Abstract
Objective
Postoperative nausea and vomiting (PONV) occurs in up to 30% of patients and its pathophysiology and mechanisms have not been completely described. Hypotension and a decrease in cardiac output are suspected to induce nausea. The hypothesis that intraoperative hypotension might influence the incidence of PONV was investigated.
Material and methods
The study was conducted as a retrospective large single center cohort study. The incidence of PONV was investigated until discharge from post anesthesia care unit (PACU). Surgical patients with general anesthesia during a 2-year period between 2018 and 2019 at a university hospital in Germany were included. Groups were defined based on the lowest documented mean arterial pressure (MAP) with group H50: MAP <50mmHg; group H60: MAP <60mmHg; group H70: MAP <70mmHg, and group H0: no MAP <70mmHg. Decreases of MAP in the different groups were related to PONV. Propensity-score matching was carried out to control for overlapping risk factors.
Results
In the 2-year period 18.674 patients fit the inclusion criteria. The overall incidence of PONV was 11%. Patients with hypotension had a significantly increased incidence of PONV (H0 vs. H50: 11.0% vs.17.4%, Risk Ratio (RR): 1.285 (99%CI: 1.102–1.498), p < 0.001; H0 vs. H60: 10.4% vs. 13.5%, RR: 1.1852 (99%CI: 1.0665–1.3172), p < 0.001; H0 vs. H70: 9.4% vs. 11.2%, RR: 1.1236 (99%CI: 1.013 − 1.2454); p = 0.0027).
Conclusion
The study demonstrates an association between intraoperative hypotension and early PONV. A more severe decrease of MAP had a pronounced effect.
1. Introduction
Postoperative nausea and vomiting, or PONV, describes a multifactorial, undesirable but frequent condition seen in patients in the first 24–48 h after surgery and general anesthesiaCitation1. To date more than 10,000 publications examining the causes, pathophysiology, and treatment of PONV have been published.
Despite intensive scientific effort concerning the “phenomenon” of PONV and its treatment, it is still one of the most common issues in clinical daily practice. If no preventive actions are taken, up to 30% of patients suffer from PONV following an operative procedure. Individual and procedural risk factors for PONV have been identified and transposed into scoring systemsCitation2. The fear of PONV is one of the most common concerns patients have before surgeryCitation3. Besides discomfort, PONV can directly impair the surgical outcome e.g. by rupturing bowel anastomoses or compromising wound closure. As a result, PONV can cause prolonged hospital stay, unplanned hospitalization of outpatients and increased treatment costsCitation4,Citation5. Successful PONV prophylaxis is cheaper than the treatment of PONV itself, and by far cheaper than a prolonged hospitalization caused by PONVCitation6,Citation7.
To date, the pathophysiology and mechanisms of action of PONV are still not fully understood. However, in recent years, significant progress has been made in understanding PONV followed by the development of a multi-causal model that combines the influence of multiple intrinsic patient and procedural factors.
Hypotension and a decrease in cardiac output are known to induce nausea, quite independently of anesthesiaCitation8. Medical research, especially perioperative medicine, has so far barely addressed this aspect. Several studies were carried out in obstetric anesthesia concerning the influence of hypotension on the occurrence of peri-interventional nausea after spinal anesthesiaCitation9,Citation10. Studies in more generalized, less specific populations are rare and, so far, have reported heterogeneous resultsCitation11–13.
Therefore, the present study investigated the hypothesis of an association between intraoperative arterial hypotension and the occurrence of PONV in the early postoperative period, up to the time of transfer from the PACU.
2. Methods
2.1. Data collection and subject selection
After approval by the ethics committee of the Ludwig-Maximilians University (No.: 20-211), which waved the need for written informed consent, the software used to generate the department’s electronic anesthesia records (NarkoData®, IMESO® GmbH, Giessen, Germany) was accessed. This software collects anesthesia-relevant perioperative data for all patients undergoing surgery. In addition, the hospital’s patient data management system (KAS®, SAP Deutschland SE & Co. KG, Walldorf, Germany) was used. This software package captures administrative data and some medical information like patients’ comorbidities. Data were collected from 01.01.2018 to 31.12.2019 at the university hospital.
