was OS, defined as the time from randomization (RESONATE-2) or the time from treatment initiation (PHEDRA) to death from any cause. Patients from both datasets who were alive at the data cut-off were censored on the last day known to be alive.
Trial emulation and statistical analyses
A target trial comparing treatment strategies was emulated, with patients starting on ibrutinib as 1L treatment (referred to as “1L ibrutinib” hereafter) or on a CT/CIT as 1L therapy and receiving ibrutinib as a 2L treatment after disease progression (referred to as “1L CT/CIT-2L ibrutinib” hereafter) ().
Figure 1. Schematic overview of three scenarios and their data sources.
1L, first line; 2L, second line; CT/CIT, chemotherapy/chemoimmunotherapy.
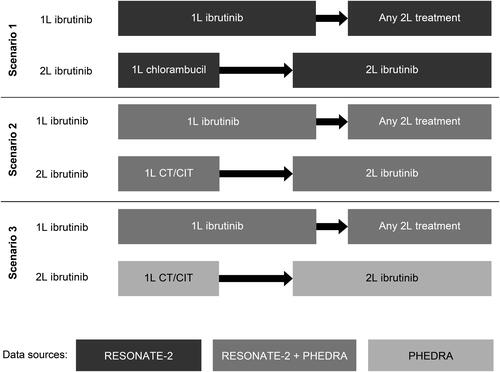
Three analysis scenarios were examined. In Scenario 1, only data from RESONATE-2, that provided comprehensive information on time-varying covariates, were used to simulate the target trial design. This allowed for a comparison of outcomes between patients initiated on ibrutinib in 1L and patients who received chlorambucil as 1L treatment followed by ibrutinib in 2L. The data for the ibrutinib arm were used as observed in the trial. Data for the chlorambucil-treated patients who did not initiate 2L therapy or who initiated ibrutinib in 2L were used as observed. Patients from the chlorambucil arm who initiated any treatment other than ibrutinib in 2L were censored at the start of their 2L therapy. However, this is a type of informative censoring that could induce a biased comparative analysis. To address this issue, the causal inference framework was used to adjust for this bias by upweighting non-censored patients with similar characteristics at baseline and over time, thus representing the censored patients, while downweighting othersCitation9. Retaining all trial patients in the analysis ensured that the randomization at baseline was preserved. The time-varying variables that were adjusted for in this scenario were creatinine clearance, Eastern Cooperative Oncology Group performance status (ECOG PS) score, Functional Assessment of Cancer Therapy score, absolute neutrophil count, hemoglobin level, platelet count, and lactate dehydrogenase level.
Scenarios 2 and 3 used data from both the RESONATE-2 trial and the PHEDRA dataset. Since two different datasets were used in these scenarios, it was necessary to adjust for differences in patient and disease characteristics at baseline between the 1L ibrutinib and 1L CT/CIT-2L ibrutinib arms. To account for this lack of randomization at baseline, propensity score-based weights were employed to balance the 1L ibrutinib and comparator cohorts at baseline, with the propensity scores representing the probability for a patient to be on 1L ibrutinib treatment versus 1L CT/CIT-2L ibrutinib treatment, conditional on the baseline characteristics.
In Scenario 2, combined data from the RESONATE-2 trial and PHEDRA dataset were used for both treatment strategies. The use of time-dependent variables in this scenario was limited to those that were captured in both the RESONATE-2 trial and the PHEDRA databases. The baseline patient and disease characteristics that were adjusted for in this scenario were age, sex, Binet/Rai stage, presence of deletion in the long arm of chromosome 11 (del11q), presence of del17p, and immunoglobulin heavy-chain variable region (IGHV) mutation status. The only time-varying variable that was commonly available in both databases, and thus adjusted for in this scenario, was ECOG PS score.
In Scenario 3, outcomes for 1L ibrutinib treatment were assessed using combined data from the RESONATE-2 trial and the PHEDRA dataset, while using data solely from the PHEDRA dataset for the 1L CT/CIT-2L ibrutinib treatment strategy. The advantage of this scenario was the ability to use all time-dependent covariates captured in the PHEDRA databases to apply to the inverse probability of censoring weighting (IPCW) analysis. The baseline patient and disease characteristics adjusted for in this scenario were age, sex, Binet/Rai stage, presence of del11q, presence of del17p, and IGHV mutation status. The time-varying variables adjusted for in this scenario were ECOG PS score, Binet/Rai stage, presence of del11q, presence of del17p, and IGHV mutation status.
