Abstract
Objective
To describe the clinical burden and healthcare resource utilization associated with managing transfusion-dependent β-thalassemia (TDT) in France.
Methods
We used the French National Health Data System (système national des données de santé) to identify eligible patients from January 1, 2012, to March 1, 2019. Inclusion criteria were a diagnosis of β-thalassemia, ≥8 red blood cell (RBC) transfusion episodes per year in ≥2 consecutive years following the diagnosis, and ≥1 year of follow-up data. Patients were excluded if medical records showed evidence of sickle cell disease, α-thalassemia, hereditary persistence of fetal hemoglobin, or hematopoietic stem cell transplant. Clinical complications, mortality, treatment use, and healthcare resource utilization were evaluated.
Results
Overall, 331 eligible patients with TDT were identified. Mean age was 26.1 (standard deviation [SD]: 18.0) years, and 50.5% were male. Common clinical complications were endocrine (26.0%), hepatobiliary (22.7%), and cardiopulmonary (18.7%). Fifteen (4.5%) patients died during follow-up, with a mortality rate of 1.16 deaths per 100 person-years (mean age of death: 52.5 years [SD: 22]). Patients had a mean of 13.5 (SD: 5.2) RBC transfusion episodes and 11.2 (SD: 5.3) iron chelation therapy treatments per year. Healthcare resource utilization was substantial, with a mean of 14.8 inpatient hospitalizations (including 13.8 mean inpatient day cases) and 16.9 outpatient prescriptions per patient per year.
Conclusions
Patients with TDT in France experience significant clinical complications, elevated mortality, and substantial healthcare resource utilization driven by frequent RBC transfusion episodes and inpatient hospitalizations. These results reinforce the need for disease-modifying therapies for this patient population.
Introduction
β-thalassemia is a rare inherited blood disorder characterized by defects in β-globin chain synthesisCitation1. Mutations in the β-globin gene (HBB) that cause β-thalassemia lead to reduced (β+) or absent (β0) β-globin chain synthesis and excessive unbound α-globin chain synthesisCitation1,Citation2. These unbound, unstable α-globin chains precipitate in erythroid precursors, resulting in ineffective erythropoiesis, which can contribute to clinical complications, such as iron overload and anemia, and detrimental effects on growth as well as organ and vascular functionCitation1,Citation2.
Some patients with β-thalassemia have severe symptomatology and must rely on lifelong, regular red blood cell (RBC) transfusions to manage the disease; this is known as transfusion-dependent β-thalassemia (TDT)Citation2,Citation3. Frequent RBC transfusions and the disease pathology of TDT itself can lead to iron overload in patients with TDT, leading to clinical complications such as endocrinopathy, cardiomyopathy, osteoporosis, and diabetes and decreasing life expectancy. Iron chelation therapy, which is used to alleviate iron overload, can decrease the incidence of these clinical complications and improve life expectancyCitation2. Given these clinical complications, it is important to fully understand clinical and economic burdens of TDT in different geographies around the world.
While there have been two previous studies that investigated the clinical and economic burden associated with managing TDT in France, these studies either used less robust definitions for TDTCitation4 or focused only on clinical outcomes in patients aged <18 yearsCitation5. Contemporary data obtained in a well-defined cohort across all ages are needed to fully understand the clinical complications, mortality, treatment use, and healthcare resource utilization among patients with TDT in France. Therefore, this retrospective, real-world claims database analysis examined the clinical and economic burden associated with managing TDT in France.
Methods
Study design and data source
This retrospective, observational study identified patients with TDT in the French National Health Data System database, the système national des données de santé (SNDS), between January 1, 2012, and March 1, 2019. The overall study period was from January 1, 2012, to March 1, 2020.
The SNDS is a pseudonymized, longitudinal, French national claims database that includes data on 65 million insurees, accounting for approximately 99% of the French populations living in national and overseas territoriesCitation6. SNDS data include all reimbursed primary care and hospital pharmacy records in France linked via social security numbers, compiled from databases of primary care, hospital, pharmacy, and death registration details. Patient-level data from medical claims were pseudonymized. These data included treatment history, treatment patterns, healthcare resource utilization and clinical outcomes (based on International Classification of Diseases, Tenth Revision [ICD-10] diagnosis codes), procedure codes, and reimbursed outpatient drug prescriptions. Complete data capture was available for individuals in the database until the end of December 2021.
