Abstract
Objective
To compare safety and efficacy of centanafadine versus methylphenidate hydrochloride extended release (ER; Concerta) in adults with ADHD.
Methods
Without head-to-head trials, anchored matching-adjusted indirect comparisons (MAIC) of adverse event rates reported across trials and mean change from baseline in Adult ADHD Investigator Symptom Rating Scale (AISRS) score between centanafadine and methylphenidate hydrochloride ER were conducted. Pooled patient-level data from two centanafadine trials (NCT03605680/NCT03605836) and aggregate data from one published methylphenidate hydrochloride ER trial (NCT00937040) were used. Characteristics of individual patients from the centanafadine trials were matched to aggregate baseline characteristics from the methylphenidate hydrochloride ER trial using propensity score weighting. A sensitivity analysis assessed the robustness of the results to the capping of extreme weights (i.e. >99th percentile).
Results
Compared with methylphenidate hydrochloride ER, centanafadine was associated with significantly lower risk of dry mouth (risk difference [RD] in percentage points: −11.95), initial insomnia (−11.10), decreased appetite (−8.05), anxiety (−5.39), palpitations (−5.25), and feeling jittery (−4.73) though a significantly smaller reduction in AISRS score (4.16-point). In the sensitivity analysis, the safety results were consistent with the primary analysis but there was no significant difference in efficacy between centanafadine and methylphenidate hydrochloride ER.
Conclusion
In this MAIC, centanafadine had better safety and possibly lower efficacy than methylphenidate hydrochloride ER. While safety results were robust across analyses, there was no efficacy difference between centanafadine and methylphenidate hydrochloride ER in the sensitivity analysis. Considering its favorable safety profile, centanafadine may be preferred among patients for whom treatment-related adverse events are a concern.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) remains one of the most commonly diagnosed neurodevelopmental disorders in children and adolescents in the United States (US)Citation1, persisting into adulthood in up to two-thirds of casesCitation2,Citation3. Among adults 18-44 years old in the US, the overall prevalence of ADHD is estimated to be 4.4% with a total lifetime prevalence of 8.1%Citation4,Citation5. ADHD is characterized by inattention, hyperactivity, impulsivity, and impairments in executive function, emotional regulation, and motivationCitation6. Due to the chronic and persistent nature of these symptoms, ADHD can be associated with a wide range of psychosocial and occupational problemsCitation7. Consequently, adults with ADHD experience low health-related quality of life, impairment of interpersonal relationships, poor educational attainment, and are likely to exhibit high-risk behaviors such as substance abuse, criminal activity, and reckless drivingCitation8–16.
Although there are no curative treatments for ADHD, symptom management can be achieved through the use of pharmacological interventionsCitation17. Currently, the treatment landscape for adults with ADHD in the US includes stimulants, such as methylphenidate- or amphetamine-based medications which are known for their rapid onset and high response rate (∼70%), and non-stimulants, such as the norepinephrine reuptake inhibitor, atomoxetine, which are typically used as an alternative for patients who cannot tolerate stimulants or for whom the misuse of stimulants is a concernCitation18–23. Centanafadine, a serotonin-norepinephrine-dopamine, triple-reuptake inhibitor, is a novel treatment option being investigated in adults with ADHDCitation24. Two large Phase 3 clinical trials of centanafadine (NCT03605680 and NCT03605836) recently examined its safety and efficacy compared to placeboCitation25.
In the absence of head-to-head trials comparing the efficacy and safety of centanafadine to other ADHD treatments, there is no direct comparative evidence between ADHD treatment options. Recently, a matching-adjusted indirect comparison (MAIC) evaluated the safety and efficacy of centanafadine compared to lisdexamfetamine dimesylate (Vyvanse), one of the most commonly used amphetamine-based stimulants, atomoxetine (Strattera), one of the most commonly used non-stimulants, and viloxazine (Qelbree), a recently approved non-stimulantCitation26–30. Relative to all three comparators, centanafadine was associated with a significantly lower incidence of adverse events, which may be associated with better outcomes and quality of life, lower healthcare costs, and improved adherence and persistence to treatmentCitation16,Citation31–33. Its efficacy was comparable to atomoxetine and viloxazine (i.e. the non-stimulants), but lower than lisdexamfetamine dimesylate (i.e. the amphetamine-based stimulant)Citation30. Nevertheless, there is currently no comparative evidence on centanafadine relative to methylphenidate-based stimulants. Given the difference in efficacy and safety profiles between methylphenidate- and amphetamine-based stimulantsCitation26,Citation34, the current study aimed to fill this gap in knowledge by performing a MAIC to evaluate the safety and efficacy of centanafadine compared to methylphenidate hydrochloride extended release (ER; Concerta), a well-established and commonly used methylphenidate-based stimulant in adults with ADHD, after adjusting for differences across trials.