Every patient aged over 18 years who received general anesthesia or general anesthesia combined with regional anesthesia preceding general surgery, urologic surgery, orthopedic surgery, gynecologic surgery, or maxillofacial surgery was included. Patients undergoing neurosurgery were excluded because these patients usually underwent special positioning maneuvers and/or were usually transferred directly to the ICU; ENT patients were also excluded because PONV prophylaxis differed from the clinic’s standard in most cases (patients received a broader preoperative PONV prophylaxis including atropine to suppress hypersalivation and marked vagal stimuli). Additionally, patients experiencing procedures with extreme intraoperative positioning, for example Trendelenburg position in robot-assisted urologic surgery could not participate in the study, because these positions may increase the risk of PONVCitation14. Patients undergoing urologic procedures requiring high amounts of intravesical flushing solutions were excluded as well because the calculation of an exact fluid balance was critical in these cases. Patients who could not be extubated after surgery or had to be transferred to an ICU were also excluded from the investigation. Patients’ length of stay in the postoperative anesthesia care unit (PACU) had to be longer than 20 min but not to exceed 360 min. Stays longer than 360 min were interpreted to be equivalent to an intermediate care (IMC) or ICU stay, and the corresponding patients were excluded as well. Only the first surgery for each patient in the corresponding quarter of the year was included. For the resulting patient cohort, data was extracted from the NarkoData® database using Python 3 (Version 3.6.9) Citation15. Patient characteristics included age, body mass index (BMI), sex and American Society of Anesthesiology(ASA)-classification. Time between induction of anesthesia and extubation was collected as well as the balance of fluids. For each opioid given, the total dose was recorded, and a corresponding morphine equivalent dose was calculatedCitation16. Concerning maintenance of general anesthesia, differentiation was made between total intravenous anesthesia (TIVA) and balanced anesthesia. The smoker status of the patient was also recorded. If a patient received one of the following substances prior to extubation, this was considered as administration of a PONV prophylaxis: dimenhydrinate, ondansetron, haloperidol, metoclopramide, dexamethasone, and droperidol, irrespective of the dose. All patients received propofol for anesthesia induction, regardless of whether maintenance of anesthesia was carried out as balanced anesthesia or TIVA.
To examine a possible relationship with PONV, three “severity levels” of arterial hypotension were definedCitation17–19. First, a single MAP measurement below 70 mmHg (Group: H70), second, a single MAP measurement below 60 mmHg (Group: H60) and third, a single MAP measurement below 50 mmHg (Group: H50)Citation17–19. As there is no generally accepted definition of hypotension, we have oriented ourselves on previously published studies and clinically relevant blood pressure limitsCitation17. If patients experienced at least one of these predefined episodes (regardless of its duration) they were assigned to the corresponding hypotension group. The time duration was not added as a variable since this is only inaccurately reflected in the anesthesia protocol (documentation of the value every 3 min automatically regardless of non-invasive or invasive measurement). An H50 episode was defined as most important, followed by H60 and H70. Thus, patients were assigned to the H50 group if an H50 episode occurred, regardless of additional H60 and/or H70 episodes during the same operation. Similarly, patients were assigned to the H60 group if an H60 episode was recorded, regardless of additional H70 episodes. If no hypotensive event according to the definitions H50 to H70 was recorded, the patient was assigned to the H0 group.
Blood pressure measurement was done using non-invasively (cuff) or invasively, mostly using radial artery.
The occurrence of PONV after surgery was defined as the primary endpoint of the study. Patients were considered PONV-positive if the mandatory documentation of PONV at the end of PACU showed PONV and/or if a patient had received a medication against nausea and vomiting (PONV prophylaxis as defined above) after extubation (in order to close the electronic combined anesthesia/PACU protocol an entry concerning the presence or absence of PONV needs to be submitted). The incidence of the primary endpoint in cohorts H0 to H70 was examined.
2.2. Statistical analysis
For all data analyses SPSS version 23 (IBM Corporation, Armonk, New York, USA), Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA, USA), the free software package “R©” version 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria) including the packages “matchit,” “cor” and “car” as well as the “glm”-function and Python 3 (Version 3.6.9, Python Software Foundation, Delaware, USA) with the libraries Sklearn 0.23.2, Matplotlib 3.0.3, and Numpy 1.18.1. were used.