We obtained approval to access SNDS data from the relevant authorities and conducted our analyses via remote access on the Caisse nationale de l’assurance maladie (CNAM) portal to comply with référentiel de sécurité (national security guidance) guidelines. The finalized protocol was approved by the National Informatics and Liberty Commission (CNIL, decision DR-2022-065, 2 March 2022) and a scientific committee, and all patient data were anonymized. According to applicable jurisdictional legal requirements, anonymized data are exempt from privacy laws; therefore, informed consent from patients was neither required nor obtained for this study. Statistical analyses were conducted using SAS (version 9.4 TS1M4).
Population
Patient eligibility required an inpatient claim for β-thalassemia or registration in the affection longue durée or long-term disease (LTD) database with a diagnosis of β-thalassemia between January 1, 2012, and March 1, 2019. Additionally, patients must have had ≥8 RBC transfusion episodes per year in ≥2 consecutive years after the date of the first qualifying diagnosis code for β-thalassemia and had ≥1 year of follow-up data available from the index date. The index date was defined as the date of the eighth RBC transfusion episode in the second year of 2 consecutive years. Patients were excluded from the study if α-thalassemia, hereditary persistence of fetal hemoglobin, sickle cell disease, or hematopoietic stem cell transplant were identified at any time in their medical records. Eligible patients of all ages who met the inclusion criteria, and none of the exclusion criteria, were included in the analysis. Eligible patients were followed from the index date until the first occurrence of a censoring event, defined as either death or study period end (March 1, 2020).
Study outcomes
Patient demographics, including age, sex, and region of residence, were assessed at the index date. Mortality in terms of rate (death per 100 person-years), proportion (%), and mean age of death was calculated in the follow-up period. The proportions of clinical complications (%) as well as the annualized rates of treatment use and healthcare resource utilization (per patient per year) were also calculated in the follow-up period.
Statistical analysis
Patient demographics, clinical complications, mortality, and healthcare resource utilization were analyzed descriptively. In all reporting, patient numbers <10 were masked (i.e. reported as “-”) to protect patient confidentiality. Categorical variables were tabulated as number and percentage of patients or events in each category. Continuous variables were summarized using mean and standard deviation (SD) values.
Subgroup analyses were conducted for age at the index date (<18 years and ≥18 years) for all outcomes. Additional subgroup analyses were conducted based on the number of RBC transfusion episodes per patient per year (<8, 8–16, and >16) in the follow-up period for treatment use and healthcare resource utilization.
Results
Demographics
Overall, 331 patients with TDT were identified and met enrollment criteria (). Mean age at the index date was 26.1 years (SD: 18.0; range: 1–88), and 50.5% of the patients were male (). Most patients (94.9%) lived in metropolitan France and few (3.9%) lived in overseas French territories; 87.3% were registered in the LTD database ().
Figure 1. Patient attrition. Abbreviations: HPFH, hereditary persistence of fetal hemoglobin; HSCT, hematopoietic stem cell transplant; LTD, long-term disease; RBCT, red blood cell transfusion; SCD, sickle cell disease; SNDS, système national des données de santé; TDT, transfusion-dependent β-thalassemia. Note: Values presented in parentheses represent the proportion of patients with ≥1 inpatient claim or LTD database registration during the study period.
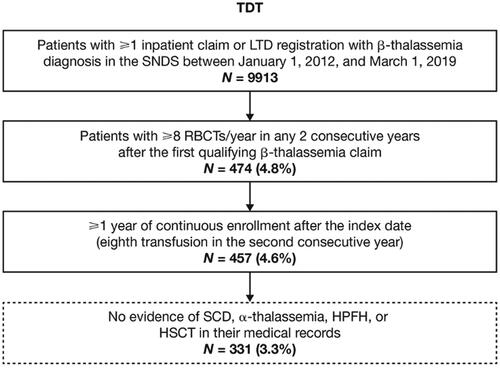
Table 1. Baseline demographics of patients with TDT.
Clinical complications
Patients with TDT had significant clinical complications, the most prevalent being endocrine complications (26.0%), hepatobiliary complications (22.7%), cardiopulmonary complications (18.7%), and splenomegaly (8.8%) (). The most common endocrine complications were diabetes (10.6%), hypogonadotropic hypogonadism (9.1%), hypoparathyroidism (7.6%), and osteoporosis (6.7%) ().
Table 2. Clinical complications in the follow-up period in patients with TDT.
