Abstract
Objective
This retrospective study using claims data compared demographics, clinical characteristics, treatment patterns, healthcare resource utilization, and clinical outcomes in Black and White patients with pulmonary arterial hypertension (PAH) in the United States.
Methods
Patients (aged ≥18 years) had ≥1 pharmacy claim for PAH medication, ≥6 months’ continuous healthcare plan enrollment, ≥1 inpatient/outpatient medical claim with a pulmonary hypertension diagnosis ≤6 months before first PAH medication, and race recorded.
Results
This analysis included 836 Black and 2896 White patients. Black patients were younger, with lower levels of education and annual household income, and higher comorbidity scores versus White patients. Only ∼14% of Black and White patients received index combination therapy. Lower adherence to index treatment was observed in Black patients. Although adjusted regression analysis in the overall population showed no differences in outcomes between groups, Black patients <65 years were 36% less likely to receive index combination therapy (odds ratio [OR] 0.64; 95% confidence interval [CI] 0.41–0.99), and 46% less likely to adhere to index treatment (OR 0.54; 95% CI 0.33–0.90). Other disparities included 24% higher all-cause health care resource utilization, 75% higher all-cause costs, and higher risk of clinical composite outcome. Social determinants of health (education, income, health insurance plan) partially mediated these race effects.
Conclusions
Differences in demographics, clinical characteristics, and treatment patterns between Black and White patients with PAH were observed. Disparities between Black and White patients <65 years were only partially mediated through social determinants of health variables, suggesting other factors may be involved.
Introduction
Pulmonary arterial hypertension (PAH) is a rare, progressive, life-threatening disease characterized by increasing pulmonary vascular resistance, which ultimately leads to right heart ventricular failure and death. Access to specialized care and regular risk assessment is essential for ensuring that patients with PAH receive appropriate treatment to optimize their long-term outcomesCitation1. In treatment-naive and newly diagnosed patients with PAH, initial combination therapy, thereby targeting multiple pathogenic pathways, can improve symptoms, exercise capacity, and outcomes compared with initial monotherapy aloneCitation1–4. Adherence to PAH medication is an important factor for maintaining or improving patients’ functional capacity and for reducing the need for hospitalization and other healthcare servicesCitation5–7. However, there are multiple barriers to treatment, including high cost and the requirement for prior approval and use of specialty pharmacies, which can lead to access issues and impact patient adherence to treatmentCitation8–10.
Studies indicate that there are racial and ethnic differences in PAH disease characteristics and, more concerningly, health disparities in the management of PAH between White and non-White patients, as between patients with high versus low socioeconomic statusCitation11–18. Many differences are likely associated with access to care, household income, and health insuranceCitation13,Citation19. In 2016, the American Thoracic Society issued an official statement highlighting these health disparities, among others, and made recommendations to increase research efforts to improve the quality of care provided to those belonging to vulnerable populations who are living with PAHCitation15.
One of the challenges of understanding the differences in outcomes among patients of different races and ethnicities is the under-representation of non-White patients in key studies that guide the management of rare diseases. For example, fewer than 13% of patients in the large Registry to Evaluate Early and Long-term PAH Disease Management (REVEAL) in the United States were Black or HispanicCitation11. Although an analysis of REVEAL did not demonstrate that a patient’s race or ethnicity was a significant predictor of mortality, this conclusion was based on the small proportion of Black, Asian, and Hispanic patients enrolled in REVEALCitation20. Furthermore, potential confounding variations in social determinants of health on patient outcomes were not explored because relevant data were not captured in REVEAL. Several other studies have evaluated the differences in mortality across patient groups of differing races or ethnicitiesCitation12,Citation20–22. One study suggested that non-Hispanic Black patients and Asian patients had a higher likelihood of all-cause transplant-free inpatient mortality compared with non-Hispanic White patientsCitation21. The impact of social determinants of health on the relationship between race/ethnicity and mortality was demonstrated in another study that identified a higher mortality risk in Black patients versus White patients with newly diagnosed pulmonary hypertension, which was attenuated after adjusting for insurance statusCitation22. Overall, there are few studies describing health disparities among patients with PAH who have different races or ethnicities, which may contribute to differential outcomes.
Here we report the results of a retrospective, observational, longitudinal analysis of Black patients and White patients with PAH treated in the United States. The aim of the study was to describe PAH treatment patterns (i.e. use of and adherence to combination therapy at index) stratified by race, patient demographics, and clinical characteristics, including social determinants of health. The study also evaluated healthcare resource utilization and relevant clinical outcomes (e.g. lung transplant, atrial septostomy, oxygen initiation, and hospitalization) stratified by race and other potential effect modifiers such as age groups. We also conducted sensitivity analyses to evaluate whether the racial disparity in index treatment patterns differed before versus during COVID-19 (i.e. prior to 2020 versus 2020 and beyond).
