ABSTRACT
Purpose: Although osteoarthritis is widely viewed as a disease of the whole joint, relatively few studies have focused on interactions among joint tissues in joint homeostasis and degeneration. In particular, few studies have examined the effects of the infrapatellar fat pad (IFP) on cartilaginous tissues. The aim of this study was to test the hypothesis that co-culture with healthy IFP would induce degradation of cartilage and meniscus tissues. Materials and Methods: Bovine articular cartilage, meniscus, and IFP were cultured isolated or as cartilage-fat or meniscus-fat co-cultures for up to 14 days. Conditioned media were assayed for sulfated glycosaminoglycan (sGAG) content, nitrite content, and matrix metalloproteinase (MMP) activity, and explants were assayed for sGAG and DNA contents. Results: Co-cultures exhibited increased cumulative sGAG release and sGAG release rates for both cartilage and meniscus, and the cartilage (but not meniscus) exhibited a substantial synergistic effect of co-culture (sGAG release in co-culture was significantly greater than the summed release from isolated cartilage and fat). Fat co-culture did not significantly alter the sGAG content of either cartilage or meniscus explants, indicating that IFP co-culture stimulated net sGAG production by cartilage. Nitrite release was increased relative to isolated tissue controls in co-cultured meniscus, but not the cartilage, with no synergistic effect of co-culture. Interestingly, MMP-2 production was decreased by co-culture for both cartilage and meniscus. Conclusions: This study demonstrates that healthy IFP may modulate joint homeostasis by stimulating sGAG production in cartilage. Counter to our hypothesis, healthy IFP did not promote degradation of either cartilage or meniscus tissues.
Introduction
The infrapatellar fat pad (IFP), also known as Hoffa’s fat pad, is found in the anterior knee joint, intra-capsular but external to the synovium. Several studies suggest a protective role for the IFP with respect to the development of knee osteoarthritis (OA), with IFP size associated with reduced knee pain and lateral tibial cartilage loss over a two year period in initially non-OA adults (Citation1), negatively correlated with radiographic evidence of OA and knee pain in a large, cross-sectional study (Citation2), and associated with greater cartilage volume and fewer cartilage defects among knee OA patients (Citation3). However, other studies reported no difference in IFP size between OA patients and controls (Citation4) or an increased IFP size in patients with patellofemoral OA, with a positive relationship between IFP volume and pain (Citation5). The overall role of the IFP in joint health and OA is thus unclear (Citation6), motivating specific studies on metabolic interactions with other tissues.
Degenerative disorders of articular joints are now widely viewed as “whole joint” diseases, and both the maintenance and degeneration of joints involve direct and indirect interactions among multiple joint tissues (Citation6, Citation7). In vitro models allow exploration of potential interactions between tissues, and have been useful for studying effects of joint capsular cells and tissues on articular cartilage metabolism. In co-culture and conditioned medium models, OA and rheumatoid arthritis (RA) synovium both inhibited cartilage anabolism and stimulated cartilage degradation (Citation8–Citation10). Interestingly, healthy synovium and capsular tissue have also been reported to inhibit cartilage anabolism (Citation11) and stimulate cartilage matrix breakdown (Citation12), although co-culture with isolated synoviocytes reduced the severity of the catabolic response to interleukin-1 (Citation13) and mechanical overload (Citation14).
In contrast to synovium, interactions between IFP and other tissues in the joint have received relatively little attention (Citation15). Like synovium, IFP can be a major source of pro-inflammatory factors associated with OA (Citation16–Citation19). OA IFP produces inflammatory mediators such as interleukin-6, tissue necrosis factor-α, and various adipokines associated with tissue degradation at greater concentrations than does subcutaneous adipose tissue (Citation18–Citation20), suggesting that the IFP has a distinct secretory profile that may play an important role in the local environment of the knee joint. Conditioned media from osteoarthritic IFP induced cartilage collagen release and increased MMP activity in chondrocytes (Citation21), and induced migration, proliferation, and catabolic enzyme expression by synovial fibroblasts (Citation20, Citation22).
Unlike synovium, however, interactions between non-degenerative IFP and cartilage have not been reported. Furthermore, despite a growing appreciation that meniscal degeneration is commonly involved in knee OA pathogenesis (Citation23), effects of other joint tissues on meniscal metabolism have received little attention. The goal of this study was to use a tissue explant co-culture model to assess effects of healthy IFP tissue on articular cartilage and meniscal fibrocartilage proteoglycan metabolism. As IFP is known to be a source of multiple proinflammatory factors that are individually capable of inducing cartilage and meniscus catabolism, we hypothesized that co-culture with IFP tissues would induce degradation of both tissues.