The existence of a Gaussian distribution of data was evaluated using the Kolmogorov-Smirnov test; skewed data are displayed as median ± interquartile range, otherwise they are given as mean ± standardized deviation of the mean. For group comparisons, the Mann-Whitney U-test was used if data was not normally distributed, otherwise, a Student’s T-test or Welch’s test was applied. Associations regarding categorical demographic and outcome variables were assessed using Pearson’s chi-square test or Fisher’s exact test if necessary. Concerning the primary outcome parameter, the risk ratio and their corresponding 99% confidence intervals (CI), as well as the corresponding p-values were calculated. P-values were used as a measure of overall significance. For all comparisons, a value of p < 0.05 was considered significant.
In order to eliminate overlapping risk factors and differences in the frequency of the already known risk factors for PONV in the individual hypotension and control groups, propensity score matching was carried out following analysis of the uncorrected data set. Prior to the matching process, potential confounders influencing PONV frequency were identified with the help of a systematic literature analysis. Only risk factors that are well accepted in the majority of studies (gender, age, PONV history, total intravenous anesthesia (versus balanced anesthesia), duration of anesthesia, PONV prophylaxis given (versus not given), dose of opioids given, fluid balance, smoker (versus non-smoker)) were used as confounding variables in the matching process, whereas risk factors labeled as “unproven” or “conflicting” (for example BMI) were not includedCitation20. In order to get the best matching results, the “nearest neighbor method” was applied. In addition, a caliper of 0.2 was applied to further harmonize study groups. Matching was done with a ratio of 1:3 with replacementCitation21–23. Data concerning the quality of the matching process is displayed in the supplemental material (/1–A1/3).
Table 1. Patients’ characteristics.
In a second step, we tried to answer the question whether a PONV-prophylaxis given to patients experiencing a relevant drop in blood pressure (H50, H60, and H70 groups) was suitable to prevent PONV. Because six different antiemetic drugs were administered to the complete study cohort in various combinations, we did not aim to analyze the effect of a single drug or drug combination in this context but concentrated on the number of antiemetics a patient had received. Thus, we examined patients who had received up to three antiemetics, one antiemetic and two antiemetic drugs versus any antiemetic drug, respectively. Patients who had received three antiemetic drugs were not processed solely, because numbers were too small to do reasonable statistics (n = 171). First, a chi-square test was carried out to examine unadjusted data. Second, to gain a set of adjusted data, a further propensity score matching (nearest neighbor method, 1:1 ratio, no caliper) was initiated with PONV-prophylaxis being the binary variable to test in the H50, H60 and H70 groups. Gender, age, PONV history, total intravenous anesthesia (versus balanced anesthesia), duration of anesthesia, dose of opioids given, fluid balance, smoker (versus non-smoker) were considered as risk factors. Data concerning the quality of the matching process are displayed in the appendix (). To test for significant differences in the resulting adjusted groups, a chi-Square test was used, too. In this case we abstained from forming subgroups and only examined patients who did receive an antiemetic medication (regardless of the number of antiemetics) versus those who had received none, because, again, patient numbers, especially in the H50-group were too small to perform a valid propensity score matching in the potential subgroups.
Table 2. Statistical results in the different study groups after propensity score matching.
Table 3. Influence of PONV prophylaxis on incidence of PONV in the different groups of hypotension.
3. Results
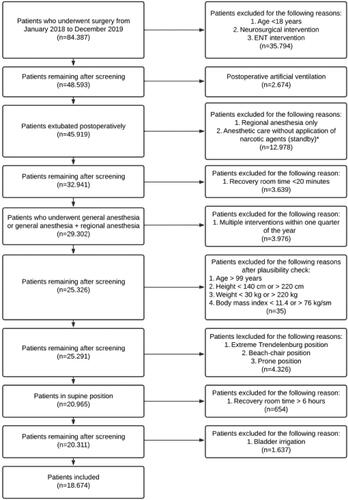
In the 2-year time frame 84.387 surgeries were registered at the University Hospital of Munich. Out of these 18.674 matched the inclusion criteria and were enrolled in the study. The complete process of patient inclusion is demonstrated in . Patient characteristics are described in .
Figure 1. Process of patient inclusion.
*Central venous catheter installation, dialysis catheter installation, vascular embolization, etc.
ENT: ear, nose and throat surgery.