The IPCW method was used to estimate the treatment effect of interest. The method represents an observational-based approach, whereby data for patients who initiated 2L treatment (switchers) are censored at the time of 2L treatment initiation (switch) to the treatment to be adjusted for, and remaining observations are weighted with the aim of removing any censoring-related selection biasCitation15,Citation16. The method has been mainly used to adjust for the initiation of a new treatment in RCTs, where patients transition from the control arm to the experimental arm after disease progression.Citation16–18. In such context, standard intention-to-treat analyses may underestimate the OS benefit of the new treatment in a clinical setting where the experimental treatment is not (yet) available in the next line. The IPCW approach allows for mimicking the target RCT, where this switching after progression to the comparator treatment in the active arm did not occur. As an example, the IPCW method was previously applied to estimate the benefit of adding abiraterone acetate + prednisone to androgen-deprivation therapy (ADT) in the LATITUDE trial, where patients receiving placebo + ADT could switch to abiraterone acetate + prednisone + ADT upon disease progressionCitation19.
The same IPCW framework was used in this study. However, instead of adjusting for the survival benefit resulting from the subsequent treatment with ibrutinib in the comparator arm, the opposite was done by adjusting for the potentially lower efficacy of non-ibrutinib therapy as subsequent 2L therapy. This adjustment was done to emulate the target RCT of interest, which involved comparing the two treatment sequences. Therefore, patients in the comparator arm who started non-ibrutinib 2L therapy were censored at that time, while patients with similar patient characteristics (both at baseline and over time) who started ibrutinib in 2L were upweighted to represent the censored patients. This was done to remove bias caused by informative censoring.
To perform the IPCW analysis, a panel dataset was created, with a window size of 1 day to estimate the time-dependent stabilized weights. Time-dependent weights were estimated by modeling the probability of starting 2L ibrutinib rather than starting non-ibrutinib 2L therapy after disease progression using a repeated logistic regressionCitation20. These time-dependent weights (multiplied with the baseline weights for Scenarios 2 and 3) were then used in the final weighted Cox proportional hazards model (referred to as the “weighted Cox PH model” hereafter). This adjustment aimed to obtain the hazard ratio (HR) and 95% confidence interval (CI) for 1L ibrutinib treatment versus 1L CT/CIT-2L ibrutinib treatment while accounting for both the differences in baseline characteristics between both treatment cohorts and between patients starting ibrutinib in 2L versus alternative treatments. Additionally, weight truncation at 10 was used to address the potential overrepresentation of certain patients in the model estimates. Furthermore, the doubly robust approach was used as a sensitivity analysis in the weighted Cox PH model by additionally including a set of baseline covariates in the model on top of the weighting, to further balance both patient cohorts at baselineCitation21,Citation22.
Results
Baseline characteristics
Data were analyzed from the RESONATE-2 trial, which included data with a follow-up period of 82.7 months, as published by Barr et al. in 2022Citation8. This dataset consisted of data from 266 patients. Additionally, RW data from the PHEDRA databases were analyzed for a total of 1360 patients. The data cut for the CLL dataset from PHEDRA was completed in 2019 and published by Doubek et al. in 2021Citation23. The PHEDRA data were cut off at the same follow-up time as the RESONATE-2 data because including additional data would not provide further insights into the comparison of the two treatment strategies.
Baseline demographics and disease characteristics were well balanced between patients in Scenario 1, as expected, due to randomization in RESONATE-2 (). However, in Scenarios 2 and 3, differences in age, disease stage, performance status, and genetic mutations were observed between both treatment strategies before reweighting due to the inclusion of patients from the PHEDRA databases (). Two patients from RESONATE-2 (one from each treatment arm) were excluded from the analysis because they did not receive any treatment. Another two patients (one from the RESONATE-2 control arm and one from the PHEDRA databases) were removed from the analysis as they received ibrutinib combination therapy in 2L.
Table 1. Baseline characteristics for cohorts of Scenarios 1, 2, and 3.