Materials and methods
Materials
Juvenile bovine stifles were from Research 87 (Marlborough, MA). High glucose Dulbecco’s modified Eagle’s medium (DMEM) was from HyClone (Logan, UT). Non-essential amino acids (NEAA), proteinase K, casein and gelatin zymogram gels, Tris-glycine sodium dodecyl sulfate (SDS) sample and running buffers, Triton X-100 renaturing buffer, Tris-HCl developing buffer, coomassie blue, and molecular weight standards were from Life Technologies (Carlsbad, CA). N-(2-hydroxyethyl)-piperazine-N’-2-ethanesulfonic acid (HEPES) and gentamicin were from Mediatech (Manassas, VA). Insulin-transferrin-selenous acid (ITS+) premix was from BD Biosciences (Franklin Lakes, NJ). L-ascorbic acid 2-phosphate, protease inhibitor cocktail, ammonium acetate, 1,9-dimethyl-methylene blue (DMMB) dye, shark chondroitin sulfate, sodium nitrite, bisbenzimide (Hoechst 33258), and calf thymus DNA were from Sigma (St. Louis, MO). 1 mM 4-aminophenylmercuric acetate (APMA) was from EMD Millipore (Billerica, MA). Biopsy punches were from Miltex (York, PA).
Tissue harvest and culture
Full thickness articular cartilage (AC) explants from the medial femoral condyles and meniscal fibrocartilage (MFC) explants from the tibial aspect of the medial menisci were aseptically harvested with a 4 mm biopsy punch from immature bovine stifles of two animals within one day of slaughter. Infrapatellar fat pad (IFP) tissue was excised from each leg with care to avoid the synovial membrane. Tissues were separately cultured at 37 °C, 5% CO2, and 95% relative humidity in serum-free medium consisting of high glucose DMEM with 0.1 mM NEAA, 10 mM HEPES, 50 μg/mL L-ascorbic acid-2-PO4, 50 μg/ml gentamicin and 1% ITS+ premix (6.25 μg/ml insulin, 6.25 μg/ml transferrin, 6.25 ng/ml selenous acid, 1.25 mg/ml bovine serum albumin, 5.35 μg/ml linoleic acid). After overnight culture, cartilage and meniscus explants were trimmed to 2 mm thickness, leaving the articular surfaces intact. IFP tissue was cut into pieces to fill a 6 mm diameter, 3 mm deep well to obtain a consistent fat volume of approximately 85 mm3.
Tissue explants were then distributed equally (n = 8/group) into five groups with co-culture samples paired from the same animal (): AC, MFC, fat, AC co-culture with fat, and MFC co-culture with fat. Based on the kinetics of anabolic and catabolic responses observed in multiple previous studies from our lab involving cartilage and meniscus explants (Citation24–Citation31), samples were cultured for 14 days with medium exchanges every 48 hours. Additional, non-cultured samples (n = 8/tissue) were stored frozen in protease inhibitor for comparisons with baseline composition. A second, independent experiment using tissues from an additional harvest was run to generate media samples for gelatin and casein zymography.
Biochemical analysis
At the end of culture, explants and fat tissue were weighed, lyophilized overnight and re-weighed dry. Samples were digested in 1 mL proteinase K (0.2–0.5 mg/mL, 1 mg/40 mg of cartilage, 1 mg/20 mg of meniscus) buffered with 100 mM ammonium acetate pH 7.0 at 60 °C overnight. Sulfated glycosaminoglycan (sGAG) contents of digested tissues and conditioned media were assayed using the DMMB assay (pH 3, 525 nm) with chondroitin sulfate standards (Citation32). To indicate production of nitric oxide (NO), a cell signaling mediator in inflammation (Citation33, Citation34), nitrite release was measured in conditioned media using the Griess assay (Citation35) with sodium nitrite standards. Tissue DNA contents were measured using the Hoechst dye assay with calf thymus DNA standards.