Methods
Study design
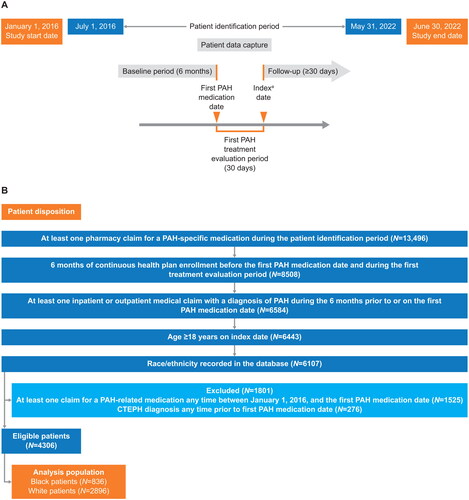
This was a retrospective, observational, cohort study of adult patients with PAH treated in the United States, using data captured between January 1, 2016, and June 30, 2022, from Optum’s de-identified Clinformatics Data Mart – Socioeconomic Status database. Patients with at least one prescription for PAH medications were identified in the period between July 1, 2016, and May 31, 2022. Index PAH treatment therapy was determined using a 30-day window following the first PAH medication prescription (index treatment evaluation period). Baseline was defined as the 6-month period prior to the first PAH medication prescription. The index date was set to the thirty-first day following the first PAH medication prescription. Patients were followed up until the earlier of either the health plan disenrollment date or the end of available data. The study design is shown in .
Figure 1. (A) Study design; (B) study eligibility and patient disposition. aIndex PDE5i dosage, dosage form, and route of administration for PDE5i claims must indicate PAH use and ratio of pill count to days of supply must be ≥1. Abbreviations. CTEPH, chronic thromboembolic pulmonary hypertension; PAH, pulmonary arterial hypertension; PDE5i, phosphodiesterase (Type 5) enzyme inhibitor.
This study was conducted according to the principles in the Declaration of Helsinki. As the study did not involve the collection, use, or transmission of any identifiable patient data, institutional review board and ethical approval were not required.
Data source
The study was conducted using data from the Optum Clinformatics Data Mart – Socioeconomic Status database, which is a database of administrative healthcare claims, including commercial and Medicare Advantage enrollees, from across all census regions in the United States. Optum Clinformatics Data Mart – Socioeconomic Status comprises patient demographics, medical claims for inpatient and outpatient procedures, outpatient pharmacy dispensing claims, and standardized costs. It also includes information on patients’ socioeconomic characteristics, such as race and ethnicity, education level, and annual household income. As of this analysis, there were a total of 95 million patient lives in this Optum Clinformatics Data Mart – Socioeconomic Status dataset.
Study population
The inclusion criteria for eligible patients were as follows: (1) patients aged ≥18 years with at least one pharmacy claim for a PAH-specific medication during the patient identification period; (2) continuous health plan enrollment during baseline and the index treatment evaluation period; (3) at least one inpatient or outpatient medical claim with a diagnosis of pulmonary hypertension (i.e. International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] 416.0, ICD-9-CM 416.8; International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] I27.0, ICD-10-CM I27.20, ICD-10-CM I27.21, ICD-10-CM I27.89) during the baseline period; and (4) race/ethnicity recorded in the Clinformatics Data Mart – Socioeconomic Status database (). Patients who had already received PAH-specific medication any time before July 1, 2016, patients diagnosed with chronic thromboembolic pulmonary hypertension (identified from ICD-10-CM I27.24), or patients who participated in a clinical trial prior to the first PAH medication date were excluded. Patients who met the above inclusion and exclusion criteria were stratified into one of two cohorts (Black or White race). The race variable in the Optum Clinformatics Data Mart – Socioeconomic Status dataset was derived based upon the last names and ZIP-code level information of patients, which is a method for imputing race that previously has been validated and demonstrated positive predictive value for identifying Black raceCitation23.
Analysis and outcome variables
Variables analyzed in this study and their corresponding codes are described in Tables S1–S5. The index treatment regimen was captured as the PAH-related medication class(es) recorded, comprising phosphodiesterase (Type 5) enzyme inhibitor, soluble guanylate cyclase stimulator, endothelin receptor antagonist (ERA), and prostacyclin pathway agents. Table S1 lists the medication codes used. A monotherapy regimen was identified by a pharmacy claim for only one PAH-related medication class during the 30-day PAH index treatment evaluation period; combination therapy was identified by pharmacy claims for more than one PAH-related medication class during the 30-day PAH index treatment evaluation period.