In Scenario 1 (RESONATE-2), 135 and 131 patients received 1L therapy with ibrutinib (1L ibrutinib treatment strategy) or chlorambucil (1L CT/CIT-2L ibrutinib treatment strategy), respectively. In the ibrutinib arm, fewer patients initiated 2L treatment than in the chlorambucil arm (17% and 73%, respectively). In Scenario 2 (RESONATE-2 and PHEDRA), 145 and 1481 patients received 1L therapy with either ibrutinib (1L ibrutinib treatment strategy) or CT/CIT (1L CT/CIT-2L ibrutinib treatment strategy), respectively. The most common 1L treatment in the 1L CT/CIT-2L ibrutinib treatment strategy was fludarabine + cyclophosphamide + rituximab (FCR; 37%), followed by other anti-CD20–based treatment regimens (19%) and bendamustine and rituximab (BR; 18%; ). A lower proportion of patients treated with ibrutinib than with CT/CIT in 1L had initiated 2L treatment (18% and 40%, respectively). In Scenario 3, 145 and 1350 patients received initial therapy with either ibrutinib (1L ibrutinib treatment strategy; RESONATE-2 and PHEDRA) or CT/CIT (1L CT/CIT-2L ibrutinib treatment strategy; PHEDRA only), respectively. The most common 1L treatment in the CT/CIT group was FCR (40%), followed by other anti-CD20–based treatment regimens (21%) and BR (20%; ). A lower proportion of patients treated with ibrutinib than with CT/CIT in 1L had initiated 2L treatment (18% and 38%, respectively).
Table 2. Treatment regimens for cohorts of Scenarios 1, 2, and 3.
Comparison of outcomes in patients treated with ibrutinib in 1L versus 2L after 1L CT/CIT
In Scenario 1 (cohort with RESONATE-2 data only), patients with missing baseline characteristics were excluded from the analyses, resulting in a sample size of 106 for the 1L ibrutinib arm and 104 for the 1L CT/CIT-2L ibrutinib arm. Using the weighted Cox PH model, an HR of 0.35 (95% CI 0.20–0.62; p = .0003) was obtained, indicating that patients who received 1L ibrutinib had a 65% lower risk of death compared with patients who received 2L ibrutinib after 1L chlorambucil. This difference was statistically significant. The estimated 5-year OS was 84% for patients who received 1L ibrutinib, whereas it was 62% for patients who received 1L CT/CIT-2L ibrutinib. As the two arms were well balanced due to randomization, and correction for any baseline characteristic was not required, the doubly robust weighted Cox PH model did not apply.
The sample size was reduced to 119 patients for the 1L ibrutinib and 807 patients for the 1L CT/CIT-2L ibrutinib strategy in Scenario 2, and to 119 patients for the 1L ibrutinib and 692 patients for the 1L CT/CIT-2L ibrutinib strategy in Scenario 3, due to missing data for baseline characteristics used in the different analyses. Using the weighted Cox PH model, patients who received 1L ibrutinib had a 65% and 36% lower risk of death compared with patients who received 1L CT/CIT-2L ibrutinib in Scenarios 2 and 3, respectively (HR 0.35; 95% CI 0.21–0.61; p = .0002; and HR 0.64; 95% CI 0.39–1.04; p = .0722), which was statistically significant in Scenario 2. In the weighted Cox PH model, the estimated 5-year OS was 87% and 89% for patients who received 1L ibrutinib, while it was 63% and 82% for patients who received 1L CT/CIT-2L ibrutinib, in Scenarios 2 and 3, respectively. The doubly robust method, further adjusting for potential imbalances at baseline, confirmed the results obtained in the weighted Cox PH model and showed a statistically significant OS advantage for patients treated with 1L ibrutinib compared with 1L CT/CIT-2L ibrutinib in both Scenarios 2 and 3 (HR 0.31; 95% CI 0.18–0.55; p < .0001; and HR 0.56; 95% CI 0.33–0.94; p = .0270). The results for all scenarios are summarized in , and the Kaplan–Meier curves are shown in .
Figure 2. Kaplan–Meier curves for Scenario 1 (A), Scenario 2 (B), and Scenario 3 (C). Overall survival analysis of observed data and adjusted data using the weighted Cox PH model.
1L, first line; 2L, second line; CT/CIT, chemotherapy/chemoimmunotherapy; CI, confidence interval; HR, hazard ratio; PH, proportional hazards.