MMP activity in conditioned media was determined via zymography on gelatin and casein substrates. Conditioned media from day 2 (n = 6/tissue/group) were mixed 1:1 in Tris-Glycine SDS sample buffer containing bromophenol blue and loaded into 0.1% gelatin-substrate in 10% polyacrylamide gels or 0.1% casein-substrate in 12% polyacrylamide gels. Electrophoresis was conducted at 125 V constant (100 minutes for gelatin and 115 minutes for casein) in a Tris-Glycine SDS running buffer. After electrophoresis, gels were removed from their casing, rinsed in deionized water, and scanned on a flatbed scanner to image the molecular weight standard. Gels were then incubated in a Triton X-100 renaturing buffer for 30 minutes at room temperature and in a Tris-HCl and CaCl2 developing solution at 37 °C (17 hours for gelatin and 42 hours for casein). Developed gels were rinsed in deionized water, stained using coomassie blue for 1 hour, rinsed again in deionized water to remove excess dye and then scanned on a flatbed scanner to image bands. To determine total MMP levels, an additional set of media samples was incubated with 1 mM 4-aminophenylmercuric acetate (APMA) for three hours at 37 °C to activate latent MMPs prior to loading into gels. Scanned images were analyzed using TotalLab Quant (Version 12.5, TotalLab, Ltd., Newcastle upon Tyne, UK). After background subtraction, Gaussian models were fit to the intensity curve profiles and the net intensity of the Gaussian for each band was used for each datum.
Data analysis
sGAG and nitrite release rates were calculated for each sample as the slope of the linear regression of cumulative release vs. time. Statistical analyses were performed with Minitab (version 17, Minitab, Inc., State College, PA). As data were generally not normally distributed, data were subjected to optimal Box-Cox transformation prior to analysis via general linear models (GLMs). Significance was set at p < 0.05 and Bonferroni’s method was used for pairwise comparisons. The results are presented as mean ± SEM.
Media cumulative release data at each time point and release rates were analyzed with GLMs treating tissue as a main effect and co-culture group nested within tissue. To examine synergistic effects of co-culture, media release rates and day 14 cumulative release values were also analyzed via linear regression using categorical predictors (fat, cartilage, meniscus) and interactions (cartilage+fat, meniscus+fat) but excluding an intercept term. Baseline compositions of cartilage and meniscus explants were compared using one factor GLMs. Due to large compositional differences among tissues, changes in composition relative to baseline were analyzed separately for each tissue, with baseline vs. culture as the main effect and co-culture group nested within culture. Composition of cultured tissues was analyzed with GLMs treating tissue as a main effect and co-culture group nested within tissue. Zymography results were analyzed via two-factor (tissue, co-culture) GLMs, and effects of APMA activation were examined via three-factor (tissue, co-culture, APMA) GLMs.
Results
sGAG release
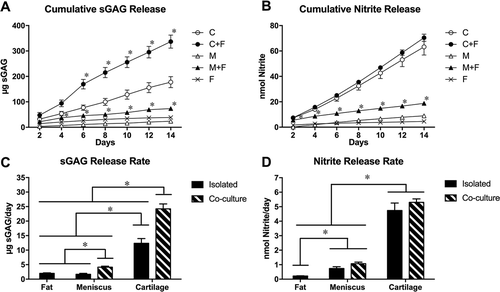
Throughout the culture duration, cartilage explants had higher cumulative sGAG release than other tissues, reflecting the intrinsically higher sGAG content and basal sGAG synthesis rate in cartilage tissue (). Although cartilage explants had higher DNA contents than meniscus explants (), the greater sGAG release from cartilage explants was disproportionately larger than the implied difference in cellularity. Cartilage co-cultured with fat had significantly higher cumulative sGAG release than cartilage cultured alone beginning on day 6 of culture and lasting through the culture duration, while meniscus co-cultured with fat had significantly higher cumulative sGAG release than meniscus alone at all time points. Meniscus and cartilage explants co-cultured with fat had significantly higher sGAG release rates than corresponding tissue explants cultured alone (). The sGAG release rate of co-cultured cartilage and fat (24.2 ± 1.7 µg/explant/day) was significantly greater than the combined rates of release from isolated cartilage (12.3 ± 1.7 µg/explant/day) and isolated fat (2.01 ± 0.17 µg/explant/day), indicating a synergistic effect of fat on sGAG release from cartilage. For meniscus, however, the sGAG release rate from co-cultured meniscus and fat (4.13 ± 0.33 µg/explant/day) was not significantly greater than the combined release rates of isolated meniscus (1.67 ± 0.34 µg/explant/day) and isolated fat (2.01 ± 0.17 µg/explant/day). Similarly, the cumulative sGAG release at day 14 () exhibited a synergistic response to co-culture for cartilage but not for meniscus.
Table 1. Baseline explant composition.
Figure 2. Conditioned media analysis. Cumulative levels of (A) sGAG and (B) nitrite released to conditioned media and average (C) sGAG and (D) nitrite release rates for fat, meniscus and cartilage explants cultured in isolation or in co-culture with fat. *p < 0.05 relative to isolated tissue controls (A,B) or as indicated between groups (C,D). Mean ± SEM.