Patient characteristics included age, sex, race/ethnicity, US census–level division (based on state), health plan type, insurance type (commercial or Medicare Advantage), education level, annual household income at index date, and calendar year of index date. The following were described during the 6-month baseline period: Quan-Charlson Comorbidity Index (QCCI), comorbidities of interest, PAH-related symptoms, all-cause and PAH-specific health care resource utilization (emergency department visits, outpatient visits, hospitalizations, and intensive care unit stays) and associated costs, and right heart catheterization (Table S2). ICD-9-CM/ICD-10-CM codes used for the identification of comorbidities, PAH-related symptoms, and codes captured for PAH-related clinical outcomes are provided in Tables S3–S5. The QCCI score was calculated using the 19-condition algorithmCitation24.
Adherence to the index regimen was assessed using the proportion of days covered, which is calculated by dividing the number of days of available medication by the number of days between the index date and discontinuation of the index regimen, plan disenrollment, end of data availability, or end of the study period, whichever was earliest. Patients were excluded if their initial treatment with PAH medication was discontinued within 30 days. Among patients on combination therapy, proportion of days covered was based on possession of all medications in the regimen. Proportion of days covered was corrected for inpatient events with the assumption that medications were supplied by the facility during the stay. The threshold for adherence was proportion of days covered ≥0.8, with proportion of days covered <0.8 considered not adherent.
A composite clinical outcome endpoint evaluated during the follow-up period comprised lung transplant, atrial septostomy, oxygen therapy, and all-cause hospitalization. Health care resource utilization and associated costs were also calculated during the follow-up period.
Statistical analyses
All study variables, including patient demographics, clinical characteristics, and clinical outcomes, were reported descriptively. Numbers and percentages are provided for dichotomous and polychotomous variables. Continuous variables were summarized as the mean (standard deviation [SD]) or median (interquartile range [IQR]). All relevant variables were stratified by White versus Black race. Index treatment regimen was stratified by the index year.
Health care resource utilization per patient per month (PPPM) was calculated by dividing the occurrences or costs of health care resource utilization by the personal follow-up months to account for the varying length of follow-up.
A propensity score for binary exposureCitation25 was used to account for confounding when comparing outcomes for the groups of White versus Black patients. Confounding variables included patient demographics, social determinants of health variables at index, QCCI, PAH-related symptoms, and procedure use assessed during baseline. Logistic regression was used to calculate the propensity score weight for each patient. The balance of baseline variables was assessed through standardized mean difference (SMD) after propensity score weighting: SMD <0.1 was considered to indicate covariate balance. All comparative analyses of outcomes between the two race groups were conducted with propensity score weighting. Logistic regression was used for binary outcomes (i.e. index treatment strategy and index treatment adherence). Negative binomial regression was used to analyze rate ratio of health care resource utilization. Generalized linear regression with gamma distribution and log link was used to model the healthcare cost ratio during follow-up. A robust standard error was obtained for the point estimates of interest obtained from all regression analyses.
Because age groups (<65 years versus ≥65 years) can serve as an effect modifier as patients transition into Medicare-eligible age, all analyses were repeated in the subgroups of patients aged <65 years and ≥65 years on the index date. As post hoc sensitivity analyses, the extent to which the observed effect is mediated through social determinants of health known to mediate the effect of race (education level, insurance type, and annual household income level) was evaluated by including these mediators in the regression analyses. This analysis enabled the direct effect of race not mediating through these factors to be reportedCitation26.
A sensitivity analysis was also conducted to evaluate whether index treatment patterns in the Black patients and White patients differed before versus during the COVID-19 pandemic (i.e. prior to January 2020 versus after January 2020).
Results
Study population, baseline demographics, and follow-up
The disposition of patients is shown in . A total of 836 Black patients and 2896 White patients were included in the analysis. Black patients were younger (mean age 67 years versus 71 years for White patients [SMD 0.259]; 34% of Black patients were aged <65 years on the index date versus 26% of White patients [SMD 0.273]) (). Most patients had Medicare Advantage insurance (85% of Black patients versus 81% of White patients [SMD 0.101]). Among younger patients (aged <65 years), 64% of Black patients had Medicare Advantage insurance compared with 46% of White patients (SMD 0.366) (Table S6). By contrast, in the older group (aged ≥65 years), almost all patients had Medicare Advantage insurance (96% of Black patients and 94% of White patients [SMD 0.102]).
Table 1. Demographics at baseline in the Black patients and White patients with PAH.
The majority of patients were female and lived in the US South (), although the Black patient cohort included a numerically higher proportion of females (75% in the Black group versus 65% in the White group [SMD 0.233]) and people living in the South (72% of Black patients versus 40% of White patients [SMD 0.751 for US region]). Black patients generally had a lower level of education than White patients (53.0% of Black patients had a high school diploma or lower education level versus 24.9% of White patients [SMD 0.65 for educational level]) and lower annual household income (57.8% of Black patients versus 30.0% of White patients had an annual income < US$40,000 [SMD 0.76 for annual household income]) ().
