ABSTRACT
We investigated the effects of cyclic muscle twitch contraction caused by neuromuscular electrical stimulation (NMES) on immobilization-induced muscle contracture and fibrosis in rats. Twenty-nine rats were divided into control, immobilization, and immobilization with muscle contraction groups. The ankle joints of the immobilization and muscle contraction rats were fixed in full plantar flexion with a plaster cast for 4 weeks. In the muscle contraction group, cyclic muscle twitch contraction of the soleus muscle was induced using a commercial device (1 Hz, 4 ± 2 mA, 60 min/day, 5 times/week) with the ankle joint immobilized. The dorsiflexion range of ankle joint motion in the muscle contraction group was significantly greater than that in the immobilization group. The expressions of fibrosis-related genes (i.e., hypoxia inducible factor-1α, transforming growth factor-β1, α-smooth muscle actin, and types I and III collagen) were significantly decreased in the muscle contraction group compared to the immobilization group. The fluorescence intensities of type I and type III collagen in the perimysium and endomysium in the muscle contraction group were significantly decreased compared to the immobilization group. These results suggest that cyclic muscle twitch contraction induced by NMES might alleviate skeletal muscle fibrosis, reducing immobilization-induced muscle contracture.
Introduction
Skeletal muscle fibrosis, which is characterized by excessive fibrous tissue accumulation (Citation1), causes organ dysfunction such as a decrease of skeletal muscle extensibility (Citation2,Citation3) that is believed to be related to limiting the range of joint motion known as muscle contracture (Citation4). One of the main factors leading to muscle contracture is joint immobilization (e.g., bed rest, plaster cast, or external fixation), which is commonly required as part of medical treatment during the acute phase of musculoskeletal disorders as well as during the postoperative phase to decrease inflammation.
Some studies have shown that fibrosis, i.e., the overexpression and accumulation of collagen in the perimysium and endomysium, is strongly related to the occurrence and progression of immobilization-induced muscle contracture (Citation5,Citation6). Additionally, Honda et al. revealed the biological processes underlying immobilization-induced skeletal muscle fibrosis (Citation7). One process is the overexpression of transforming growth factor (TGF)-β1, a known promoter of collagen deposition. Chen et al. also reported that TGF-β1 signaling played a crucial role in skeletal muscle-derived stem cells synthesizing collagen proteins in vitro (Citation8).
The other biological process underlying immobilization-induced skeletal muscle fibrosis is skeletal muscle hypoxia, as indicated by markedly increased hypoxia-inducible factor (HIF)-1α, which was confirmed after 4 weeks of immobilization (Citation7). Concurrent with these changes, the number of myofibroblasts, characterized by the expression of α-smooth muscle actin (SMA), was increased in the immobilized muscle (Citation7). Type I and type III collagen expression in the perimysium and endomysium was also increased in the immobilized muscle (Citation7). Future therapeutic approaches for treating muscle contracture should thus address ways to inhibit the overexpression of TGF-β1 and hypoxia in the immobilized skeletal muscle. The biological effects of a fibrosis marker-based therapeutic approach are not well understood. Blaauboer et al. (Citation9) demonstrated that cyclic mechanical stimulation, representing a normal breathing cycle, with the application of 10 ng/mL TGF-β1 to primary normal human lung fibroblasts inhibited the expression of a number of genes (including TGF-β1, α-SMA, COL1A1, and COL1A2) compared with those of non-stimulated cells. These findings indicate the possibility that an appropriate mechanical stimulation of fibroblasts decelerates TGF-β1 mRNA overexpression and the subsequent fibrotic alteration. We speculated that mechanical stimulation of immobilized skeletal muscle regulates fibrotic alteration through TGF-β1, as suggested by previous findings (Citation7,Citation9), and we therefore hypothesized that cyclic muscle contraction may be an appropriate mechanical stimulation for fibroblasts in the skeletal muscle and that this may inhibit immobilization-induced muscle fibrosis. A number of studies have investigated the effect of neuromuscular electrical stimulation (NMES) on blood flow in the skeletal muscle, and these studies’ results suggested that the muscle pump action of contracting muscle increases blood flow (Citation10,Citation11). Sandberg et al. demonstrated that twitch contraction at low-frequency (2 Hz) NMES, but not tetanic contraction at high-frequency (80 Hz) NMES, appears to be a prerequisite for increasing blood flow in the trapezius muscle of healthy subjects (Citation12). We thus hypothesized that the cyclic muscle twitch contraction induced by NMES inhibits muscle fibrosis by reducing TGF-β1 overexpression and hypoxia, which alleviates the development of muscle contracture induced by immobilization. Our aim in the present study was to determine the effects of cyclic muscle twitch contraction induced by NMES on immobilization-induced muscle contracture and fibrosis.