Nitrite Release
Beginning on day 4, cartilage explants exhibited higher cumulative nitrite release than did meniscus (as with sGAG release, disproportionately greater than the difference in cellularity), but there was no significant effect of fat co-culture on cumulative cartilage nitrite release (). Cumulative nitrite release of meniscus co-cultured with fat was significantly greater than that of meniscus cultured alone throughout the 14-day culture. The average nitrite release rate was greatest for cartilage and least for fat (), but neither cartilage nor meniscus exhibited a synergistic effect of co-culture on nitrite release rate or on day 14 cumulative nitrite release (e.g., values for co-cultured groups did not significantly differ from the combined values for isolated tissue and fat groups). The apparent discrepancy between these two views of nitrite release is related to elevated nitrite release on day 2 in the meniscus-fat co-culture group compared to either tissue cultured alone.
Explant composition
At baseline (), wet mass did not significantly differ between cartilage and meniscus explants, while cartilage explants exhibited significantly lower dry mass and significantly higher water fraction, DNA content and sGAG content. Cultured fat explants from all groups () lost approximately 60% of their initial mass over the two-week culture period, with relatively small compositional differences among cultured groups. While neither the wet mass nor dry mass of cultured fat tissue varied significantly among groups, the water fraction of fat co-cultured with meniscus was significantly lower than that of fat co-cultured with cartilage (). Both the DNA () and sGAG () contents of fat tissue were significantly lower than those of both meniscus and cartilage, with no significant differences among cultured fat tissue groups.
Figure 3. Explant analysis. (A) Wet mass, (B) dry mass, (C) water content, (D) DNA content and (E) tissue sGAG content for cultured fat, meniscus and cartilage explants. *p < 0.05 between groups, ˆ p < 0.05 relative to corresponding tissue at baseline (see ). Note that explant sGAG data (E) are plotted on a logarithmic scale due to order-of-magnitude differences among tissues. Mean ± SEM.
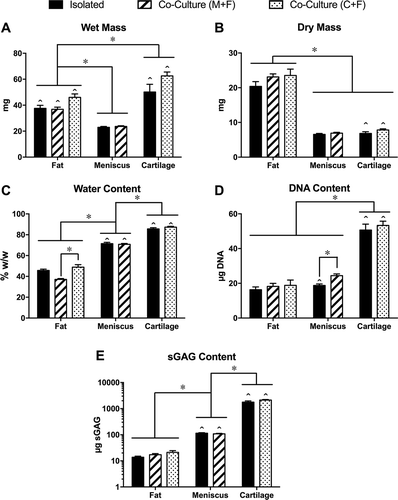
Both the wet mass () and water fraction () of meniscus explants were significantly lower than those of cartilage explants. Neither the wet nor dry mass (of cultured meniscus explants differed significantly from non-cultured baseline tissue or among culture groups (–), but the fractional water content was significantly lower in cultured explants. In contrast, the wet mass, dry mass and water fraction of cultured cartilage explants were all significantly greater than those of non-cultured baseline tissue (with the wet mass nearly doubling), with no significant difference between cartilage explants cultured alone or co-cultured with fat. The DNA content of meniscus explants cultured in isolation was significantly lower than that of both baseline and co-cultured explants, with no difference between non-cultured baseline and co-cultured tissue (). In contrast, the DNA content of cultured cartilage explants was significantly greater than that of baseline explants, with no significant difference between isolated and co-cultured cartilage explants.
There were significant, order of magnitude differences among the sGAG contents of the different tissues (). The sGAG content of cultured fat tissue was very low (below 0.1% of dry mass) and did not significantly vary among culture groups. The sGAG contents of cultured meniscus explants were significantly lower (by nearly half) than that of non-cultured baseline tissue (typical of serum-free culture), while the sGAG contents of cultured cartilage explants were significantly higher (nearly double) than that of non-cultured baseline tissue; neither tissue exhibited a significant difference in sGAG content between isolated and co-cultured groups.
Zymography
Gelatin zymograms (,) had two bright bands in all conditioned media groups at approximately 60 kDa, likely associated with proMMP-2 (66 kDa) and active MMP-2 (62 kDa) which have high specific activity against gelatin (Citation36). Combined MMP-2 activity (pro-MMP2 plus MMP-2) in conditioned media was significantly greater for cartilage than for meniscus and was significantly lower for co-cultured groups, with no significant interaction between tissue and co-culture (). The active form generated 1%–3% of the total MMP-2 signal, and was significantly greater for cartilage than for meniscus with a trend (p = 0.054) toward lower levels with co-culture. APMA activation of latent MMPs significantly increased the overall MMP-2 signal somewhat for cartilage but not for meniscus, and total (active + latent) MMP-2 activity was significantly greater in cartilage than in meniscus with no effect of co-culture ().