Prevalence data for a substantial number of clinical complications were masked in the younger age subgroup (<18 years), as <10 patients had each complication. For complications that were not masked in both age subgroups (<18 years and ≥18 years), the prevalence of clinical complications was substantially higher in older patients than in younger patients (Supplementary Table 1). In addition, the prevalence of clinical complications was higher in older patients than in the overall study cohort (N = 331). In older patients, the most prevalent complications were endocrine (37.6%), hepatobiliary (28.8%), and cardiopulmonary (26.8%) (Supplementary Table 1).
Mortality
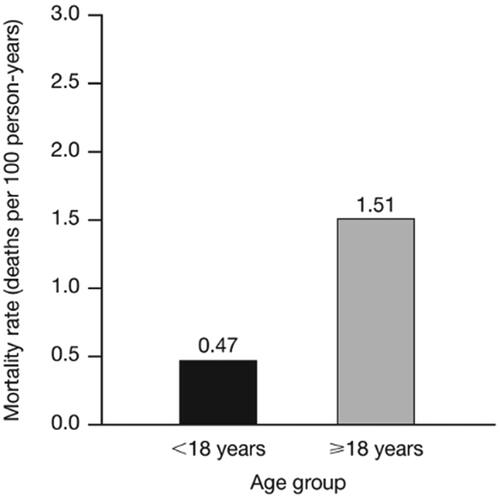
The mortality rate among patients with TDT was substantial (1.16 per 100 person-years). Of the patients who died during the follow-up period (n = 15 [4.5%]), both within and outside of the hospital setting, the mean age of death was 52.5 (SD: 22.0) years. The mortality rate among patients aged ≥18 years was approximately three times higher than that among patients aged <18 years (1.51 vs. 0.47 deaths per 100 person-years) ().
Treatment use
Patients with TDT had a mean of 13.5 (SD: 5.2) RBC transfusion episodes per patient per year. Most patients (97.3%) received iron chelation therapy in the follow-up period, with a mean rate of 11.2 (SD: 5.3) iron chelation therapy treatments per patient per year (). Older patients used pain medications, especially non-steroidal anti-inflammatory drugs and opioids, at a higher rate per patient per year than younger patients (). Both age subgroups received RBC transfusions (mean [SD]) at a similarly frequent rate per patient per year in the follow-up period (<18 years: 12.6 [3.9] vs. ≥18 years: 14.1 [5.7]) (). The use of iron chelation therapy and pain medications per patient per year increased with higher frequencies of RBC transfusion episodes per patient per year (Supplementary Table 2).
Table 3. Treatment use in the follow-up period in patients with TDT.
Table 4. Treatment use in the follow-up period by age group.
Healthcare resource utilization
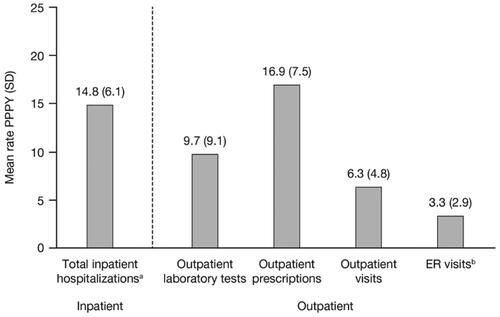
Patients with TDT had a mean rate of 14.8 (SD: 6.1) inpatient hospitalizations per patient per year, most of which lasted <1 day (13.8 [SD: 6.1]) (), as well as 26 total mean days of hospitalizations (SD: 49.4) per patient per year. Patients had a mean rate of 3.3 emergency room (ER) visits, 16.9 outpatient prescriptions, and 6.3 outpatient visits per patient per year ().
Figure 3. HCRU during follow-up in patients with TDT. Abbreviations: ER, emergency room; HCRU, healthcare resource utilization; PPPY, per patient per year; SD, standard deviation; TDT, transfusion-dependent β-thalassemia. aPatients had a mean rate of 13.8 (SD: 6.1) day cases (<1 day), 1.0 (SD: 2.7) overnight stays (≥1 day), and 26.0 (SD: 49.4) total days hospitalized PPPY. bThe mean rate of ER visits PPPY that were followed by an inpatient hospitalization was 0.2 (SD: 0.4).
All healthcare resource utilization measures (per patient per year) were higher among patients aged ≥18 years than among patients aged <18 years, including outpatient visits (7.1 vs. 4.9) and inpatient hospitalizations (15.5 vs. 13.7); inpatient hospitalizations were mostly driven by those lasting <1 day (14.5 vs. 12.5) (Supplementary Table 3). The mean rate of inpatient hospitalizations per patient per year increased as the number of RBC transfusion episodes per patient per year increased (<8: 6.5; 8–16: 13.3; >16: 21.3), which was driven primarily by hospitalizations lasting <1 day (Supplementary Table 3).