Methods
Data sources
The anchored MAIC relied on deidentified individual patient data (IPD) from the centanafadine trials (NCT03605680 and NCT03605836) as well as aggregate statistics from a comparable methylphenidate hydrochloride ER trial (NCT00937040) which was identified through a review of the literature. Relative to unanchored MAICs where the active treatment arms are compared across trials, in an anchored MAIC, the differences between the active treatments and a common comparator are compared across trials, thus adjusting further for cross trial heterogeneity.
As the two centanafadine trials employed the same study designCitation25, IPD from these two trials were pooled and analyzed together. Both were Phase 3, randomized, double-blind, multicenter, placebo-controlled, parallel-group trials evaluating the efficacy, safety, and tolerability of centanafadine in adult patients 18-55 years old. The trials included eligible patients who had a confirmed diagnosis of ADHD based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) criteria, a score of ≥28 on the Adult ADHD Investigator Symptom Rating Scale (AISRS; a semi-structured clinical interview that assesses each symptom domain of ADHD using an 18-item scale that directly corresponds to the DSM symptoms with a between-treatment minimally clinical difference of 10 pointsCitation35) and a score of ≥4 on the Clinical Global Impression-Severity of Illness Scale (CGI-S) at baseline. Patients with comorbid psychiatric disorders were excluded. The trial started with a single-blind run-in period whereby all patients were given matched-placebo tablets twice daily for 7 days. Subjects were administered the AISRS before the start of the single-blind placebo run-in period (Day −7) and immediately before randomization (Day −1). Those with a ≥30% improvement in their Adult ADHD Self-report Symptom Checklist (ASRS) score versus the previous test were not eligible for the study. Baseline measures were evaluated after the run-in period. In both trials combined, 301 patients were randomized to receive 200 mg of centanafadine daily, 303 patients were randomized to receive 400 mg of centanafadine daily, and 302 patients were randomized to receive placebo daily for 6 weeks following the run-in period.
To identify potential comparator clinical trials, a literature review was conducted on the ClinicalTrials.gov registry on April 24, 2023 based on the following criteria: ADHD (condition or disease); methylphenidate hydrochloride ER (intervention); completed (recruitment status); adult (18-64; eligibility criteria); Phase 3 or 4 (study phase). All aspects of the trial design, including the patient populations, interventions, and outcomes of interest, were reviewed. Relative to the two centanafadine clinical trials, any potential comparators that used a different design (e.g. open-label), focused on a specific subpopulation (e.g. only male), compared centanafadine to any intervention other than placebo (e.g. a combination of drugs), or did not include the main outcomes of interest (i.e. AISRS scores and adverse events) were excluded. The assessment yielded one potential comparator trial for methylphenidate hydrochloride ERCitation36.
The NCT00937040 clinical trial was a Phase 3, randomized, double-blind, multicenter, placebo-controlled, parallel-group trial evaluating the safety and efficacy of methylphenidate hydrochloride ER in adults 18-65 years old. Eligible patients had a confirmed diagnosis of ADHD based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria and a score >24 on the AISRS at baseline. Patients with mild depression according to the Hamilton Depression Rating Scale (i.e. score <18) or mild anxiety according to the Hamilton Anxiety Rating Scale (i.e. score <21) were eligible, but those with moderate or severe depression or anxiety were excluded. Patients with a history of substance dependence or a history of stimulant or atomoxetine use were also excluded. A total of 178 patients were randomized to receive 18 mg daily and 179 patients were randomized to receive placebo daily. Treatment doses were increased to 36 mg, 54 mg, and 72 mg until the patient reached an AISRS score <18 or a limit of tolerability.
Patient selection and characteristics
According to the eligibility criteria for the centanafadine trials and the comparator trial for methylphenidate hydrochloride ER, patients included in this MAIC were 18-65 years old (with a maximum age of 55 years for centanafadine and 65 years for methylphenidate hydrochloride ER), had a confirmed diagnosis of ADHD per the DSM-IV/V criteria, had moderate to severe ADHD at baseline (patients included in the centanafadine trials had an AISRS score ≥28 at screening and baseline if not receiving any pharmacological treatment, and ≥28 at baseline and ≥22 at screening for those receiving pharmacological treatment for ADHD; patients included in the comparator methylphenidate hydrochloride ER trial had to have an AISRS score >24 at screening and at baseline), had no comorbid psychiatric diagnoses (patients with comorbid psychiatric disorders, including Axis I and Axis II disorders, were excluded from the centanafadine trials, and patients with moderate or severe comorbid psychiatric diagnoses were excluded from the methylphenidate hydrochloride ER trial), and were not taking prohibited medications (the centanafadine trials excluded patients who used psychotropic medications, and the methylphenidate hydrochloride ER trial excluded patients who used stimulants or atomoxetine within 5 years of the trial or other ADHD medications within 30 days of the trial). While the centanafadine trial excluded placebo responders during the run-in period, the methylphenidate hydrochloride ER trial did not have a run-in period.