Figure 4. Representative zymograms. Scanned sections of (A,C) gelatin and (B,D) casein zymograms of (A,B) cartilage and (C,D) meniscus conditioned media samples.
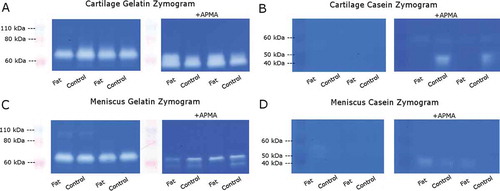
Figure 5. Zymography of conditioned media. Levels of (A,B) gelatinase activity typical of MMP-2 and (C,D) caseinase activity typical of MMP-3 in (A,C) untreated media samples and (B,D) media samples incubated with APMA to activate latent MMPs. Band intensities for each substrate are normalized to the mean combined (pro- plus active) activity for the cartilage control group. *p < 0.05 between groups for total MMP. Mean ± SEM.
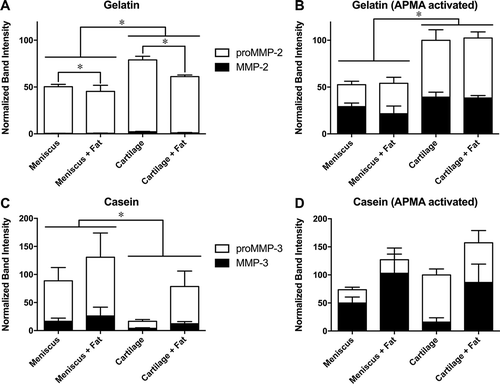
Casein zymograms (,) exhibited two relatively faint bands at approximately 50 kDa, likely associated with proMMP-3 (57 kDa) and active MMP-3 (45 kDa) which have high specific activity against casein (Citation36). In contrast to MMP-2 activity, combined MMP-3 activity (pro-MMP-3 plus MMP-3) in conditioned media was significantly greater for meniscus than for cartilage (), with a trend (p = 0.058) toward greater values with co-culture. The active form generated 18%–30% of the total MMP-3 signal, and was significantly greater for meniscus than for cartilage with no effect of co-culture. Again, APMA activation significantly increased the overall MMP-3 signal for cartilage but not for meniscus, and there were no significant differences among culture groups in total (latent + active) MMP-3 levels ().
Discussion
This study demonstrated that IFP can interact with cartilage and meniscus tissues in a co-culture system to alter their extracellular matrix production, and provides a basis for studying the role of IFP tissue in knee joint homeostasis and degeneration. Contrary to our hypothesis, co-culture with IFP did not induce degradation of either cartilage or meniscus explants. Fat co-culture increased total sGAG release from cartilage, increased the rates of sGAG release from both cartilage and meniscus, decreased total MMP-2 production in both tissues and did not significantly affect MMP-3 production in either tissue. As explant sGAG content was not significantly reduced by co-culture in either tissue, it appears that the increased sGAG release from cartilage explants was in fact due to a stimulation of sGAG production in fat coculture.
While cultured meniscus explants had decreased DNA content compared to freshly harvested tissue, meniscus co-cultured with fat had greater DNA content than meniscus cultured alone. A similar trend was observed for cartilage, with tissue co-cultured with fat having greater DNA content than freshly harvested tissue (although there was no significant difference between cultured groups). Thus, it appears that factors secreted by fat may enhance viability and/or proliferation of cartilage and meniscus cells in these juvenile tissues, although more detailed studies are required to firmly establish and explain these effects.
Nitric oxide is a cell mediator in inflammatory responses that can inhibit proteoglycan and collagen synthesis in cartilage and enhance chondrocyte apoptosis (Citation33, Citation34). Consistent with previous observations of juvenile tissues (Citation30), nitrite release was greater in cartilage than meniscus, perhaps due to greater cellularity of cartilage tissue (as evidenced by greater DNA content). While cumulative nitrite release was greater in co-cultured meniscus than in meniscus cultured alone, the nitrite release rate was unaffected. This can be seen visually on as fat co-culture with meniscus increased the very early release on day 2 (curve offset) but not the subsequent rate (slope) of nitrite release. We saw similar trends in the responses to various adipokines and interleukin-1α (Citation37), indicating that meniscus tissue responds quickly to biochemical perturbations. However, it is not clear if subsequent quiescence in nitric oxide release is due to an adaptive response such as inhibition of NO synthase or due to decreased meniscus sensitivity with time.