Materials and methods
Animals
The animal care and experimental procedures were performed in accordance with Nagasaki University’s guidelines for animal experimentation, with approval from the institutional Animal Care and Use Committee (approval no. 1404181138). Eight-week-old male SPF Wistar rats (weighing 325 ± 25 g) obtained from Kyudo Corp. (Saga, Japan) were randomly divided into three groups: (Citation1) control (n = 10); (Citation2) immobilization (n = 11); and (Citation3) immobilized with cyclic muscle twitch contraction caused by NMES (muscle contraction) (n = 11).
Animals in whom edema was detected in the hind paw in the immobilization and muscle contraction groups were excluded from the experiment. We analyzed a final total of 29 rats: the control group (n = 10), the immobilization group (n = 8), and the muscle contraction group (n = 11). The rats were housed in individual cages in a temperature-controlled room (22 ± 1 °C) with the lights on for 12 h each day (0700 to 1900) and provided unrestricted access to tap water and food pellets.
Immobilization
The rats in the immobilization and muscle contraction groups were anesthetized with sodium pentobarbital (40 mg/kg), and the bilateral ankle joints were fixed in full plantar flexion using plaster casts for 4 weeks. Surface electrodes (7 mm × 10 mm) were placed on the rats of the muscle contraction group, with electrical leads on the medial and lateral sides of both posterior lower legs. The ankle joints were then fixed using plaster casts. The end of each lead was exposed from the plaster cast to connect to the NMSE device. The plaster cast from above the knee joint to the distal foot was replaced at least every 2 or 3 days to prevent loosening and edema in the hind paw.
Neuromuscular electrical stimulation
Cyclic muscle twitch contraction was performed using a commercial NMSE device (Torio 300; Ito Physiotherapy and Rehabilitation, Tokyo, Japan). The rats in the muscle contraction group were anesthetized, and the electrical stimulator was connected to the electrode leads. The bilateral soleus muscles were subjected to NMES (frequency, 1 Hz; pulse width, 250 μs) and given a stimulus intensity of 4 ± 2 mA with the cast on. In our preliminary experiment, we confirmed soleus muscle contraction by visual examination and by palpating the Achilles tendon with a fingertip with ankle joint movement in the absence of a cast. Electrical stimulation was applied for 60 min once a day, 5 days a week, for 4 weeks.
Measurement of range of motion of ankle joint dorsiflexion
At the end of the immobilization period, the rats were anesthetized with pentobarbital sodium (40 mg/kg). Following a body weight measurement, the range of motion (ROM) of ankle joint dorsiflexion was determined with a goniometer as described in our previous study (Citation7). Briefly, ROM was defined as the angle (0–180°) to the straight line connecting the fifth metatarsal and malleolus lateralis of the fibula to a line connecting the malleolus lateralis of the fibula and the center of the knee joint. After confirming that the minimum tension required to achieve maximum dorsiflexion in the foot joint of control rats was 0.3 N (Citation7), we determined the ROM of ankle joint dorsiflexion when the tension of pressing on the plantar surface with a tension gauge to achieve dorsiflexion reached 0.3 N.
Tissue sampling and preparation
After the anesthetized rat’s ROM was measured, the right soleus muscle was excised and embedded in tragacanth gum. Subsequently, the muscle samples were frozen in isopentane, cooled to the freezing point with liquid nitrogen, and stored in a freezer at −80 °C. Serial frozen cross-sections (7 μm and 10 μm) of the muscle were collected using a cryostat (CM1950; Leica, Wetzlar, Germany) and mounted on glass slides for histological and immunofluorescence staining.