Discussion
This retrospective, real-world claims database analysis reports the clinical and economic burden associated with managing TDT in France. In the follow-up period, patients with TDT experienced significant clinical complications, early mortality, and increased treatment use and healthcare resource utilization. Older patients (≥18 years) and those with a higher frequency of RBC transfusion episodes per patient per year showed evidence of more severe disease and higher healthcare resource utilization.
This study supports the progressive nature of TDTCitation3,Citation7, as higher proportions of older patients (aged ≥18 years) experienced clinical complications compared with younger patients (aged <18 years). Our masking of prevalence data for clinical complications in younger patients may have limited our ability to compare these data between younger and older patients. However, we hypothesize that a larger sample size would yield a similar finding that TDT-related clinical complications increase with age, as other published studies on patients with TDT and associated pathophysiology have reportedCitation8,Citation9. Although older patients are more likely to experience clinical complications, children and adolescents with TDT experience disease-related complications as wellCitation5,Citation9, which underlines the urgency of treating patients with TDT as early as possible in the disease courseCitation3.
Our study showed significant mortality among patients with TDT, whose mean age of death was 52.5 years, which is consistent with another published study that reported premature mortality among these patients in France (median age of death: 37 years)Citation4. Mortality is high among this patient population, and the mean age of death observed in this study is 30 years lower than the mean life expectancy of the general population of France at birth (82.3–82.5 years)Citation10–12. Although another French study reported similar mortality rates to those reported here among patients with TDT, differences in study design may explain the variations in these rates and the mean ages of deathCitation4. The substantial mortality observed in this study further highlights the need for effective, curative treatments for patients with TDT in France.
In line with the current treatment landscapeCitation3,Citation13, patients in this study relied on frequent RBC transfusion episodes (mean rate per patient per year: 13.5) and additional disease-specific medications (e.g. iron chelation therapy) to manage TDT. The mean rate of RBC transfusion episodes reported here is consistent with treatment guidelines that recommend that patients with TDT undergo RBC transfusions every 2 to 5 weeksCitation3. Similar rates of RBC transfusion episodes among younger and older patients with TDT were likely driven by our use of the same criteria across age subgroups to define transfusion-dependence (i.e. ≥8 RBC transfusion episodes per year for 2 consecutive years). Consistent with reports conducted outside of FranceCitation9, most French patients with TDT in this study required iron chelation therapy (97.3%). The mean rate of iron chelation therapy per patient per year (11.2) suggests that these patients received daily iron chelation therapy (orally, subcutaneously, or intravenously) per treatment guidelines for iron overloadCitation3 but that some of them may have been non-adherent. Despite the high rates of RBC transfusions and iron chelation therapy use, patients with TDT experienced clinical complications, such as endocrine, hepatobiliary, and cardiopulmonary complications, that are known to develop as a result of iron overloadCitation3. Given that TDT is a progressive and lifelong disease, older patients often present with more severe disease than younger patients due to the accumulation of excess iron and associated organ complications, irrespective of whether they receive RBC transfusions at a similar rate as seen in this studyCitation2,Citation14. This notion was supported by the clinical complications’ prevalence data from the age subgroup analysis. However, additional analysis on a larger sample size of patients would allow for more granular subgroups detailing change in prevalence of complications as patients age.
As previously reported, healthcare resource utilization among patients with TDT in France is substantial, and inpatient hospitalization rates observed in our study were similar to those observed in the Brousse et al. studyCitation4. Although the number of inpatient hospitalizations reported here was considerable, the majority were for day cases (length of stay <1 day), which is consistent with healthcare use due to frequent RBC transfusion episodes. This correlation between RBC transfusion episodes and healthcare resource utilization is evident among the subgroup of patients with the highest number of RBC transfusion episodes, who experienced the highest frequency of inpatient hospitalizations, outpatient prescriptions, and ER visits. These findings support the linear relationship previously reported between healthcare resource utilization and disease severity among patients with TDT in FranceCitation4. Although our more recent analysis provides more robust healthcare resource utilization data compared with the Brousse et al. study, our study observed higher rates of inpatient hospitalizations (13.0 per patient per year in Brousse et al. vs. 14.8 per patient per year in our analysis), including day cases, which may reflect the difference in how each study identified patients with TDT (≥8 RBC transfusion episodes in 1 year in Brousse et al. vs. ≥8 RBC transfusion episodes-per year in ≥2 consecutive years in our analysis). Our study also confirms that older patients have higher mean rates of healthcare resource utilization (15.5 inpatient hospitalizations per patient per year) and RBC transfusion episodes (14.1 per patient per year) than younger patients (13.7 vs. 12.6 per patient per year, respectively). These trends were seen across additional healthcare resource utilization measures such as outpatient prescriptions, outpatient laboratory tests, outpatient visits, and ER visits. The significant healthcare resource utilization and clinical burden reported here reflect the substantial humanistic burden among patients managing TDT and TDT’s negative impact on their quality of lifeCitation15,Citation16. Taken together, this study provides a comprehensive analysis of healthcare resource utilization inclusive of hospitalizations, outpatient visits, laboratory tests, outpatient prescriptions, and ER visits, and highlights the significant utilization of health resources among patients with TDT in France.