Juvenile bovine tissues have been widely used to study metabolism and degeneration in cartilaginous tissues, and generally behave similarly to human tissues of interest. OA human IFP secretes a complex mix of factors capable of influencing tissue homeostasis, including pro-inflammatory cytokines such as interleukin-6 (IL-6), IL-8 and tissue necrosis factor-α (TNF-α) (Citation18–Citation20), adipokines such as leptin, adiponectin, visfatin, resistin and adipsin (Citation16, Citation17, Citation19, Citation20), and prostoglandins such as PGE2 and PGF2α (Citation20, Citation22). However, differences in the secretome between bovine and human IFP (or even between healthy and diseased human IFP) have not been described, and further study is required to strongly relate these observations to human joint physiology. While convenient, co-culture systems lack a defined medium and cannot fully decouple responses of the individual tissues, making it difficult to identify specific mechanisms of interaction. These limitations can be ameliorated through use and characterization of fat-conditioned medium, as well as through controlled experiments exploring the individual and combined effects of specific factors (particularly those differentially produced by diseased and healthy IFP).
Previous studies examining articular cartilage co-culture with synovium show that synovium produces MMPs (Citation9) and aggrecanases (Citation12) and can reduce protein synthesis (Citation38) and proteoglycan production (Citation9, Citation38) in cartilage. Adipose tissues can also produce a wide range of signaling molecules, and considerable recent interest has focused on the effects of fat-secreted factors on other tissues. In studies on the isolated effects of individual adipokines that can be produced by IFP, we found that several adipokines (most notably resistin) stimulated catabolic and anti-anabolic responses in cartilage tissues, with meniscus exhibiting greater sensitivity than articular cartilage (Citation30). In contrast, the current co-culture study found that both cartilage and meniscus tissues displayed a modest anti-catabolic response to co-culture with fat (lower MMP-2 production) and cartilage but not meniscus exhibited a substantial anabolic response. Interestingly, culture of bovine cartilage explants cultured in conditioned medium from human OA infrapatellar fat pad resulted in decreased NO release, proteoglycan release, and MMP-1 expression, which could indicate decreased catabolism or generally depressed metabolism. Differences between these studies may reflect secretory differences between healthy bovine IFP and OA human IFP, but further study is required to identify differences in individual factors and interactions among secreted factors that regulate tissue metabolism. Simulation of an early inflammatory environment, for example by adding low levels of pro-inflammatory factors such as IL-1 or TNF-α to a healthy tissue coculture system, may also be a valuable extension. Collectively, these studies demonstrate that factors released from other joint tissues (including IFP) can modulate cartilage and meniscus homeostasis and underscore the importance of viewing joint health and degeneration from a holistic, multi-tissue perspective.
In summary, these studies demonstrated that co-culture with non-diseased IFP can modulate cartilage and meniscal sGAG metabolism, and contrary to expectations actually stimulated sGAG production and accumulation by cartilage. Combining these results with observations from other co-culture studies and investigations into the role of adipokines, there is evidence that biologic factors from adipose tissue can alter the homeostasis of cartilaginous tissues. These findings provide a reference for future studies examining changes in tissue interactions with age and disease development, and may be particularly relevant to studies aimed at identifying factors related to the pathogenesis of obesity-related osteoarthritis.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Funding
This work was supported by a grant from the National Institutes of Health (NIAMS R01AR052861), a National Science Foundation Graduate Research Fellowship (JFN) and by Stanford University through the Summer Undergraduate Research Institute (MFB).
Additional information
Funding
References
- Teichtahl AJ, Wulidasari E, Brady SR, Wang Y, Wluka AE, Ding C, Giles GG, Cicuttini FM. A large infrapatellar fat pad protects against knee pain and lateral tibial cartilage volume loss. Arthritis Res Ther 2015;17:318.
- Han W, Cai S, Liu Z, Jin X, Wang X, Antony B, Cao Y, Aitken D, Cicuttini F, Jones G, Ding C. Infrapatellar fat pad in the knee: is local fat good or bad for knee osteoarthritis? Arthritis Res. Ther. 2014;16(4):R145.
- Cai J, Xu J, Wang K, Zheng S, He F, Huan S, Xu S, Zhang H, Laslett L, Ding C. Association between infrapatellar fat pad volume and knee structural changes in patients with knee osteoarthritis. J Rheumatol 2015;42(10):1878–1884.