Outcome measures
The efficacy outcome was the mean change in the AISRS score from baseline to Week 6, which was the primary efficacy outcome across trials. The safety outcomes comprised the adverse events reported at Week 6 for both centanafadine and methylphenidate hydrochloride ER and experienced by ≥5% of patients in any treatment group with an incidence twice that of placebo (the reporting criteria for the methylphenidate hydrochloride ER trial). Based on these criteria, the adverse events included in the analyses were anxiety, decreased appetite, dry mouth, feeling jittery, insomnia (including both initial insomnia [i.e. trouble falling asleep] and any insomnia), and palpitations.
Statistical methods
Descriptive statistics were reported using frequencies and proportions for categorical variables and means and standard deviations (SDs) for continuous variables. All the available baseline prognostic factors and effect modifiers reported across trials were considered for adjustment. Baseline patient characteristics (i.e. age, sex, race, ethnicity, weight, body mass index [BMI], baseline AISRS score, baseline CGI-S score, and baseline Adult ADHD Self-Report Scale [ASRS] score) were reported when available from the centanafadine trials IPD and published for comparator methylphenidate hydrochloride ER trial. The treatment and placebo arms were compared across trials using Wald tests for categorical and continuous variables (i.e. chi square and z tests) to identify differences between the trial populations, separately for the safety and efficacy analyses.
For each patient enrolled in the centanafadine trials, the likelihood of their enrollment in the comparator methylphenidate hydrochloride ER trial was estimated. Using a propensity score approach, IPD from the centanafadine trials were reweighted to match the baseline characteristics (i.e. means, SDs, and proportions) of the methylphenidate hydrochloride ER trial. The safety and efficacy outcomes were then compared between centanafadine and methylphenidate hydrochloride ER before and after matching. Specifically, efficacy analyses reported the difference in AISRS change from baseline to Week 6 between centanafadine and methylphenidate hydrochloride ER. Safety analyses reported the risk differences between centanafadine and methylphenidate hydrochloride ER for each adverse event. All estimates were reported with corresponding confidence intervals (CIs) and p-values.
Sensitivity analysis
In a MAIC, when the overlap of patient characteristics between trials is limited due to differences across populations, some patients may receive extreme weights. These extreme weights can bias the results by overly representing those patients in the analyses. To mitigate this issue, sensitivity analyses can be conducted to assess the robustness of the results to the removal of extreme weights (i.e. to assess whether the results change when extreme weights are not allowed). In this study, following prior MAICs, a sensitivity analysis was conducted, with weights capping at the 99th percentileCitation37,Citation38. Weight capping defines a maximum weight based on the observed distribution of weights, and replaces the extreme weights by the weight capCitation37,Citation39–44. For patients with extreme weights in the entire population (both treatment and placebo arms combined), the extreme weights (i.e. weights above the 99th percentile) were replaced by the 99th percentile weight value, separately for the safety and efficacy populations.
Results
The safety analyses comprised 876 patients from the centanafadine trials (586 patients in the centanafadine arm and 290 patients in the placebo arm) as well as aggregated data for 349 patients from the methylphenidate hydrochloride ER trial (174 patients in the methylphenidate hydrochloride ER arm and 175 patients in the placebo arm). The efficacy analyses included 859 patients from the centanafadine trials (574 patients in the centanafadine arm and 285 patients in the placebo arm) as well as aggregated data for 349 patients from the methylphenidate hydrochloride ER trial (174 patients in the methylphenidate hydrochloride ER arm and 175 patients in the placebo arm).
Centanafadine versus methylphenidate hydrochloride ER
Baseline characteristics
In the safety population, before matching, there were no significant differences in age, sex, race, or ethnicity across trials. However, on average, patients who received centanafadine had a significantly higher weight and BMI than patients who received methylphenidate hydrochloride ER, with patients in the placebo arm of the centanafadine trials also having significantly higher BMI than those in the placebo arm of the methylphenidate hydrochloride ER trial. There were differences in baseline severity across trials, with CGI-S suggesting that patients who received centanafadine had less severe symptoms than patients receiving methylphenidate hydrochloride ER, and AISRS suggesting that patients in the placebo arm of the centanafadine trial had more severe symptoms than patients in the placebo arm of the methylphenidate hydrochloride ER trial. After matching on age, sex, race, ethnicity, weight, BMI, AISRS at baseline, CGI-S at baseline, and ASRS at baseline, the baseline characteristics of patients in the centanafadine trials were comparable to those in the methylphenidate hydrochloride ER trial (). In the efficacy population, the same differences were observed across trials before matching and all characteristics were similar after matching ().
Table 1. Baseline characteristic for centanafadine versus methylphenidate hydrochloride ER (base case).