There are several limitations to this study, which largely reflect our use of retrospective administrative claims data sources. Firstly, due to clinical differences across hospitals and physicians, there was the potential for misclassified, miscoded, or misreported data, as well as variations in how the data were reported. Secondly, there is no validated algorithm for β-thalassemia diagnosis reporting and the positive predictive value of ICD-10 codes for confirmed β-thalassemia is unknown. To mitigate against the potential for misclassification, we used strict eligibility criteria (e.g. ≥8 RBC transfusion episodes per year in ≥2 consecutive years after the date of the first qualifying β-thalassemia diagnosis), which likely resulted in a high specificity for identifying TDT. We used these rigorous eligibility criteria to define clinical burden and healthcare resource utilization and not to assess epidemiologic outcomes, including estimating the size of the patient population with β-thalassemia or TDT in France. Thirdly, because patients with TDT had to be continuously enrolled for ≥1 year to be eligible for inclusion in our study, those who were not continuously enrolled for this length of time were not represented; therefore, we cannot speak to the potential of systematically different clinical or economic outcomes in those patients and the potential impact or underestimation of outcomes reported here. Only outpatient prescription data were evaluated in this study and were based on reimbursement claims. As patient consumption or administration could not be confirmed, treatment use may be underestimated, including the use of treatments received during inpatient hospitalizations. Furthermore, complications in this study were identified via ICD-10 diagnosis codes documented in the inpatient setting, captured in hospital discharge records, or registration in the LTD database, which likely underestimated the overall prevalence of complications among patients in this study. Lastly, this study did not account for recently approved TDT therapies (such as luspatercept) and their potential impact on healthcare resource utilization. It should also be noted that our study did not directly analyze the costs associated with TDT treatment. However, studies have described elevated costs associated with TDT treatment in other countries and similar cost analyses for France could be an area of interest for future research. Finally, given the use of a claims database in this study, we were unable to capture patient iron levels and thus not able to understand the level of iron overload in these patients.
Conclusions
Despite the availability of supportive treatments for TDT, patients with this disease in France experienced substantial clinical complications, early mortality, and significant healthcare resource utilization driven by inpatient hospital day cases and frequent use of RBC transfusions. Innovative treatments that reduce the need for regular RBC transfusions and alleviate the clinical complications of TDT are expected to reduce the clinical and economic burden associated with TDT.
Transparency
Declaration of financial/other relationships
F Galactéros is a scientific advisor for Pfizer, Novartis, Theralia, Agios, Vertex, Novo Nordisk, and Terumo. J Baldwin, N Li, C Udeze, L Boulmerka, and L Dahal were employees of Vertex Pharmaceuticals Incorporated at the time of the study was conducted and may own stock or stock options in Vertex Pharmaceuticals Incorporated. L Boulmerka received grants or contracts from Vertex Pharmaceuticals Incorporated and Amgen. G Pesce, N Quignot, and H Jiang are employed by Certara. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors contributed to the study conceptualization, design, and interpretation of the results per ICJME guidelines. Initial data analysis was performed by H Jiang, N Quignot, and G Pesce in consultation with J Baldwin and C Udeze. All authors have reviewed the results and have read and approved the final manuscript.
Supplemental Material
Download MS Word (58.5 KB)Acknowledgements
Data were provided by the French CNAM and its staff, in particular the DEMEX team. Data application support was provided by the Health Data Hub; the Ethics and Scientific Committee for Health Research, Studies and Evaluations (CESREES); and the French Commission nationale de l’informatique et des libertés (CNIL), the administrative authority for data access and privacy laws. Medical writing and editing support were provided by Sophie Irving, Iona Linford, Jenifer Li, and Nicholas Strange of Complete HealthVizion, IPG Health Medical Communications, Chicago, IL, USA, funded by Vertex Pharmaceuticals Incorporated.