- Chuckpaiwong B, Charles HC, Kraus VB, Guilak F, Nunley JA. Age-associated increases in the size of the infrapatellar fat pad in knee osteoarthritis as measured by 3T MRI. J Orthopaedic Res: Off Publ Orthopaedic Res Soc 2010;28(9):1149–1154.
- Cowan SM, Hart HF, Warden SJ, Crossley KM. Infrapatellar fat pad volume is greater in individuals with patellofemoral joint osteoarthritis and associated with pain. Rheumatol Int 2015;35(8):1439–1442.
- Poole AR. Osteoarthritis as a whole joint disease. HSS J: Musculoskeletal J Hosp Spec Surg 2012;8(1):4–6.
- Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheumatism 2012;64(6):1697–1707.
- Steinberg JJ, Sledge CB. Synovial factors and chondrocyte-mediated breakdown of cartilage: inhibition by hydrocortisone. J Orthopaedic Res: Off Publ Orthopaedic Res Soc 1983;1(1):13–21.
- Beekhuizen M, Bastiaansen-Jenniskens YM, Koevoet W, Saris DB, Dhert WJ, Creemers LB, van Osch GJ. Osteoarthritic synovial tissue inhibition of proteoglycan production in human osteoarthritic knee cartilage: establishment and characterization of a long-term cartilage-synovium coculture. Arthritis Rheumatism 2011;63(7):1918–1927.
- Steinberg JJ, Tsukamoto S, Sledge CB. Breakdown of cartilage proteoglycan in a tissue culture model of rheumatoid arthritis. Biochimica et biophysica Acta 1983;757(1):47–58.
- Patwari P, Lin SN, Kurz B, Cole AA, Kumar S, Grodzinsky AJ. Potent inhibition of cartilage biosynthesis by coincubation with joint capsule through an IL-1-independent pathway. Scand J Med Sci Sports 2009;19(4):528–535.
- Vankemmelbeke MN, Ilic MZ, Handley CJ, Knight CG, Buttle DJ. Coincubation of bovine synovial or capsular tissue with cartilage generates a soluble “Aggrecanase” activity. Biochem Biophys Res Commun 1999;255(3):686–691.
- Gregg AJ, Fortier LA, Mohammed HO, Mayr KG, Miller BJ, Haupt JL. Assessment of the catabolic effects of interleukin-1beta on proteoglycan metabolism in equine cartilage cocultured with synoviocytes. Am J Vet Res 2006;67(6):957–962.
- Lee CM, Kisiday JD, McIlwraith CW, Grodzinsky AJ, Frisbie DD. Synoviocytes protect cartilage from the effects of injury in vitro. BMC Musculoskeletal Disord 2013;14:54.
- Ioan-Facsinay A, Kloppenburg M. An emerging player in knee osteoarthritis: the infrapatellar fat pad. Arthritis Res Ther 2013;15(6):225.
- Presle N, Pottie P, Dumond H, Guillaume C, Lapicque F, Pallu S, Mainard D, Netter P, Terlain B. Differential distribution of adipokines between serum and synovial fluid in patients with osteoarthritis. Contribution of joint tissues to their articular production. Osteoarthritis Cartilage 2006;14:690–695.
- Chen W-P, Bao J-P, Feng J, Hu P-F, Shi Z-L, Wu L-D. Increased serum concentrations of visfatin and its production by different joint tissues in patients with osteoarthritis. Clin Chem Lab Med 2010;48:1141–1145.
- Distel E, Cadoudal T, Durant S, Poignard A, Chevalier X, Benelli C. The infrapatellar fat pad in knee osteoarthritis: an important source of interleukin-6 and its soluble receptor. Arthritis Rheumatism 2009;60(11):3374–3377.
- Klein-Wieringa IR, Kloppenburg M, Bastiaansen-Jenniskens YM, Yusuf E, Kwekkeboom JC, El-Bannoudi H, Nelissen RG, Zuurmond A, Stojanovic-Susulic V, Van Osch GJ, Toes RE, Ioan-Facsinay A. The infrapatellar fat pad of patients with osteoarthritis has an inflammatory phenotype. Ann Rheumatic Dis 2011;70(5):851–857.
- Eymard F, Pigenet A, Citadelle D, Flouzat-Lachaniette CH, Poignard A, Benelli C, Berenbaum F, Chevalier X, Houard X. Induction of an inflammatory and prodegradative phenotype in autologous fibroblast-like synoviocytes by the infrapatellar fat pad from patients with knee osteoarthritis. Arthritis Rheumatol (Hoboken, NJ) 2014;66(8):2165–2174.