The duration of follow-up was similar in the Black and White cohorts (median 14.72 months [IQR 6.51–30.24] and median 15.71 months [IQR 6.80–31.29], respectively; SMD 0.031) (). There was no difference in the duration of follow-up between Black patients and White patients in the subgroups of patients aged <65 years or ≥65 years.
Table 2. Duration of follow-up.
Clinical characteristics recorded during the 6-month baseline period
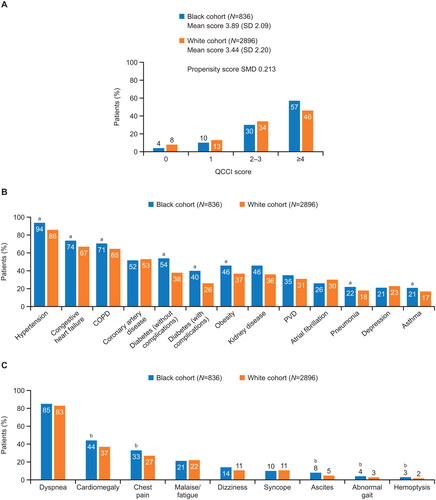
Black patients had a greater comorbidity burden than White patients, with a higher QCCI score (mean 3.89 [SD 2.09] and 3.44 [SD 2.20], respectively; SMD 0.213) (). The comorbidities recorded during the baseline period were typical of those expected for patients with PAH ( and Table S7). Hypertension, congestive heart failure, chronic obstructive pulmonary disease, diabetes (with and without complications), obesity, pneumonia, and asthma were more frequent in Black patients versus White patients (all with SMD >0.1). Among PAH-related symptoms (), cardiomegaly, chest pain, ascites, abnormal gait, and hemoptysis were also more common in Black patients versus White patients (all with SMD >0.1). The profile of PAH-related diagnostic tests was similar in both cohorts (Table S8), although a greater proportion of Black patients underwent right heart catheterization (47% of Black patients versus 41% of White patients [SMD 0.114]).
Figure 2. Clinical characteristics of patients with PAH recorded during the 6-month baseline period. (A) Quan-Charlson Comorbidity Index score; (B) most common comorbidities (>20% of patients in either group); (C) PAH-related symptoms. SMD >0.1 indicates difference in index scores between the groups. aIndicates comorbidities with SMD >0.1, indicating difference in frequency between the groups. bIndicates symptoms with SMD >0.1, indicating difference in frequency between the groups. Abbreviations. COPD, chronic obstructive pulmonary disorder; QCCI, Quan-Charlson Comorbidity Index; PAH, pulmonary arterial hypertension; PVD, peripheral vascular disease; SD, standard deviation; SMD, standardized mean difference.
PAH treatment patterns associated with index treatment
shows the index PAH treatment patterns in the Black patients and White patients. Most patients received monotherapy treatment, with few receiving an index combination treatment (14.4% of Black patients; 14.5% of White patients).
Table 3. Index PAH treatment pattern in the Black patients and White patients.
There were few differences between Black patients and White patients in terms of the proportions receiving monotherapy, combination treatment, or an ERA (SMD ≤0.1 for all these parameters).
A lower proportion of Black patients received index treatment containing a prostacyclin pathway agent (10.2% of Black patients versus 14.7% of White patients [SMD 0.137]). Furthermore, Black patients were observed to have lower adherence to index treatment (proportion of days covered median 0.96 [IQR 0.83–1.00] in Black patients versus 0.99 [IQR 0.88–1.00] in White patients; SMD 0.155). It was also observed that a lower proportion of Black patients than White patients met the criteria for adherence (proportion of days covered ≥0.8) to their index PAH treatment (85.3% of Black patients and 89.9% of White patients [SMD 0.14]).
The patterns for discontinuing index treatment were generally similar in the Black patients and White patients, with the most common reason captured being the discontinuation of PAH medication without restarting (53.7% of Black patients; 58.6% of White patients) ().
A sensitivity analysis comparing the time periods before versus during the COVID-19 pandemic showed consistent index treatment patterns, such that the differences in treatments (i.e. use of monotherapy or combination therapy) between Black patients and White patients were observed independent of the COVID-19 pandemic.
Healthcare resource use and costs during the baseline and follow-up periods
Descriptive analyses of health care resource utilization and associated costs in the Black patients and White patients in the 6-month baseline period are shown in , and in the follow-up period are shown in . During the baseline period, Black patients had higher rates of all-cause health care resource utilization, with a median PPPM utilization rate of 7.69 visits (IQR 5.52–11.03) versus 7.19 visits (IQR 5.02–10.20) in White patients (SMD 0.145). Black patients also had more visits to cardiologists than White patients (median 0.84 [IQR 0.33–1.50] and 0.67 [IQR 0.33–1.34], respectively; SMD 0.175). This descriptive analysis, unadjusted for potential confounders, showed that all-cause costs in the baseline period were almost twice as high for Black patients versus White patients (median total costs PPPM US$64,805 [IQR US$14,649–154,932] versus US$33,348 [IQR US$9217–119,368]; SMD 0.128).