Safety and efficacy outcomes
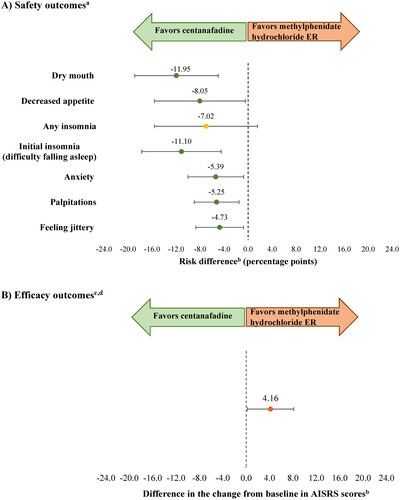
After matching and anchoring (i.e. accounting for the difference between placebo arms), the risk difference (in percentage points) in the safety analysis between centanafadine and methylphenidate hydrochloride ER was −11.95 for dry mouth (CI = −18.91, −4.98; p = 0.001), −11.10 for initial insomnia (CI = −17.72, −4.48; p = 0.001), −8.05 for decreased appetite (CI = −15.63, −0.47; p = 0.037), −5.39 for anxiety (CI = −10.03, −0.74; p = 0.023), −5.25 for palpitations (CI = −8.99, −1.51; p = 0.006), and −4.73 for feeling jittery (CI = −8.71, −0.75; p = 0.020). There was no significant difference in the risk of insomnia with centanafadine compared with methylphenidate hydrochloride ER (−7.02; CI = −15.61, 1.57; p = 0.109). In the efficacy analysis, there was a statistically significant 4.16-point difference in the AISRS score change from baseline between centanafadine and methylphenidate hydrochloride ER (CI = 0.19, 8.13; p = 0.040; ). The arm-by-arm safety and efficacy outcomes before and after matching for the base case are presented in Supplementary Figure S1.
Figure 1. Safety and efficacy outcomes for centanafadine versus methylphenidate hydrochloride ER (base case).
Abbreviations. AISRS, Adult ADHD (attention-deficit/hyperactivity disorder) Investigator Symptom Rating Scale; ASRS, Adult ADHD (attention-deficit/hyperactivity disorder) Self-Report Scale; BMI, body mass index; CGI-S, Clinical Global Impression-Severity of Illness Scale; ER, extended release.
Notes:
aAdverse events for which information was available in both trials, and which were reported by ≥5% of patients in any treatment group with an incidence twice that of placebo at Week 6 (as available in methylphenidate hydrochloride ER trial).
bAnalyses were matched on age, sex, race, ethnicity, weight, BMI, AISRS at baseline, CGI-S at baseline, and ASRS at baseline. AISRS is an 18-item clinician-administered scale with 9 inattentive and 9 hyperactive-impulsive items. The ASRS is an 18-item self-report questionnaire. Higher scores indicate more severe ADHD symptoms for both scales.
c Efficacy outcomes were compared at Week 6 (as available in methylphenidate hydrochloride ER trial).
dAISRS is a clinician-administered 18-item scale. The higher the score is, the more severe the ADHD symptoms are.
Centanafadine versus methylphenidate hydrochloride ER (sensitivity analysis with weight capping at the 99th percentile)
The 99th percentile weight across the treatment and placebo arms was 3.39 in the safety population and 3.38 in the efficacy population. The weights of nine patients in the safety population and nine patients in the efficacy population exceeded the respective weight caps and were replaced by these values.
Baseline characteristics
Before matching, the baseline characteristics in the sensitivity analyses were the same as in the base case (see above for description of differences across trials). After matching on age, sex, race, ethnicity, weight, BMI, AISRS at baseline, CGI-S at baseline, and ASRS at baseline (as in the base case) and weight-capping at the 99th percentile for the 9 patients who had received extreme weights in the base case, cohorts were still well-balanced in both the safety and efficacy population. Differences in baseline characteristics across trials were all insignificant ().
Table 2. Baseline characteristic for centanafadine versus methylphenidate hydrochloride ER (sensitivity analysis with weight capping at the 99th percentile.