In the total unmatched cohort, 2,157 (11.6%) patients met the primary endpoint of PONV. Of these, 1366 (63.3%) had PONV documented prior to PACU discharge and 1526 (70.7%) received rescue antiemetics between extubation and PACU discharge. PONV incidence in the different study groups is outlined in . PONV prophylaxis was given in 49.7% of the patients and 18.7% received at least two different agents for PONV prophylaxis. It was carried out with ondansetron (n = 3123), dexamethasone (n = 8147), droperidol (n = 203), haloperidol (n = 1361), dimenhydrinate (n = 22) and metoclopramide (n = 93).
Group comparisons after matching are displayed in . All data concerning the matching process is presented in the appendix ().
A PONV-prophylaxis given in patients suffering from relevant hypotension during anesthesia tended to be advantageous in preventing PONV in the H50 group (unadjusted data). Nevertheless, we did not observe a significantly reduced frequency of PONV, in hypotensive patients who had received anti-emetic drugs, neither in the raw, nor in the adjusted data groups (exception: in group H70 two anti-emetic drugs turned out to be more effective than none; p = 0.04, see ).
4. Discussion
The present study demonstrates an association between intraoperative hypotension and early PONV. Patients belonging to a specific group of hypotension (H50, H60, H70) showed a significant increase in the occurrence of PONV compared to patients without intraoperative hypotension.
Hypotension has been studied as a risk factor for PONV for several years. However, to date, most studies investigating this topic have focused on obstetric populations. In these populations, short-lasting hypotension frequently occurs during or immediately after spinal anesthesia. George et al. demonstrated that prophylactic administration of vasoconstrictors is able not only to minimize hypotension but also to reduce nausea and vomiting in obstetric patientsCitation10. Likewise, an evaluation of treatment quality concerning cases of maternal hypotension after spinal anesthesia from a current Cochrane analysis showed that (i) PONV is a common problem and that (ii) a reduced frequency of hypotensive events also led to fewer patients suffering from PONVCitation24.
Nevertheless, there are few studies on the relationship of PONV with intraoperative hypotension in general surgical populations. In an actual study on 247 patients undergoing thyroidectomy, a significant influence of hypotension on the occurrence of PONV was notedCitation25. Two further prospective observational studies carried out by Pusch et al. also showed a positive association between hypotension and PONVCitation11,Citation12. A significant increase in the rate of PONV was noted in patients whose systolic blood pressure dropped more than 35% compared to induction. Interestingly, it was irrelevant whether the decrease in blood pressure occurred during induction or maintenance of general anesthesia. A subsequent study with a similar group of patients observed a significantly higher rate of PONV in patients with orthostatic dysregulation. However, it must be noted that in these studies there were some group differences with regard to known PONV risk factors, which was officially commented in a reply to the authorsCitation26.
Maleczek et al. investigated the association of PONV and intraoperative hypotension in a recent retrospective analysis of more than 30.000 patients and identified a MAP < 50 mmHg to be a risk factor for PONV in the PACUCitation13.
To identify PONV risk factors (other than hypotension) in the study groups, we carried out an extensive literature research and chose only well-established factors (gender, age, PONV history, total intravenous anesthesia (versus balanced anesthesia), duration of anesthesia, PONV prophylaxis given (versus not given), dose of opioids given, fluid balance, and smoker (versus non-smoker)). Using the identified factors as “confounders,” we performed propensity score matching. As a result, the examined post-analysis groups were comparable with regard to pre-described PONV risks and demographic dataCitation27. Statistics calculated in the post-analysis groups only displayed minimal bias despite their retrospective structure. Therefore, they should be suitable to confirm the suspected connection between PONV and intraoperative hypotension in a large population.
In many studies, the definition of intraoperative hypotension is a point of criticism, especially given the growing evidence that blood pressure values should be defined more individualized than with general valuesCitation28–30. A current review found 140 different definitions of perioperative hypotension used in different trials, which, according to Bijker et al. limits the comparability of research resultsCitation17. The most common definition of perioperative hypotension is a systolic blood pressure lower than 80 mmHg or a decrease of systolic blood pressure of more than 20% compared to values before induction of anesthesiaCitation17. If MAP decreases more than 55% compared to pre-anesthetic values, damage to the myocardium and renal tissue is probableCitation19. However, besides the mere severity of a reduction in blood pressure, the duration of intraoperative hypotensive episodes is also a relevant factorCitation18.