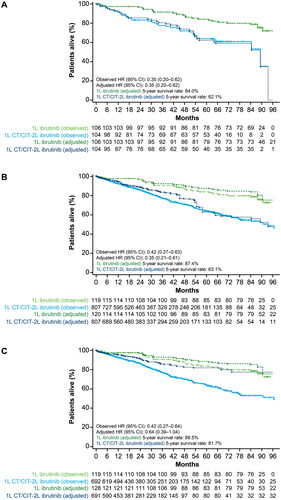
Table 3. Summary of observed and adjusted overall survival.
Discussion
This study examined whether there is a difference in OS between patients with CLL who received ibrutinib as a 1L treatment compared with those who were treated with ibrutinib in the 2L after initial CT/CIT. To our knowledge, this study is the first to explore treatment sequencing outcomes in CLL/SLL, as this specific comparison has not been previously investigated in clinical trials.
Data were used from two sources: the RCT RESONATE-2 and the real-world PHEDRA databases. RESONATE-2 provided 1L data for ibrutinib and chlorambucil, and 2L data for ibrutinib after receiving chlorambucil in 1L. The RESONATE randomized trial data, which compared ibrutinib with ofatumumab in patients with previously treated CLLCitation24, was not used in the study because it contained only data from patients treated in second or further lines of treatment and can not inform the sequence comparisons starting from 1L, as reported in this study. The ALLIANCE study, which compared ibrutinib regimens with CIT in patients with previously untreated CLLCitation25, was also not included due to lack of access to patient-level data for the analyses of interest.
The PHEDRA dataset provided data on 1L and 2L ibrutinib, as well as 1L CT/CIT, including a variety of physician’s choice treatments from 2009 onward. However, PHEDRA had limited data on the use of ibrutinib as it predates ibrutinib’s approval by the European Medicines Agency for CLL treatment in 2014. Furthermore, in the monitored regions, ibrutinib reimbursement was granted even later, which contributes to the limited availability of data.
The study used a causal inference framework to emulate an RCT, comparing two treatment strategies. Three scenarios for analysis were considered. In Scenario 1, data exclusively from the RESONATE-2 trial were used, which has a comprehensive list of time-varying covariates. This scenario represents the most robust analysis with maintained randomization and shows significantly improved OS for 1L ibrutinib compared with 2L ibrutinib after 1L chlorambucil. In Scenarios 2 and 3, the analysis was expanded by integrating data from the RESONATE-2 trial with information from the PHEDRA databases, as illustrated in . In Scenarios 2 and 3, the adjustment for baseline characteristics using propensity score-based weights and employing IPCW to account for informative censoring allowed for a meaningful OS comparison. Although differences in baseline characteristics were observed before reweighting due to the inclusion of PHEDRA data, the weighting approach effectively balanced the cohorts in both scenarios. All analyses in Scenarios 2 and 3 showed a reduced risk of death with 1L ibrutinib compared with 1L CT/CIT-2L ibrutinib. The OS advantage was statistically significant in all analyses except the weighted Cox PH model for Scenario 3. However, in Scenario 3, a relatively small number of patients (n = 42) received 2L ibrutinib. The limited sample size and low event rate in this scenario reduced statistical power and increased confidence intervals. Scenario 1 included only chlorambucil as the CT/CIT comparator, which may not be representative of a current CT/CIT choice for CLL. Scenarios 2 and 3 combined data from the PHEDRA databases, thus including a variety of physician’s choice treatments that may be more recent preferred CT/CIT comparators. The current trend is toward CT-free regimens, and CIT is only recommended for fit patients with CLL and mutated IGHV statusCitation3.