Safety and efficacy outcomes
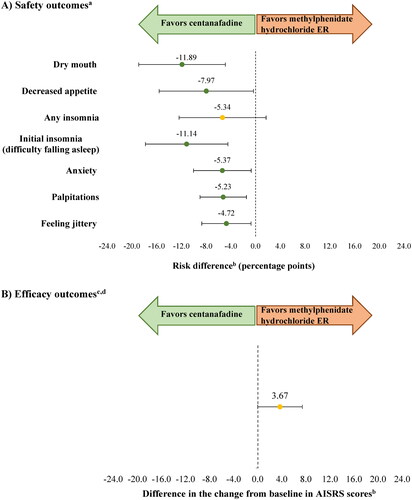
After matching, anchoring, and capping weights at the 99th percentile, the results of the sensitivity analysis for safety outcomes remained consistent with those of the base case. The risk difference (in percentage points) between centanafadine and methylphenidate hydrochloride ER was −11.89 for dry mouth (CI= −18.89, −4.89; p = 0.001), −11.14 for initial insomnia (CI= −17.81, −4.47; p = 0.001), −7.97 for decreased appetite (CI= −15.59, −0.34; p = 0.041), −5.37 for anxiety, (CI= −10.03, −0.71; p = 0.024), −5.23 for palpitations (CI= −8.98, −1.49; p = 0.006), and −4.72 for feeling jittery (CI= −8.70, −0.74; p = 0.020). There was no significant difference in the risk of any insomnia with centanafadine versus methylphenidate hydrochloride ER (-5.34; CI= −12.37, 1.70; p = 0.137). However, the results of the sensitivity analysis for efficacy were not consistent with those of the base case as there was no statistically significant difference in the AISRS score change from baseline between centanafadine and methylphenidate hydrochloride ER (3.67; CI= −0.04, 7.37; p = 0.053; ). The arm-by-arm safety and efficacy outcomes before and after matching and weight capping at the 99th percentile are presented in Supplementary Figure S2.
Figure 2. Safety and efficacy outcomes for centanafadine versus methylphenidate hydrochloride ER (sensitivity analysis with weight capping at the 99th percentile).
Abbreviations. AISRS, Adult ADHD (attention-deficit/hyperactivity disorder) Investigator Symptom Rating Scale; ASRS, Adult ADHD (attention-deficit/hyperactivity disorder) Self-Report Scale; BMI, body mass index; CGI-S, Clinical Global Impression-Severity of Illness Scale; ER, extended release.
Notes:
aAdverse events for which information was available in both trials, and which were reported by ≥5% of patients in any treatment group with an incidence twice that of placebo at Week 6 (as available in methylphenidate hydrochloride ER trial).
bAnalyses were matched on age, sex, race, ethnicity, weight, BMI, AISRS at baseline, CGI-S at baseline, and ASRS at baseline. AISRS is an 18-item clinician-administered scale with 9 inattentive and 9 hyperactive-impulsive items. The ASRS is an 18-item self-report questionnaire. Higher scores indicate more severe ADHD symptoms for both scales.
cEfficacy outcomes were compared at Week 6 (as available in methylphenidate hydrochloride ER trial).
dAISRS is a clinician-administered 18-item scale. The higher the score is, the more severe the ADHD symptoms are.
Discussion
Efficacy and safety are important aspects of the treatment decision making process and in the absence of head-to-head trials or real-world data to conduct relevant comparisons between available treatment options for ADHD, it can be challenging for physicians and patients to make informed treatment decisions. Generally, outcomes cannot be directly compared between clinical trials since cohorts have different characteristics (e.g. age, sex, race, disease severity) that can confound the results, and adjusting for confounding using standard methods, such as regression analysis, requires IPD for both therapies of interest, which is typically only available for one trial by its sponsor. Comparative evidence on centanafadine versus methylphenidate-based stimulants for adults with ADHD was not available. In this context, this study conducted an anchored MAIC of key safety and efficacy outcome measures between centanafadine and methylphenidate hydrochloride ER, one of the leading methylphenidate-based stimulants, by leveraging IPD from the Phase 3 centanafadine trials and published aggregate results for a comparable methylphenidate hydrochloride ER trial (for which IPD were not available) and adjusting for cross-trial differences that may impact the outcomes.
This study demonstrates that centanafadine has a better safety profile and possibly lower efficacy than methylphenidate hydrochloride ER, although the efficacy findings are mixed. Centanafadine was found to have a consistently better safety profile than methylphenidate hydrochloride ER in both the base case and the sensitivity analysis. However, while centanafadine had lower efficacy than methylphenidate hydrochloride ER in the base case (4.2 AISRS points, p = 0.040), there was no difference in efficacy between centanafadine and methylphenidate hydrochloride ER in the sensitivity analysis when extreme weights, received by only nine patients, were capped at the 99th percentile (3.7 points, p = 0.053). The non-robustness of the results to the capping of extreme weights suggests that the base case efficacy results may have been driven by outliers. Further studies are needed to investigate the comparative efficacy of centanafadine versus methylphenidate hydrochloride ER.
The large differences in safety outcomes found between treatments may have important health and quality of life implications. Relative to methylphenidate hydrochloride ER, centanafadine was associated with significantly lower risks of anxiety, initial insomnia, palpitations, feeling jittery, decreased appetite and dry mouth, with a risk difference ranging between 5-12 percentage points. Using initial insomnia as an example, this means that out of a hypothetical cohort of 100 patients, approximately 11 fewer patients would experience initial insomnia with centanafadine treatment compared with methylphenidate hydrochloride ER treatment. Prior research showed that adverse events are commonly reported among patients treated for ADHD, particularly among those treated with stimulantsCitation17,Citation45. Based on a survey conducted among adults receiving ADHD medications in the US, 95% reported having experienced ≥1 symptom associated with ADHD and its treatment-related adverse events in the prior monthCitation16. Together, these results underscore the importance of considering the safety outcomes associated with ADHD medications in clinical practice, as well as the potential for centanafadine to offer a safer treatment option.