In the current study, the various definitions of hypotension given in the literature were taken into account by defining three different hypotension groups. We found that all three defined types of hypotension increased the frequency of PONV. Pronounced, albeit short-lived, hypotension with a MAP <60 mmHg had a particularly pronounced effect. These results are comparable to the findings of Maleczek et al. which were carried out in a comparable study settingCitation13.
We also considered investigating the duration of hypotension as a possible influencing factor but decided not to do this because long-lasting periods of severe hypotension were very rare in this cohort (data not shown). Blood pressure values are transferred to the digital anesthesia protocol every 3 min and are thus only a very inaccurate representation of the temporal component of hypotension. All in all, despite large patient numbers, our data were not suitable to study the effect of prolonged hypotension on PONV incidence.
If one considers the negative effects of intraoperative hypotension on various organ systems, which are well known and have been repeatedly demonstrated, this study adds another negative aspectCitation30–32. PONV primarily affects postoperative patient comfort, but can also have an outcome-influencing character.
Different types of PONV prophylaxis were shown to be effective in various investigationsCitation7. In our clinic mostly dexamethasone and/or ondansetron are given as a PONV-prophylaxis. Nevertheless, the number of different antiemetic drug combinations administered was too large to carry out a differentiated analysis concerning only dexamethasone and ondansetron. Interestingly, a prophylaxis with two antiemetics was effective in the H70 group. However, in groups with a more pronounced hypotension (H50; H60), PONV-prophylaxis was not helpful and was presumably overshadowed by the low blood pressure effect.
4.1. Limitations
The current investigation has some limitations. Due to the retrospective nature of the study, it is possible that unmeasured confounders could have influenced the results despite propensity score matching. Propensity score matching can compensate for some limitations associated with retrospective investigations, but data quality is not comparable to a RCT.
Second, compared to many other studies, a PONV rate of around 11% in the current population seems rather low. This might be due to the relatively short observation interval (compared to other studies), which only lasted until the end of care in the PACU. Unfortunately, our digital documentation goes only up to this point. After that, the data was no longer accessible in a standardized and structured manner with regard to this issue. Additionally, it may be attributed to the fact that 49.6% of the patients received a PONV prophylaxis, that induction of general anesthesia was carried out with propofol (propofol was not administered in most patients during early PONV research in the 1990s) and that total intravenous anesthesia was applied in 55%Citation2,Citation33. Additionally, the exclusion criteria might have influenced the occurrence of PONV. A large number of patients who received anesthesiological care within the 2-year observation period were excluded. The reasons for doing so are given in the methods section. The inclusion of these special populations (for example patients with extreme positioning) could have altered the PONV rates and, thus, the results, but from our point of view also could have generated relevant bias with regard to more general surgical populations.
A very small number of patients might have been classified as “PONV positive” because intraoperative PONV prophylaxis was forgotten and erroneously administered immediately after extubation, which would be recognized as a rescue administration in our algorithm. This may have occurred in any group but should have been leveled out by the matching and group-building process.
Third, the number of prophylactically administered antiemetics was not considered for integration in the general propensity score matching process. In our clinic, we first administer dexamethasone prophylactically. If the PONV risk is high, ondansetron is added towards the end of the surgery. If the risk is very high, inhaled anesthetics are not used at all. Due to the high number of cases, differences in the number of prophylactics administered should almost balance out in the propensity score matching.
Fourth, the definition of hypotension is inconsistent around the world and a universal definition is still being debated. We tried to accommodate for this problem by “creating” different hypotension groups.
Furthermore, one cannot rule out the fact that – despite mandatory electronic documentation – the documentation of PONV might not have been completely consistent. To further improve documentation, we also interpreted the postoperative administration of certain medications as a sign of PONV. Finally, the reduction of PONV is defined as one of the quality goals of our clinic and is therefore a focal point for all anesthesiologists involved.
5. Conclusion
This study demonstrates an association between intraoperative hypotension per se and the occurrence of early PONV prior to transfer from the PACU. As most observed episodes of hypotension were brief, a relationship concerning the duration of these episodes and PONV frequency could not be established.