Data availability statement
Data are available from The French National Healthcare Data System. Submit requests to the National Institute of Health Data (INDS) https://www.snds.gouv.fr/SNDS/Accueil.
Additional information
Funding
References
- Galanello R, Origa R. Beta-thalassemia. Orphanet J Rare Dis. 2010;5(1):11. doi: 10.1186/1750-1172-5-11.
- Taher AT, Weatherall DJ, Cappellini MD. Thalassaemia. Lancet. 2018;391(10116):155–167. doi: 10.1016/S0140-6736(17)31822-6.
- Cappellini MD, Farmakis D, Porter J, et al. Guidelines for the management of transfusion dependent thalassaemia (TDT). 4th ed. Nicosia (Cyprus): Thalassaemia International Federation; 2021 [Internet]. 2021 [cited 23 August 2023]. Available from: https://www.thalassemia.org/wp-content/uploads/2021/06/TIF-2021-Guidelines-for-Mgmt-of-TDT.pdf.
- Brousse V, Badens C, Quignot N, et al. PRO58 clinical and economic burden of transfusion-dependent beta-thalassemia in France: a retrospective analysis of the French National Health Data System (SNDS). Value Health. 2019;22(3):S851. doi: 10.1016/j.jval.2019.09.2388.
- Donze C, Benoit A, Thuret I, et al. β-Thalassemia in childhood: current state of health in a high-income country. Br J Haematol. 2023;201(2):334–342. doi: 10.1111/bjh.18631.
- Tuppin P, Rudant J, Constantinou P, et al. Value of a national administrative database to guide public decisions: from the systeme national d‘information interregimes de l‘Assurance Maladie (SNIIRAM) to the systeme national des donnees de sante (SNDS) in France. Rev Epidemiol Sante Publique. 2017;65(Suppl 4):S149–S167. doi: 10.1016/j.respe.2017.05.004.
- Baronciani D, Angelucci E, Potschger U, et al. Hemopoietic stem cell transplantation in thalassemia: a report from the European Society for Blood and Bone Marrow Transplantation Hemoglobinopathy Registry, 2000–2010. Bone Marrow Transplant. 2016;51(4):536–541. doi: 10.1038/bmt.2015.293.
- Pinto VM, Poggi M, Russo R, et al. Management of the aging beta-thalassemia transfusion-dependent population - The Italian experience. Blood Rev. 2019;38:100594. doi: 10.1016/j.blre.2019.100594.
- Udeze C, Evans KA, Yang Y, et al. Economic and clinical burden of managing transfusion-dependent β-thalassemia in the United States. J Med Econ. 2023;26(1):924–932. doi: 10.1080/13696998.2023.2235928.
- World Health Organization (WHO) [Internet]. The Global Health Observatory: life expectancy at birth (years); 2020. [cited December 2020]. Available from: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/life-expectancy-at-birth-(years.).
- Institut national d‘études démographiques (INED) [Internet]. Life expectancy at birth and at 65 years; 2023. [cited January 2023]. Available from: https://www.ined.fr/en/everything_about_population/data/france/deaths-causes-mortality/life-expectancy/.
- The World Bank [Internet]. Life expectancy at birth (years); 2023. [cited October 2023]. Available from: https://genderdata.worldbank.org/indicators/sp-dyn-le-00-in/.
- Farmakis D, Porter J, Taher A, et al. 2021 Thalassaemia International Federation Guidelines for the Management of Transfusion-dependent Thalassemia. Hemasphere. 2022;6(8):e732. doi: 10.1097/HS9.0000000000000732.
- Taher AT, Cappellini MD. How I manage medical complications of β-thalassemia in adults. Blood. 2018;132(17):1781–1791. doi: 10.1182/blood-2018-06-818187.
- Drahos J, Boateng-Kuffour A, Calvert M, et al. Sustained Humanistic Burden and Work Impact in Adults with Transfusion-Dependent Beta-Thalassemia (TDT): Results from a Global Longitudinal Survey [abstract]. Value Health. 2023;26(6):S317. Abstract PCR29. doi: 10.1016/j.jval.2023.03.1809.
- Shah F, Telfer P, Velangi M, et al. Routine management, healthcare resource use and patient and carer-reported outcomes of patients with transfusion-dependent beta-thalassaemia in the United Kingdom: A mixed methods observational study. EJHaem. 2021;2(4):738–749. doi: 10.1002/jha2.282.