The results of this MAIC provide novel information with key implications for medical decision making. The literature suggests there is a potential unmet need with the current therapeutic options for ADHDCitation16, such that the condition often remains untreated among adult patientsCitation17,Citation46. In addition, among those receiving medications, a large proportion cycle through multiple regimens within a short timeCitation32. As such, treatment adherence and persistence remain ongoing challenges in this population, with suboptimal tolerability and high risks of adverse events often underlying these issuesCitation16,Citation31–33. To that end, new treatment options for ADHD that are associated with a favorable safety profile might have the potential to increase adherence and persistence, improve outcomes, and reduce the increased healthcare costs associated with frequent treatment changesCitation32.
Limitations
The results of this study should be considered in light of certain limitations. Some differences existed in the eligibility criteria used across trials as well as in the definitions of certain criteria. Patients included in the centanafadine trials were 18-55 years old while patients included in the methylphenidate hydrochloride ER trial were 18-65 years old. Since only aggregate data were available from the methylphenidate hydrochloride ER trial, patients between 56-65 years old could not be excluded from the analyses. Moreover, severity-based inclusion criteria (AISRS and CGI-S at baseline) were different across trials. In addition, certain exclusion criteria in the centanafadine trials were applied using a different definition or not considered in the methylphenidate hydrochloride ER trial. Specifically, the exclusion criteria related to the use of prohibited medications in the methylphenidate hydrochloride ER trial could not be applied to the centanafadine IPD as it would have resulted in the exclusion of most patients from the centanafadine trials, thereby making the analyses not feasible.
The risk of bias related to the differences in these eligibility criteria across trials was mitigated by matching on all baseline characteristics available, including age and baseline severity (using all instruments available: AISRS, CGI-S, and ASRS). However, matching based on baseline characteristics was only possible for variables that were collected in the trials. As such, there may have been other residual differences in the patients’ characteristics, such as comorbidities and concomitant medications, that were unobserved and remained unbalanced after matching.
Finally, given that the reweighting was conducted to match the population included in the methylphenidate hydrochloride ER trial, the target population of this analysis is more closely representative of the populations included in that trial than of the population included in the centanafadine trials. Therefore, generalizability to a broader population of adults with ADHD may be limited.
Conclusions
In this anchored MAIC in adults with ADHD, centanafadine had a better safety profile and possibly lower efficacy than methylphenidate hydrochloride ER. While the safety results were robust across analyses, the efficacy results should be interpreted with caution since they may be driven by a few patients receiving extreme weights and there was no difference in efficacy between centanafadine and methylphenidate hydrochloride ER in the sensitivity analysis capping extreme weights. Considering its consistently favorable safety profile, centanafadine may represent a promising treatment option for adults with ADHD, particularly those with safety concerns, with the potential to address some of the unmet needs related to treatment-related adverse events.
Transparency
Declaration of financial/other relationships
Jeff Schein is an employee of Otsuka Pharmaceutical Development & Commercialization, Inc. Martin Cloutier, Marjolaine Gauthier-Loiselle, Maryaline Catillon, Chunyi Xu, and Alice Qu are employees of Analysis Group, Inc., a consulting company that has provided paid consulting services to Otsuka Pharmaceutical Development & Commercialization, Inc. Ann Childress received research support from Allergan, Emalex, Akili, Cingulate, Corium, Lumos, Neurocentria, Otsuka, Purdue, Adlon, Sunovion, Tris, KemPharm, and Supernus; was on the advisory board of Corium, Otsuka, Tris, and Supernus; received consulting fees from Aytu, Cingulate, Corium, Lumos, Neurocentria, Noven, Otsuka, Tris, KemPharm, Supernus, and Tulex; received speaker fees from Takeda, Corium, Ironshore, Tris, and Supernus; and received writing support from Takeda, Corium, Ironshore, Purdue, and Tris.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Martin Cloutier, Marjolaine Gauthier-Loiselle, Maryaline Catillon, Chunyi Xu, and Alice Qu contributed to study conception and design, collection and assembly of data, and data analysis and interpretation. Jeff Schein and Ann Childress contributed to study conception and design, data analysis and interpretation. All authors reviewed and approved the final content of this manuscript.
Ethics statement
This was a post-hoc analysis of previously collected, anonymized trial data; thus, no ethical review was required.