Table 4. Health care resource utilization and costs during the 6-month baseline period.
Table 5. Health care resource utilization and costs during the follow-up period.
During the follow-up period, Black patients also had higher rates of all-cause health care resource utilization, with a median PPPM utilization rate of 3.91 visits (IQR 1.23–7.18) versus 3.50 visits (IQR 1.16–6.30) in White patients (SMD 0.108).
Adjusted regression analyses
In the overall population and in patients aged ≥65 years, after adjusting for patient demographics, social determinants of health, comorbidities, PAH symptoms, and procedure utilization during baseline using propensity score weighting, regression analyses showed no differences in any of the outcomes assessed between Black patients and White patients (). However, in patients aged <65 years, regression analysis showed that Black patients were 36% less likely to receive index combination therapy than monotherapy (adjusted odds ratio 0.64; 95% confidence interval [CI] 0.41–0.99) and were 46% less likely to be adherent with their index treatment (odds ratio 0.54; 95% CI 0.33–0.90) than White patients (). In addition, in the follow-up period, among patients aged <65 years, Black patients had 24% higher all-cause health care resource utilization (rate ratio 1.24; 95% CI 1.05–1.46), 75% higher all-cause costs (cost ratio 1.75; 95% CI 1.09–2.80), and a 24% higher risk of experiencing the composite clinical outcome of lung transplant, atrial septostomy, oxygen therapy or all-cause hospitalization (hazard ratio 1.24; 95% CI 1.01–1.52) compared with White patients (). The mediation analysis showed that effect of race remained after adjusting for education level, annual household income, and insurance type.
Table 6. Inverse probability of treatment-weighted regression analysis of outcomes comparing Black patients versus White patients with PAH.
Discussion
This study evaluated a comprehensive range of factors associated with clinical management and outcomes in a US population of Black patients and White patients with PAH. This study showed that Black patients were younger with a higher comorbidity burden than White patients on their first PAH medication index date. This is not surprising given that it has been established that Black patients are younger at PAH diagnosis than White patientsCitation12. Social determinants of health variables (education level and annual household income) were also less favorable in Black patients than White patients, and Black patients were observed to have lower index treatment adherence rates.
While the adjusted regression analyses found no differences in outcomes between Black patients and White patients in the overall population, differences were found when examining the impact of age at index treatment as an effect modifier. When adjusted for baseline confounders, the adjusted analysis in patients aged <65 years at index treatment showed that Black patients were less likely to receive index combination therapy than monotherapy and to have lower adherence to their index treatment. With respect to health care resource utilization, younger Black patients with PAH also had higher all-cause health care resource utilization, greater all-cause costs, and a higher risk of experiencing the composite clinical outcome of lung transplant, atrial septostomy, oxygen therapy, or all-cause hospitalization during follow-up compared with White patients.
The reasons for the differences seen in younger patients aged <65 years at index but not in patients ≥65 years are unclear. The higher all-cause health care resource utilization observed in the Black patients could be related to their higher comorbidity burden compared with the White patients. We speculate that the reason could also be partly associated with differences in social determinants of health. At 65 years of age, individuals in the US become eligible for Medicare insurance. Before the age of 65 years, Medicare is usually only available for individuals with a disability or certain medical conditions (i.e. end-stage renal disease or amyotrophic lateral sclerosis). Among the patients aged <65 years, 63.7% of Black patients and 45.8% of White patients had Medicare Advantage insurance, whereas in the older group (aged ≥65 years), almost all patients (>90%) had Medicare Advantage insurance, regardless of race. One possible reason why racial disparities were not observed in the older population, yet were observed in the younger age group, is because all patients became eligible for Medicare at age 65 years. Another study has shown that becoming eligible for Medicare coverage at age 65 years was associated with reductions in racial disparities in healthcare accessCitation29. Medicare patients cannot access manufacturer copay assistance programs, so they could incur larger out-of-pocket costs for treatment. Higher out-of-pocket costs for treatment may place a higher cost or health care resource utilization burden on Black patients compared with White patients, and may therefore contribute to the disparities seen between these race groups in patients aged <65 years. It is also possible that the 36.3% of Black patients <65 years of age who had commercial insurance were covered by plans that offered worse or less consistent coverage (including more restrictive formularies) than the commercial plans used by White patients, which could also contribute to the racial disparity seen in this age group. Other studies have shown differences in the quality of insurance plans used by Black and White patientsCitation30,Citation31.