Histological observation and measurement of the muscle fiber cross-sectional area
Some cross-sections of the muscle (7 µm thick) were stained with hematoxylin and eosin. The stained cross-sections were observed under a light microscope, and photos were obtained using a digital camera (DS-Ri1; Nikon, Tokyo) at 200× magnification. The muscle fiber cross-sectional area (CSA) was measured using the Scion Image program for Windows (Scion Image Beta 4.0.3; Scion Corp., Frederick, MD) on ≥100 fibers per muscle.
Immunofluorescence staining
The unstained cross-sections (10 µm thick) were air-dried and fixed in ice-cold acetone. The sections were blocked with 5% bovine albumin in phosphate-buffered saline (PBS; pH 7.4). The sections were then incubated overnight at 4 °C with the mouse monoclonal anti-type I collagen antibodies (1:250 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) or the rabbit polyclonal anti-type III collagen antibodies (1:1000 dilution; LSL; Shiga, Japan). The sections were rinsed in PBS, after which the secondary antibody, fluorescein isothiocyanate (FITC)-conjugated anti-mouse immunoglobulin G (1:50 dilution; Millipore, Bedford, MA) or Texas Red-conjugated anti-rabbit immunoglobulin G (1:1500 dilution; Vector Laboratories, Burlingame, CA) was applied at room temperature.
Measurement of fluorescence intensity
A semiquantitative analysis of types I and III collagen in the perimysium and endomysium was performed as described (Citation7). Images of the stained cross-sections were saved to a computer (200× magnification), and the fluorescence intensity of FITC and Texas Red in the perimysium and endomysium was measured using NIS-Elements imaging software (Nikon). The images of the perimysium or endomysium were trimmed to 150 × 150 pixels. The brightness of each pixel in the image was measured within the range of 0 (minimum) to 255 (maximum). Subsequently, all measurements were summed and then divided by the luminescence area. The data acquired by the above-mentioned method were used to calculate the relative value for the control group, which was adopted as the fluorescence intensity in the perimysium and endomysium.
Real-time reverse transcription polymerase chain reaction
We performed a real-time reverse transcription polymerase chain reaction (RT-PCR) to detect the mRNA expression of HIF-1α, TGF-β1, α-SMA, and types I and III collagen in the left soleus muscle. Total RNA was extracted using the RNeasy Fibrous Tissue Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s protocols. RNA was reverse-transcribed to cDNA using a QuantiTect Reverse Transcription Kit (Qiagen). The mRNA expression levels were determined by a real-time RT-PCR, which was performed in an optical 96-well plate with an Mx3000P and Mx3005P Real-Time QPCR System (Agilent Technologies, Santa Clara, CA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression was used for normalization.
The primers used were as follows: HIF-1α (forward primer, 5ˊ-CGAGCTGCCTCTTCGACAAG-3ˊ; reverse primer, 5ˊ-CCCAGCCGCTGGAGCTA-3ˊ); TGF-β1 (forward primer, 5ˊ-AGAAGTCACCCGCGTGCTAAT-3ˊ; reverse primer, 5ˊ-CACTGCTTCCCGAATGTCTGA-3ˊ); α-SMA (forward primer, 5ˊ-CGGGCTTTGCTGGTGATG-3ˊ; reverse primer, 5ˊ-GGTCAGGATCCCTCTCTTGCT-3ˊ); type I collagen (forward primer, 5ˊ-ATCAGCCCAAACCCCAAGGAG-3ˊ; reverse primer, 5ˊ-GCGTCCTTCCAGTCGACCTATC-3ˊ); type III collagen (forward primer, 5ˊ-TGATGGGATCCAATGAGGGAGA-3ˊ; reverse primer, 5ˊ-CTCAGAGTACCGGAACGCACAAA-3ˊ); and GAPDH (forward primer, 5ˊ-CCATTCTTCCACCTTTGARGCT-3ˊ; reverse primer, 5ˊ-ACAACGACATCGGTATAAGTAACA-3ˊ).