ADHD MAIC_supplementary materials.docx
Download MS Word (89.1 KB)Acknowledgements
Medical writing assistance was provided by professional medical writer, Loraine Georgy, PhD, MWC, an employee of Analysis Group, Inc., and was funded by Otsuka Pharmaceutical Development & Commercialization, Inc.
Additional information
Funding
References
- National Institute of Health National Institute of Mental Health. Attention-deficit/hyperactivity disorder (ADHD); 2019 [2019 Jul 11]. Available from: https://www.nimh.nih.gov/health/statistics/attention-deficit-hyperactivity-disorder-adhd.shtml
- Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006; 36(2):159–165.
- Cheung CH, Rijsdijk F, McLoughlin G, et al. Cognitive and neurophysiological markers of ADHD persistence and remission. Br J Psychiatry. 2016;208(6):548–555. doi: 10.1192/bjp.bp.114.145185.
- Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602.
- Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716–723.
- Wilens TE, Faraone SV, Biederman J. Attention-deficit/hyperactivity disorder in adults. JAMA. 2004; 292(5):619–623.
- Asherson P, Buitelaar J, Faraone SV, et al. Adult attention-deficit hyperactivity disorder: key conceptual issues. Lancet Psychiatry. 2016;3(6):568–578. doi: 10.1016/S2215-0366(16)30032-3.
- Thompson AL, Molina BS, Pelham W, Jr., et al. Risky driving in adolescents and young adults with childhood ADHD. J Pediatr Psychol. 2007;32(7):745–759.
- Kooij SJ, Bejerot S, Blackwell A, et al. European consensus statement on diagnosis and treatment of adult ADHD: the European Network Adult ADHD. BMC Psychiatry. 2010;10(1):67.
- Hodgkins P, Arnold LE, Shaw M, et al. A systematic review of global publication trends regarding long-term outcomes of ADHD. Front Psychiatry. 2011;2:84.
- Brod M, Schmitt E, Goodwin M, et al. ADHD burden of illness in older adults: a life course perspective. Qual Life Res. 2012;21(5):795–799.
- Kotsopoulos N, Connolly MP, Sobanski E, et al. The fiscal consequences of ADHD in Germany: a quantitative analysis based on differences in educational attainment and lifetime earnings. J Ment Health Policy Econ. 2013;16(1):27–33.
- Vaa T. ADHD and relative risk of accidents in road traffic: a meta-analysis. Accid Anal Prev. 2014;62:415–425.
- Pitts M, Mangle L, Asherson P. Impairments, diagnosis and treatments associated with attention-deficit/hyperactivity disorder (ADHD) in UK adults: results from the Lifetime Impairment Survey. Arch Psychiatr Nurs. 2015; 29(1):56–63.
- Mohr-Jensen C, Steinhausen HC. A meta-analysis and systematic review of the risks associated with childhood attention-deficit hyperactivity disorder on long-term outcome of arrests, convictions, and incarcerations. Clin Psychol Rev. 2016;48:32–42.
- Schein J, Cloutier M, Gauthier-Loiselle M, et al. Symptoms associated with ADHD/treatment-related adverse side effects and their impact on quality of life and work productivity in adults with ADHD. Curr Med Res Opin. 2023;39(1):149–159.
- Faraone SV, Asherson P, Banaschewski T, et al. Attention-deficit/hyperactivity disorder. Nat Rev Dis Primers. 2015;1(1):15020. doi: 10.1038/nrdp.2015.20.
- Kolar D, Keller A, Golfinopoulos M, et al. Treatment of adults with attention-deficit/hyperactivity disorder. Neuropsychiatr Dis Treat. 2008;4(1):107–121. doi: 10.2147/ndt.s1747.
- Substance Abuse and Mental Health Services Administration. Results from the 2017 National Survey on Drug Use and Health: detailed tables 2017. Available from: https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHDetailedTabs2017/NSDUHDetailedTabs2017.htm#tab1-25D.
- Cortese S, Adamo N, Del GC, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry. 2018;5(9):727–738. doi: 10.1016/s2215-0366(18)30269-4.
- Upadhyaya H, Tanaka Y, Lipsius S, et al. Time-to-onset and-resolution of adverse events before/after atomoxetine discontinuation in adult patients with ADHD. Postgrad Med. 2015;127(7):677–685. doi: 10.1080/00325481.2015.1083394.
- Posner J, Polanczyk GV, Sonuga-Barke E. Attention-deficit hyperactivity disorder. Lancet. 2020; 395(10222):450–462. doi: 10.1016/S0140-6736(19)33004-1.
- Mattingly GW, Wilson J, Rostain AL. A clinician’s guide to ADHD treatment options. Postgrad Med. 2017; 129(7):657–666.
- Otsuka announces positive top-line results from two phase 3 studies of centanafadine for the treatment of attention-deficit hyperactivity disorder (ADHD) in adult patients; 2020 [Internet]. Available from: https://www.otsuka-us.com/discover/otsuka-announces-positive-top-line-results-from-two-phase-3-studies-of-centanafadine.