Transparency
Declaration of financial/other relationships
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Sebastian Goss: This author helped to analyze the results and to draft the manuscript. Jan Jedlicka: This author helped to supervis the study and to draft the manuscript. Elisabeth Strinitz: This author helped to conduct the study, to analyze the results, and to draft the manuscript. Sebastian Niedermayer: This author helped to plan the study. Daniel Chappell: This author helped to plan and to supervise the study. Klaus Hofmann-Kiefer: This author helped to plan and to supervise the study and he helped to analyze the results and to draft the manuscript. Ludwig C. Hinske: This author helped to plan and to supervise the study. Philipp Groene: This author helped to plan, conduct and supervise the study and he helped to draft the manuscript.
Appendix_R1_clean.docx
Download MS Word (71 KB)Additional information
Funding
References
- Pierre S, Whelan R. Nausea and vomiting after surgery. Contin Educ Anaesth Crit Care Pain. 2013;13(1):28–32. doi:10.1093/bjaceaccp/mks046.
- Apfel CC, Heidrich FM, Jukar-Rao S, et al. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br J Anaesth. 2012;109(5):742–753. doi:10.1093/bja/aes276.
- Mavridou P, Dimitriou V, Manataki A, et al. Patient’s anxiety and fear of anesthesia: effect of gender, age, education, and previous experience of anesthesia. A survey of 400 patients. J Anesth. 2013;27(1):104–108. doi:10.1007/s00540-012-1460-0.
- Chung F, Mezei G. Factors contributing to a prolonged stay after ambulatory surgery. Anesth Analg. 1999;89(6):1352–1359. doi:10.1213/00000539-199912000-00004.
- Berger ER, Huffman KM, Fraker T, et al. Prevalence and risk factors for bariatric surgery readmissions: findings from 130,007 admissions in the metabolic and bariatric surgery accreditation and quality improvement program. Ann Surg. 2018;267(1):122–131. doi:10.1097/SLA.0000000000002079.
- Gress K, Urits I, Viswanath O, et al. Clinical and economic burden of postoperative nausea and vomiting: analysis of existing cost data. Best Pract Res Clin Anaesthesiol. 2020;34(4):681–686. doi:10.1016/j.bpa.2020.07.003.
- Weibel S, Rücker G, Eberhart LH, et al. Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis. Cochrane Database Syst Rev. 2020;10(10):CD012859. doi:10.1002/14651858.CD012859.pub2.
- Singh PM, Borle A, Rewari V, et al. Aprepitant for postoperative nausea and vomiting: a systematic review and meta-analysis. Postgrad Med J. 2016;92(1084):87–98. doi:10.1136/postgradmedj-2015-133515.
- Lee A, Chan SKC, Fan LTY. Stimulation of the wrist acupuncture point PC6 for preventing postoperative nausea and vomiting. Cochrane Database Syst Rev. 2015;2015(11):CD003281. doi:10.1002/14651858.CD003281.pub4.
- George RB, McKeen DM, Dominguez JE, et al. A randomized trial of phenylephrine infusion versus bolus dosing for nausea and vomiting during Cesarean delivery in obese women. Can J Anaesth. 2018;65(3):254–262. doi:10.1007/s12630-017-1034-6.
- Pusch F, Berger A, Wildling E, et al. Preoperative orthostatic dysfunction is associated with an increased incidence of postoperative nausea and vomiting. Anesthesiology. 2002;96(6):1381–1385. doi:10.1097/00000542-200206000-00017.
- Pusch F, Berger A, Wildling E, et al. The effects of systolic arterial blood pressure variations on postoperative nausea and vomiting. Anesth Analg. 2002;94(6):1652–1655, table of contents. doi:10.1213/00000539-200206000-00054.
- Maleczek M, Laxar D, Geroldinger A, et al. Intraoperative hypotension is associated with postoperative nausea and vomiting in the PACU: a retrospective database analysis. J Clin Med. 2023;12(5). doi:10.3390/jcm12052009.
- Yonekura H, Hirate H, Sobue K. Comparison of anesthetic management and outcomes of robot-assisted vs pure laparoscopic radical prostatectomy. J Clin Anesth. 2016;35:281–286. doi:10.1016/j.jclinane.2016.08.014.
- van Rossum G, Fl D. Jr. 1995. Python reference manual. Centrum voor Wiskunde en Informatica Amsterdam.
- Thiel Roewer H N. Anästhesiologische pharmakotherapie: von den Grundlagen der Pharmakologie zur Medikamentenpraxis. Stuttgart: Georg Thieme Verlag KG; 2021. 4 unveränderte Auflage.