These results are of clinical importance as CLL remains incurable, and the primary treatment goals are enhancing patient quality of life and prolonging survivalCitation3. Consequently, OS is still considered the gold standard endpoint and the most important outcome for evaluating treatment efficacyCitation26, even if minimal residual disease is examined to determine the treatment duration of targeted therapiesCitation3. Previous studies have reported improved OS with ibrutinib treatments compared with CT/CIT when administered to patients with newly diagnosed CLL. The RESONATE-2 trial showed that for ibrutinib-treated patients, the median OS was not estimable at up to 8 years of follow-up, and 78% of patients were still alive at 7 years (HR for ibrutinib vs. chlorambucil, 0.45; 95% CI 0.28–0.74)Citation8. It has also been demonstrated that OS outcomes for 1L ibrutinib in RESONATE-2 are similar to an age-matched general US populationCitation27. To our knowledge, neither CT/CIT nor any other targeted agent has evidence of offering patients with previously untreated CLL the chance of a similar life expectancy to that of the general age-matched population. Additionally, the E1912 phase 3 RCT showed that patients receiving ibrutinib in combination with rituximab (IR) had a significantly longer OS than those receiving FCR (HR 0.47; 95% CI 0.25–0.89; p = .018). The 5-year OS was 95% for IR and 89% for FCRCitation28. In addition, an integrated analysis of RESONATE-2 and RESONATE patients demonstrated that 1L ibrutinib improved OS compared with the use of ibrutinib in later treatment linesCitation29. The clinical benefits of ibrutinib in 1L CLL, when compared with CT/CIT, are further reinforced by recent analyses of other real-world dataCitation30–32.
The main limitation of the study is the partial reliance on RW patient data from the PHEDRA databases. As with any non-randomized study, it is not possible to completely exclude the possibility of residual confounding in Scenarios 2 and 3 (unlike Scenario 1, which involved the RCT), even though the analyses assume no residual confounding at baseline. The patient population included in the PHEDRA dataset was not randomized, selected, or controlled in the same manner as in an RCT. Factors such as geographical representation and local reimbursement criteria may impact physician’s treatment choices and influence the patient populations included. However, OS as the endpoint of interest in this study is seen as an objective endpoint, and the date of death is well reported in the PHEDRA datasetCitation13. It is important to acknowledge that RW data may be subject to missing data and unmeasured confounders. Additionally, information on patient baseline characteristics in PHEDRA is not as detailed as in RESONATE-2. This resulted in a high number of patients being excluded due to missing baseline characteristics, which are needed for IPCW analysis. In the PHEDRA dataset, time-varying covariates were not captured frequently, only at the start of each line of treatment for all patients. In Scenario 2, the number of time-varying covariates was the most limited; however, more patients received 2L ibrutinib treatment than in other scenarios. In Scenario 3, only two out of the five time-varying covariates available in PHEDRA actually varied over time. Therefore, compared with Scenario 2, we gained one additional time-varying covariate. However, the number of patients receiving 2L ibrutinib treatment was lower compared with Scenarios 1 and 2.
Our study provides important insights into ibrutinib treatment sequencing by integrating data from both RCTs and RW sources. The consistent results obtained across all scenarios highlight the reliability of the findings, and the methodology used here can be employed in similar future analyses.
Conclusions
The analyses, considering data from RCT and RW settings, consistently show a reduced risk of death when ibrutinib is used as a 1L treatment in CLL compared with delaying its use until 2L after disease progression on 1L CT/CIT regimens. These findings suggest that initiating ibrutinib at 1L in the treatment sequence for CLL is advantageous for improving survival outcomes.
Transparency
Declaration of financial/other relationships
T Robak received research grants from Janssen, AbbVie, AstraZeneca, BeiGene, and Lilly, and served on advisory boards for Janssen, AbbVie, AstraZeneca, and BeiGene. M Doubek received research grants from AbbVie and AstraZeneca, and served on advisory boards for Janssen, AbbVie, AstraZeneca, AOP Orphan, and BeiGene. E Ferrant received honoraria from Janssen, congress funding from Janssen and Gilead, training funding from AstraZeneca, and served on an advisory board for AbbVie. L Neumayr is an employee of AbbVie and owns stock in AbbVie. J Diels, L Andersone, S Wilbertz, NC Healy, and S van Sanden are employees of Janssen. J Diels and S van Sanden own stock in Johnson & Johnson. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
J Diels, S van Sanden, L Andersone, S Wilbertz, and NC Healy were involved in the design of the study; J Diels and S van Sanden were responsible for methodology, data analyses, and interpretation. All authors reviewed the draft during development, provided comments for revision, and approved the final manuscript. All authors agree to be accountable for all aspects of the work.
Acknowledgements
The authors would like to thank Jedelyn Cabrieto of Janssen Pharmaceutica NV for her preparation of the PHEDRA dataset. Writing assistance was provided by Izabela Bombik, of Parexel, and funded by Janssen Pharmaceutica NV.