Factors other than social determinants of health could also explain the observed effects, as the mediation analysis indicated that the direct effect of race remained after adjusting for education level, annual household income, and insurance type. One such factor could be healthcare provider bias. Implicit bias among healthcare providers has been shown to negatively impact clinical interactions with Black patientsCitation32,Citation33, and educational initiatives have been developed to support healthcare professionals in recognizing and addressing inequities in healthcareCitation34–36. Other social determinants of health, such as housing instability, and ease of access to PAH specialists could also explain the observed disparity. However, we have no information from our database on provider bias and limited information on mediators for the effect on race beyond education level, annual household income, and insurance type. It is likely that any provider bias or mediator would apply equally to the ≥65 years age group where we saw no differences between Black and White patients. Further research is warranted to identify the factors underlying the racial differences seen in this study in order to address and overcome these inequities in care.
In this study, few patients (∼14% overall) in both race groups were initiated on combination treatment and fewer than a quarter of patients received an ERA-containing index therapy within a 30-day window. For context, the 2015 European Society of Cardiology (ESC)/European Respiratory Society (ERS) guidelines, which reflect the years this study took place, recommend that the use of combination therapy to treat PAH can improve symptoms, exercise capacity, and outcomes compared with monotherapyCitation4,Citation37. Since World Health Organization functional class assessments were not recorded in the claims data, it was not possible to determine the influence of World Health Organization functional class on the decision to initiate combination therapy, nor to conclude whether patients were treated in accordance with guidelines in place at the time. However, other real-world data studies indicate that 46–75% of patients with idiopathic PAH receive combination therapyCitation38,Citation39. The current 2022 ESC/ERS guidelines recommend initial combination treatment with the phosphodiesterase (Type 5) enzyme inhibitor tadalafil plus an ERA, either ambrisentan or macitentan, for patients with PAH without cardiopulmonary comorbiditiesCitation1.
Limitations
The data source in this study was a claims dataset, which may introduce bias due to incomplete or missing data, limited longitudinal data, and potential coding errors and inconsistencies. As no PAH-specific diagnostic code exists, identification of patients with PAH in the Optum Clinformatics Data Mart – Socioeconomic Status database and in real-world data in general is highly challenging. Patients included in our study could also have been diagnosed with pulmonary hypertension of Groups 2–5, reflecting the challenge in clinical practice for the diagnosis and management of patients in whom pulmonary hypertension can have several possible causes that are not always mutually exclusiveCitation40. In our study, identification relied upon a diagnostic code for pulmonary hypertension together with a claim for a PAH-specific medication. This approach has been validated in previous database studies and has high specificity; moreover, the inclusion of a right heart catheterization requirement does not increase the algorithm’s positive predictive value in identifying patients with PAHCitation41. Misclassification of individuals with chronic thromboembolic pulmonary hypertension was possible prior to the implementation of a specific diagnostic code, which was introduced in 2017. Race and ethnicity in the Optum Clinformatics Data Mart – Socioeconomic Status database is imputed based on last names and ZIP code–level information of patients, and some misclassification may occur. The database contained limited information on mediators for the effect on race beyond education level, insurance type, and annual household income and it might have been subject to unknown biases. While the results of this study are representative of the commercial and Medicare Advantage population in the United States, they may not be generalizable to other patient populations. A claim for a dispensed prescription does not indicate the actual date when the medication was started or that the medication was taken as prescribed. The Optum Clinformatics Data Mart – Socioeconomic Status database uses standardized costs, which may not reflect the true costs that were incurred. Furthermore, certain data such as clinical and laboratory results, risk scores reflective of PAH severity, and etiological PAH subtypes are not readily available in claims data.
Conclusions
This study identified differences in the clinical characteristics and disparities between Black patients and White patients with PAH. Black patients were younger than White patients, and also had lower levels of education and annual household income and higher comorbidity scores. Black patients were observed to have lower treatment adherence than White patients. Among patients aged <65 years at index treatment, Black patients were less likely than White patients to receive index combination therapy than monotherapy and less likely to be adherent with their index treatment; they also had higher all-cause health care resource utilization, higher all-cause costs, and a higher risk of experiencing the composite clinical outcome of lung transplant, atrial septostomy, oxygen therapy, or all-cause hospitalization during follow-up compared with White patients. In this group of patients aged <65 years, the direct impact of race was only partly mediated through education, income, and health insurance plan type, indicating that other factors may play a role.
Transparency
Declaration of funding
This study was sponsored by Janssen Scientific Affairs, LLC, a Johnson & Johnson Company.