- Hui W, Litherland GJ, Elias MS, Kitson GI, Cawston TE, Rowan AD, Young DA. Leptin produced by joint white adipose tissue induces cartilage degradation via upregulation and activation of matrix metalloproteinases. Ann Rheumatic Dis 2012;71(3):455–462.
- Bastiaansen-Jenniskens YM, Wei W, Feijt C, Waarsing JH, Verhaar JA, Zuurmond AM, Hanemaaijer R, Stoop R, van Osch GJ. Stimulation of fibrotic processes by the infrapatellar fat pad in cultured synoviocytes from patients with osteoarthritis: a possible role for prostaglandin f2alpha. Arthritis Rheumatism 2013;65(8):2070–2080.
- Sun Y, Mauerhan DR, Honeycutt PR, Kneisl JS, Norton JH, Hanley EN, Jr, Gruber HE. Analysis of meniscal degeneration and meniscal gene expression. BMC Musculoskeletal Disord 2010;11:19.
- Imler SM, Doshi AN, Levenston ME. Combined effects of growth factors and static mechanical compression on meniscus explant biosynthesis. Osteoarthritis Cartilage/OARS, Osteoarthritis Res Soc 2004;12(9):736–744.
- Wilson CG, Palmer AW, Zuo F, Eugui E, Wilson S, Mackenzie R, Sandy JD, Levenston ME. Selective and non-selective metalloproteinase inhibitors reduce IL-1-induced cartilage degradation and loss of mechanical properties. Matrix Biol 2007;26(4):259–268.
- Wilson CG, Vanderploeg EJ, Zuo F, Sandy JD, Levenston ME. Aggrecanolysis and in vitro matrix degradation in the immature bovine meniscus: mechanisms and functional implications. Arthritis Res Ther 2009;11(6):R173.
- Wilson CG, Nishimuta JF, Levenston ME. Chondrocytes and meniscal fibrochondrocytes differentially process aggrecan during de novo extracellular matrix assembly. Tissue Eng Part A 2009;15(7):1513–1522.
- Palmer AW, Wilson CG, Baum EJ, Levenston ME. Composition-function relationships during IL-1-induced cartilage degradation and recovery. Osteoarthritis Cartilage/OARS, Osteoarthritis Res Soc 2009;17(8):1029–1039.
- Nishimuta JF, Levenston ME. Response of cartilage and meniscus tissue explants to in vitro compressive overload. Osteoarthritis Cartilage/OARS, Osteoarthritis Res Soc 2012;20(5):422–429.
- Nishimuta JF, Levenston ME. Meniscus is more susceptible than cartilage to catabolic and anti-anabolic effects of adipokines. Osteoarthritis Cartilage/OARS, Osteoarthritis Res Soc 2015;23(9):1551–1562.
- Ling CH, Lai JH, Wong IJ, Levenston ME. Bovine meniscal tissue exhibits age- and interleukin-1 dose-dependent degradation patterns and composition-function relationships. J Orthopaedic Res: Off Publ Orthopaedic Res Soc 2016;34(5):801–811.
- Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochimica et biophysica acta 1986;883:173–177.
- Weinberg J, Fermor B, Guilak F. Nitric oxide synthase and cyclooxygenase interactions in cartilage and meniscus: relationships to joint physiology, arthritis, and tissue repair. Sub-Cell Biochem 2007;42:31–62.
- Amin AR, Abramson SB. The role of nitric oxide in articular cartilage breakdown in osteoarthritis. Curr Opin Rheumatol 1998;10:263–268.
- Hensley K, Mou S, Pye QN. Nitrite determination by colorimetric and fluorometric griess diazotization assays. In: Hensley K, Floyd R, eds. Methods in Pharmacology and Toxicology: Methods in Biological Oxidative Stress Totowa, NJ: Humana Press; 2003. pp. 185–193.
- Manicourt DH, Lefebvre V. An assay for matrix metalloproteinases and other proteases acting on proteoglycans, casein, or gelatin. Anal Biochem 1993;215:171–179.
- Nishimuta JF. Degradation of cartilage and meniscus tissues: an in vitro investigation in osteoarthritis development. Stanford, CA: Stanford University; 2013.
- Steinhagen J, Bruns J, Niggemeyer O, Fuerst M, Rüther W, Schünke M, Kurz B. Perfusion culture system: Synovial fibroblasts modulate articular chondrocyte matrix synthesis in vitro. Tissue Cell 2010;42(3):151–157.