- Adler LA, Adams J, Madera-McDonough J, et al. Efficacy, safety, and tolerability of centanafadine sustained-release tablets in adults with attention-deficit/hyperactivity disorder: results of 2 phase 3, randomized, double-blind, multicenter, placebo-controlled trials. J Clin Psychopharmacol. 2022;42(5):429–439. doi: 10.1097/JCP.0000000000001575.
- Takeda Pharmaceuticals America Inc. Vyvanse (lisdexamfetamine dimesylate): Prescribing information; 2022.
- Lilly USA LLC. Strattera (atomoxetine): Prescribing information; 2020.
- Children and Adults with Attention-Deficit/Hyperactivity Disorder (CHADD). Medication management Lanham, MD2023; 2023 [cited Mar 23]. Available from: https://chadd.org/for-parents/managing-medication/.
- Supernus Pharmaceuticals Inc. Qelbree (viloxazine extended-release capsules): Prescribing information; 2022.
- Schein J, Cloutier M, Gauthier-Loiselle M, et al. Assessment of centanafadine in adults with attention-deficit/hyperactivity disorder: a matching-adjusted indirect comparison vs lisdexamfetamine dimesylate, atomoxetine hydrochloride, and viloxazine extended-release. J Manag Care Spec Pharm. 2024;30(6):528–540.
- Gajria K, Lu M, Sikirica V, et al. Adherence, persistence, and medication discontinuation in patients with attention-deficit/hyperactivity disorder - a systematic literature review. Neuropsychiatr Dis Treat. 2014;10:1543–1569.
- Schein J, Childress A, Adams J, et al. Treatment patterns among adults with attention-deficit/hyperactivity disorder in the United States: a retrospective claims study. Curr Med Res Opin. 2021;37(11):2007–2014.
- Schein J, Childress A, Cloutier M, et al. Reasons for treatment changes in adults with attention-deficit/hyperactivity disorder: a chart review study. BMC Psychiatry. 2022;22(1):377.
- Janssen Pharmaceuticals Inc. Concerta (methylphenidate HCl extended-release tablets): Prescribing information; 2023.
- Spencer TJ, Adler LA, Meihua Q, et al. Validation of the adult ADHD investigator symptom rating scale (AISRS). J Atten Disord 2010;14(1):57–68.
- Goodman DW, Starr HL, Ma YW, et al. Randomized, 6-week, placebo-controlled study of treatment for adult attention-deficit/hyperactivity disorder: individualized dosing of osmotic-release oral system (OROS) methylphenidate with a goal of symptom remission. J Clin Psychiatry. 2017;78(1):105–114.
- Remiro-Azócar A. Two-stage matching-adjusted indirect comparison. BMC Med Res Methodol. 2022; 22(1):217.
- Petzold A, Steeb T, Wessely A, et al. Is tebentafusp superior to combined immune checkpoint blockade and other systemic treatments in metastatic uveal melanoma? A comparative efficacy analysis with population adjustment. Cancer Treat Rev. 2023 ;115:102543. doi: 10.1016/j.ctrv.2023.102543.
- Li F, Thomas LE, Li F. Addressing extreme propensity scores via the overlap weights. Am J Epidemiol. 2019; 188(1):250–257.
- Crump RK, Hotz VJ, Imbens GW, et al. Dealing with limited overlap in estimation of average treatment effects. Biometrika. 2009;96(1):187–199. doi: 10.1093/biomet/asn055.
- Ross ME, Kreider AR, Huang YS, et al. Propensity score methods for analyzing observational data like randomized experiments: challenges and solutions for rare outcomes and exposures. Am J Epidemiol. 2015;181(12):989–995.
- Zeng S, Li F, Wang R, et al. Propensity score weighting for covariate adjustment in randomized clinical trials. Stat Med. 2021;40(4):842–858.
- Ma X, Wang J. Robust inference using inverse probability weighting. J Am Stat Assoc. 2020;115(532):1851–1860. doi: 10.1080/01621459.2019.1660173.
- Lee BK, Lessler J, Stuart EA. Weight trimming and propensity score weighting. PLoS One. 2011;6(3):e18174. doi: 10.1371/journal.pone.0018174.
- Kis B, Lücke C, Abdel-Hamid M, et al. Safety profile of methylphenidate under long-term treatment in adult ADHD patients - results of the COMPAS study. Pharmacopsychiatry. 2020;53(6):263–271.
- Ginsberg Y, Quintero J, Anand E, et al. Underdiagnosis of attention-deficit/hyperactivity disorder in adult patients: a review of the literature. Prim Care Companion CNS Disord. 2014;16(3):PCC.13r01600. doi: 10.4088/PCC.13r01600.