Declaration of financial/other relationships
LM-G reports receiving the following fees and support: United Therapeutics Corporation (research, consulting, advisory board), Janssen (research, consulting), Gossamer Bio (research, consulting), Merck (research, consulting), Bayer (research, consulting). KS, WT, and ZL are employees of Janssen Scientific Affairs, LLC and own shares of Johnson & Johnson stock. At the time of this study, HDG was an employee of Janssen Scientific Affairs, LLC and owns shares of Johnson & Johnson stock. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
WT and ZL analyzed the data. LM-G, KS, WT, ZL, and HDG were involved in conception and design of the study, interpretation of the data, agree to be accountable for all aspects of the work and have critically reviewed and approved the final version for publication.
Acknowledgements
Medical writing support was provided by Kathryn Quinn and Ify Sargeant on behalf of Twist Medical, and was funded by Janssen Scientific Affairs, LLC.
PAH Health Equity MS_M-Groves_CMRO_Supplemental_13Jun2024.docx
Download MS Word (45.4 KB)Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43(38):3618–3731. doi: 10.1093/eurheartj/ehac237.
- Klinger JR, Elliott CG, Levine DJ, et al. Therapy for pulmonary arterial hypertension in adults: update of the CHEST Guideline and Expert Panel Report. Chest. 2019;155(3):565–586. doi: 10.1016/j.chest.2018.11.030.
- Galiè N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;53(1):1801889. doi: 10.1183/13993003.01889-2018.
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37(1):67–119. doi: 10.1093/eurheartj/ehv317.
- Narechania S, Torbic H, Tonelli AR. Treatment discontinuation or interruption in pulmonary arterial hypertension. J Cardiovasc Pharmacol Ther. 2020;25(2):131–141. doi: 10.1177/1074248419877409.
- Frantz RP, Hill JW, Lickert CA, et al. Medication adherence, hospitalization, and healthcare resource utilization and costs in patients with pulmonary arterial hypertension treated with endothelin receptor antagonists or phosphodiesterase type-5 inhibitors. Pulm Circ. 2020;10(1):2045894019880086. doi: 10.1177/2045894019880086.
- Qadus S, Naser AY, Ofori-Asenso R, et al. Adherence and discontinuation of disease-specific therapies for pulmonary arterial hypertension: a systematic review and meta-analysis. Am J Cardiovasc Drugs. 2023;23(1):19–33. doi: 10.1007/s40256-022-00553-6.
- Housten T, Brown AM. PH professional network: the burden of prior authorization for pulmonary hypertension medications: a practical guide for managing the process. Adv Pulm Hypertens. 2018;17(3):126–131. doi: 10.21693/1933-088X-17.3.126.
- Cantres-Fonseca O, Kennedy JLW. Where’s the easy button? The many barriers to care for patients with pulmonary arterial hypertension. J Am Heart Assoc. 2022;11(22):e027967. doi: 10.1161/JAHA.122.027967.
- Helgeson SA, Meno D, Helmi H, et al. Psychosocial and financial burden of therapy in USA patients with pulmonary arterial hypertension. Diseases. 2020;8(2):22. doi: 10.3390/diseases8020022.
- Frost AE, Badesch DB, Barst RJ, et al. The changing picture of patients with pulmonary arterial hypertension in the United States: how REVEAL differs from historic and non-US contemporary registries. Chest. 2011;139(1):128–137. doi: 10.1378/chest.10-0075.
- Medrek SK, Sahay S. Ethnicity in pulmonary arterial hypertension: possibilities for novel phenotypes in the age of personalized medicine. Chest. 2018;153(2):310–320. doi: 10.1016/j.chest.2017.08.1159.
- Al-Naamani N, Paulus JK, Roberts KE, et al. Racial and ethnic differences in pulmonary arterial hypertension. Pulm Circ. 2017;7(4):793–796. doi: 10.1177/2045893217732213.
- Talwar A, Sahni S, Talwar A, et al. Socioeconomic status affects pulmonary hypertension disease severity at time of first evaluation. Pulm Circ. 2016;6(2):191–195. doi: 10.1086/686489.
- Talwar A, Garcia JGN, Tsai H, et al. Health disparities in patients with pulmonary arterial hypertension: a blueprint for action. An official American Thoracic Society statement. Am J Respir Crit Care Med. 2017;196(8):e32–e47. doi: 10.1164/rccm.201709-1821ST.
- McLaughlin VV, Langer A, Tan M, et al. Pulmonary Arterial Hypertension-Quality Enhancement Research Initiative (PAH-QuERI) investigators. Contemporary trends in the diagnosis and management of pulmonary arterial hypertension: an initiative to close the care gap. Chest. 2013;143(2):324–332. doi: 10.1378/chest.11-3060.
- Gillmeyer KR, Rinne ST, Qian SX, et al. Socioeconomically disadvantaged veterans experience treatment delays for pulmonary arterial hypertension. Pulm Circ. 2022;12(4):e12171. doi: 10.1002/pul2.12171.