Data availability statement
Data used for this study were based on the RESONATE-2 study and PHEDRA database. PHEDRA is a non-interventional, secondary-use project which collaborates with the owners of existing databases of electronic health records in the Europe, Middle East, and Africa (EMEA) region and gathers deidentified patient-level data on CLL, mantle cell lymphoma, and Waldenström macroglobulinemia centrally. The real-world databases utilized in this study are not owned by Janssen Pharmaceutica NV. The Lyon-Sud data included in this study are based on patient-level information collected by Lyon-Sud University Hospital in the French routine care setting. The CLLEAR data included in this study are based on patient-level information collected by contributing local hospitals at seven academic centers in the Czech Republic, located in Brno, Hradec Králové, Nový Jičín, Olomouc, Ostrava, Plzeň, and Prague. Requests for access to data from Lyon-Sud University Hospital and the CLLEAR registry should be sent directly to the respective owners of the databases. RESONATE-2 data sharing is governed by Pharmacyclics LLC, an AbbVie Company. Requests for access to individual participant data from clinical studies conducted by Pharmacyclics LLC, an AbbVie Company, can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
Additional information
Funding
References
- IMBRUVICA® (ibrutinib) [SmPC]. Janssen-Cilag International NV. Beerse, Belgium; 2023.
- IMBRUVICA® (ibrutinib) [prescribing information]. Pharmacyclics LLC. Sunnyvale, CA: Janssen Biotech, Inc., Horsham, PA; 2023.
- Eichhorst B, Robak T, Montserrat E, et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(1):23–33. doi: 10.1016/j.annonc.2020.09.019.
- Špaček M, Šimkovič M, Pospíšilová Š, et al. 7. Chronická lymfocytární leukemie [Chronic lymphocytic leukemia]. In: Doubek M, Mayer J, editors. Léčebné postupy v hematologii 2023 [Treatments in hematology 2023]. Brno, Czech Republic: Česká hematologická společnost ČLS JEP; 2023.
- Ghia P, Pluta A, Wach M, et al. Acalabrutinib versus investigator’s choice in relapsed/refractory chronic lymphocytic leukemia: final ASCEND trial results. Hemasphere. 2022;6(12):e801. doi: 10.1097/HS9.0000000000000801.
- Sharman JP, Egyed M, Jurczak W, et al. Efficacy and safety in a 4-year follow-up of the ELEVATE-TN study comparing acalabrutinib with or without obinutuzumab versus obinutuzumab plus chlorambucil in treatment-naive chronic lymphocytic leukemia. Leukemia. 2022;36(4):1171–1175. doi: 10.1038/s41375-021-01485-x.
- Tam CS, Brown JR, Kahl BS, et al. Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): a randomised, controlled, phase 3 trial. Lancet Oncol. 2022;23(8):1031–1043. doi: 10.1016/S1470-2045(22)00293-5.
- Barr PM, Owen C, Robak T, et al. Up to 8-year follow-up from RESONATE-2: first-line ibrutinib treatment for patients with chronic lymphocytic leukemia. Blood Adv. 2022;6(11):3440–3450. doi: 10.1182/bloodadvances.2021006434.
- Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183(8):758–764. doi: 10.1093/aje/kwv254.
- Gomes M, Latimer N, Soares M, et al. Target trial emulation for transparent and robust estimation of treatment effects for health technology assessment using real-world data: opportunities and challenges. Pharmacoeconomics. 2022;40(6):577–586. doi: 10.1007/s40273-022-01141-x.
- Merz M, Goldschmidt H, Hari P, et al. Adjusted comparison of outcomes between patients from CARTITUDE-1 versus multiple myeloma patients with prior exposure to PI, IMiD and anti-CD-38 from a German registry. Cancers (Basel). 2021;13(23):5996. doi: 10.3390/cancers13235996.
- Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425–2437. doi: 10.1056/NEJMoa1509388.
- Garside J, Healy N, Besson H, et al. PHEDRA: using real-world data to analyze treatment patterns and ibrutinib effectiveness in hematological malignancies. J Comp Eff Res. 2018;7(1):29–38. doi: 10.2217/cer-2017-0046.
- Salles G, Bachy E, Smolej L, et al. Single-agent ibrutinib in RESONATE-2 and RESONATE versus treatments in the real-world PHEDRA databases for patients with chronic lymphocytic leukemia. Ann Hematol. 2019;98(12):2749–2760. doi: 10.1007/s00277-019-03830-8.