- Bijker JB, van Klei WA, Kappen TH, et al. Incidence of intraoperative hypotension as a function of the chosen definition: literature definitions applied to a retrospective cohort using automated data collection. Anesthesiology. 2007;107(2):213–220. doi:10.1097/01.anes.0000270724.40897.8e.
- Monk TG, Saini V, Weldon BC, et al. Anesthetic management and one-year mortality after noncardiac surgery. Anesth Analg. 2005;100(1):4–10. doi:10.1213/01.ANE.0000147519.82841.5E.
- Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology. 2013;119(3):507–515. doi:10.1097/ALN.0b013e3182a10e26.
- Gan TJ, Belani KG, Bergese S, et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2020;131(2):411–448. doi:10.1213/ANE.0000000000004833.
- Franklin JM, Eddings W, Austin PC, et al. Comparing the performance of propensity score methods in healthcare database studies with rare outcomes. Stat Med. 2017;36(12):1946–1963. doi:10.1002/sim.7250.
- Austin PC, Stuart EA. The performance of inverse probability of treatment weighting and full matching on the propensity score in the presence of model misspecification when estimating the effect of treatment on survival outcomes. Stat Methods Med Res. 2017;26(4):1654–1670. doi:10.1177/0962280215584401.
- Staffa SJ, Zurakowski D. Five steps to successfully implement and evaluate propensity score matching in clinical research studies. Anesth Analg. 2018;127(4):1066–1073. doi:10.1213/ANE.0000000000002787.
- Chooi C, Cox JJ, Lumb RS, et al. Techniques for preventing hypotension during spinal anaesthesia for caesarean section. Cochrane Database Syst Rev. 2020;7(7):CD002251. doi:10.1002/14651858.CD002251.pub4.
- Nakatani H, Naito Y, Ida M, et al. Association between intraoperative hypotension and postoperative nausea and vomiting: a retrospective analysis of 247 thyroidectomy cases. Braz J Anesthesiol. 2023;73(5):635–640. doi:10.1016/j.bjane.2021.02.029.
- Kranke P, Roewer N, Rüsch D, et al. Arterial hypotension during induction of anesthesia may not be a risk factor for postoperative nausea and vomiting. Anesth. Analg. journals.lww.com. 2003;96(1):302–303; author reply 303. doi:10.1213/00000539-200301000-00064.
- Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. doi:10.1093/biomet/70.1.41.
- Futier E, Lefrant J-Y, Guinot P-G, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA. 2017;318(14):1346–1357. doi:10.1001/jama.2017.14172.
- Sessler DI, Bloomstone JA, Aronson S, et al. Perioperative Quality Initiative consensus statement on intraoperative blood pressure, risk and outcomes for elective surgery. Br J Anaesth. 2019;122(5):563–574. doi:10.1016/j.bja.2019.01.013.
- Wijnberge M, Schenk J, Bulle E, et al. Association of intraoperative hypotension with postoperative morbidity and mortality: systematic review and meta-analysis. BJS Open [Internet]. 2021;5(1). doi:10.1093/bjsopen/zraa018.
- Bijker JB, Persoon S, Peelen LM, et al. Intraoperative hypotension and perioperative ischemic stroke after general surgery: a nested case-control study. Anesthesiology. 2012;116(3):658–664. doi:10.1097/ALN.0b013e3182472320.
- Sun LY, Wijeysundera DN, Tait GA, et al. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology. 2015;123(3):515–523. doi:10.1097/ALN.0000000000000765.
- Apfel CC, Läärä E, Koivuranta M, et al. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. 1999;91(3):693–700. doi:10.1097/00000542-199909000-00022.
Appendix
1. /1–A1/3 matching protocols
1.1. Group H50
Table A1/1. Summary of balance for all data before and after matching.
1.2. Group H60
Table A1/2. Summary of balance for all data before and after matching.
1.3. Group H70
Table A1/3. Summary of balance for all data before and after matching.
2. /1–A2/3 matching protocols regarding PONV prophylaxis
2.1. Group H50
Table A2/1. Summary of balance for all data before and after matching.
2.2. Group H60
Table A2/2. Summary of balance for all data before and after matching.
2.3. Group H70
Table A2/3. Summary of balance for all data before and after matching.