- Wu WH, Yang L, Peng FH, et al. Lower socioeconomic status is associated with worse outcomes in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2013;187(3):303–310. doi: 10.1164/rccm.201207-1290OC.
- Schikowski EM, Swabe G, Chan SY, et al. Association between income and likelihood of right heart catheterization in individuals with pulmonary hypertension: a US claims database analysis. Pulm Circ. 2022;12(3):e12132. doi: 10.1002/pul2.12132.
- Medrek S, Saha S, Zhao C, et al. Impact of race on survival in pulmonary arterial hypertension: results from the REVEAL registry. J Heart Lung Transplant. 2020;39(4):321–330. doi: 10.1016/j.healun.2019.11.024.
- Karnes JH, Wiener HW, Schwantes-An T-H, et al. Genetic admixture and survival in diverse populations with pulmonary arterial hypertension. Am J Respir Crit Care Med. 2020;201(11):1407–1415. doi: 10.1164/rccm.201907-1447OC.
- Parikh KS, Stackhouse KA, Hart SA, et al. Health insurance and racial disparities in pulmonary hypertension outcomes. Am J Manag Care. 2017;23(8):474–480.
- DeFrank JT, Bowling JM, Rimer BK, et al. Triangulating differential nonresponse by race in a telephone survey. Prev Chronic Dis. 2007;4: a 60.
- Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5.
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786.
- Jiang Z, VanderWeele TJ. When is the difference method conservative for assessing mediation? Am J Epidemiol. 2015;182(2):105–108. doi: 10.1093/aje/kwv059.
- OptumInsight. Clinformatics data mart user manual. 2007. Version 7.1. Initial Release November 2017.
- Buntin MB, Escarce J, Goldman D, et al. Determinants of Increases in Medicare Expenditures for Physicians’ Services. Rockville (MD): Agency for Healthcare Research and Quality (US); 2003 Oct. (Technical Reviews, No. 7.) Appendix A, Payment for Physicians’ Services Under the Resource Based Relative Value Scale [cited 2023 Sep 10]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK43879/pdf/Bookshelf_NBK43879.pdf
- Wallace J, Jiang K, Goldsmith-Pinkham P, et al. Changes in racial and ethnic disparities in access to care and health among US adults at age 65 years. JAMA Intern Med. 2021;181(9):1207–1215. doi: 10.1001/jamainternmed.2021.3922.
- Linde S, Egede LE. Association of county race and ethnicity characteristics with number of insurance carriers and insurance network breadth. JAMA Netw Open. 2022;5(4):e227404. doi: 10.1001/jamanetworkopen.2022.7404.
- Dean LT, Zhang Y, McCleary R, et al. Health care expenditures Black and White US adults living under similar conditions. JAMA Health Forum. 2023;4(11):e233798. doi: 10.1001/jamahealthforum.2023.3798.
- Sabin JA. Tackling implicit bias in health care. N Engl J Med. 2022;387(2):105–107. doi: 10.1056/NEJMp2201180.
- FitzGerald C, Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics. 2017;18(1):19. doi: 10.1186/s12910-017-0179-8.
- American Association of Medical Colleges. Unconscious Bias Resources for Health Professionals [cited 2023 Sep 10]. 2023. Available from: https://www.aamc.org/about-us/equity-diversity-inclusion/unconscious-bias-training.
- Johnson & Johnson. Advancing Unconscious Bias Training for Healthcare Professionals. [cited 2023 Sep 10]. 2023. Available from: https://www.jnj.com/our-race-to-health-equity/advancing-unconscious-bias-training-for-healthcare-professionals.
- Cooper LA, Saha S, van Ryn M. Mandated implicit bias training for health professionals—a step toward equity in health care. JAMA Health Forum. 2022;3(8):e223250. doi: 10.1001/jamahealthforum.2022.3250.
- Bai Y, Sun L, Hu S, et al. Combination therapy in pulmonary arterial hypertension: a meta-analysis. Cardiology. 2011;120(3):157–165. doi: 10.1159/000334431.
- Ling Y, Johnson MK, Kiely DG, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med. 2012;186(8):790–796. doi: 10.1164/rccm.201203-0383OC.
- Ogawa A, Ejiri K, Matsubara H. Long-term patient survival with idiopathic/heritable pulmonary arterial hypertension treated at a single center in Japan. Life Sci. 2014;118(2):414–419. doi: 10.1016/j.lfs.2014.01.077.
- Lang IM, Palazzini M. The burden of comorbidities in pulmonary arterial hypertension. Eur Heart J Suppl. 2019;21(Suppl K):K21–K28. doi: 10.1093/eurheartj/suz205.
- Sprecher VP, Didden EM, Swerdel J, et al. Evaluation of code-based algorithms to identify pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension patients in large administrative databases. Pulm Circ. 2020;10(4):2045894020961713. doi: 10.1177/2045894020961713.