- Robins JM, Finkelstein DM. Correcting for noncompliance and dependent censoring in an AIDS clinical trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics. 2000;56(3):779–788. doi: 10.1111/j.0006-341x.2000.00779.x.
- Latimer NR, Abrams KR, Lambert PC, et al. Adjusting survival time estimates to account for treatment switching in randomized controlled trials–an economic evaluation context: methods, limitations, and recommendations. Med Decis Making. 2014;34(3):387–402. doi: 10.1177/0272989X13520192.
- Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11(5):561–570. doi: 10.1097/00001648-200009000-00012.
- Ishak KJ, Proskorovsky I, Korytowsky B, et al. Methods for adjusting for bias due to crossover in oncology trials. Pharmacoeconomics. 2014;32(6):533–546. doi: 10.1007/s40273-014-0145-y.
- Feyerabend S, Saad F, Perualila NJ, et al. Adjusting overall survival estimates for treatment switching in metastatic, castration-sensitive prostate cancer: results from the LATITUDE study. Target Oncol. 2019;14(6):681–688. doi: 10.1007/s11523-019-00685-x.
- Hernán MA, Robins JM. Causal inference: what if. Boca Raton, FL: Chapman & Hall/CRC; 2020.
- Syriopoulou E, Rutherford MJ, Lambert PC. Inverse probability weighting and doubly robust standardization in the relative survival framework. Stat Med. 2021;40(27):6069–6092. doi: 10.1002/sim.9171.
- Funk MJ, Westreich D, Wiesen C, et al. Doubly robust estimation of causal effects. Am J Epidemiol. 2011;173(7):761–767. doi: 10.1093/aje/kwq439.
- Doubek M, Smolej ML, Šimkovič M, et al. Single-agent ibrutinib versus real-world (RW) treatments for patients with previously untreated chronic lymphocytic leukemia (CLL): adjusted comparison of RESONATE-2™ with the CLLEAR and Lyon-Sud RW databases. Blood. 2021;138(Supplement 1):4679–4679. doi: 10.1182/blood-2021-147927.
- Byrd JC, Brown JR, O'Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–223. doi: 10.1056/NEJMoa1400376.
- Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379(26):2517–2528. doi: 10.1056/NEJMoa1812836.
- Delgado A, Guddati AK. Clinical endpoints in oncology - a primer. Am J Cancer Res. 2021;11(4):1121–1131.
- Ghia P, Owen C, Barrientos JC, et al. Initiating first-line (1L) ibrutinib (Ibr) in patients (pts) with chronic lymphocytic leukemia (CLL) improves overall survival (OS) outcomes to rates approximating an age-matched population of ≥65 years. Blood. 2022;140(Supplement 1):4159–4161. doi: 10.1182/blood-2022-163257.
- Shanafelt TD, Wang XV, Hanson CA, et al. Long-term outcomes for ibrutinib-rituximab and chemoimmunotherapy in CLL: updated results of the E1912 trial. Blood. 2022;140(2):112–120. doi: 10.1182/blood.2021014960.
- Woyach J, Tedeschi A, Munir T, et al. Using ibrutinib in earlier lines of treatment results in better outcomes for patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Leuk Lymphoma. 2021;62(13):3278–3282. doi: 10.1080/10428194.2021.1957871.
- Huang Q, Deering KL, Harshaw Q, et al. Real-world clinical outcomes of first-line ibrutinib or chemoimmunotherapy in patients with chronic lymphocytic leukemia by risk status. Adv Ther. 2022;39(7):3292–3307. doi: 10.1007/s12325-021-01991-5.
- Levi S, Bronstein Y, Goldschmidt N, et al. Efficacy of front-line ibrutinib versus fludarabine, cyclophosphamide, and rituximab in patients with chronic lymphocytic leukemia: a retrospective multicenter “real-world” study. Am J Hematol. 2023;98(2):E24–E27. doi: 10.1002/ajh.26779.
- Ghosh N, Sharman JP, Barrientos JC, et al. Real-world outcomes with first-line ibrutinib (Ibr) versus chemoimmunotherapy (CIT) in patients with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL): final analysis results from the InformCLL registry. Blood. 2022;140(Supplement 1):7047–7049. doi: 10.1182/blood-2022-155649.