Statistical analysis
All data are presented as mean ± standard deviation (SD). We assessed the differences between groups by performing a one-way analysis of variance (ANOVA), followed by Scheffe’s method. The differences were considered significant at p < 0.05.
Results
The ROM in ankle joint dorsiflexion
The data for the ROM in dorsiflexion in all groups are summarized in . The ROM during dorsiflexion in the immobilization and muscle contraction groups was significantly less than that in the control group. The ROM in the muscle contraction group was significantly higher than that in the immobilization group.
Table 1. ROM of the ankle joint during dorsiflexion and CSA of the myofibers of the soleus muscle.
Histological observations and the muscle fiber CSA
No muscle fiber necrosis or regenerating fibers were seen in the muscles of any group. Atrophic changes in the muscle fibers were observed in the immobilization and muscle contraction groups (). The quantitative analysis revealed that the muscle fiber CSAs of the immobilization and muscle contraction groups were significantly decreased compared to those of the control group. There was no significant difference in the muscle fiber CSAs between the immobilization and muscle contraction groups ().
Figure 1. Histological observation of the soleus muscle stained with hematoxylin and eosin. No muscle fiber necrosis or regenerating fibers were observed in the muscles. In the immobilization and muscle control groups, atrophic changes in the muscle fibers were observed and compared with that of the control group. A: Control group. B: Immobilization group. C: Muscle contraction group. Magnification 100×, scale bar = 100 µm.

Expression of types I and III collagen in immunofluorescence staining
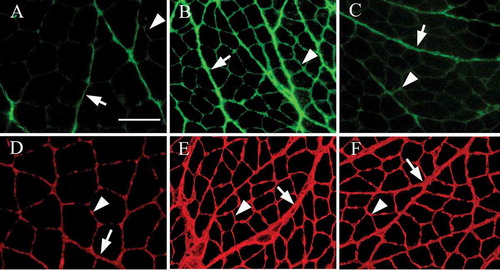
In the immobilization and muscle contraction groups, the immunofluorescence staining of types I and III collagen showed fibrotic changes. In these rats, the expressions of types I and III collagen in the perimysium were markedly stronger and thickened, more notably in the immobilization group than in the control group (,,,). In the endomysium, greater expression of type I collagen was observed in the immobilization group compared to the control group (,). However, in the muscle contraction group, the expression of type I collagen in the endomysium was similar to that in the control group (,).
Figure 2. Fluorescence immunostaining for type I (A–C) and type III (D–F) collagen in the rat soleus muscle. In the perimysium (arrow), the expressions of type I and type III collagen in the immobilization (B,E) and muscle contraction groups (C,F) were markedly stronger and thickened, more notably in the immobilization group, compared with the control group. In the endomysium (arrowhead), a higher expression of type I collagen was obvious in the immobilization group (B) compared to the control group (A). However, in the muscle contraction group (C), the expression of type I collagen in the endomysium was similar to the findings in the control group (A). Magnification 100×, scale bar = 100 µm.
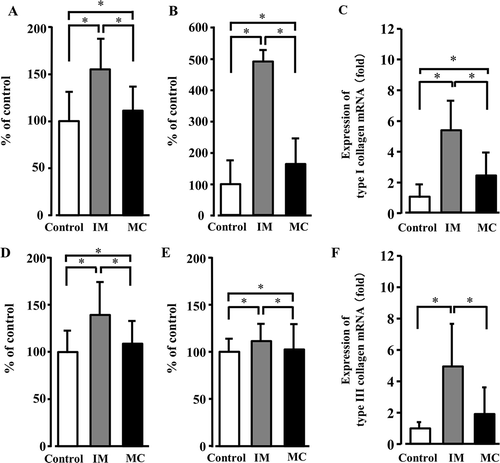
The results of the semiquantitative analysis of the expression levels of types I and III collagen in the perimysium and endomysium are shown in . The fluorescence intensity of types I and III collagen in the perimysium and endomysium in the immobilization and muscle contraction groups was significantly increased compared to the control group (,,,), whereas the fluorescence intensity of both collagen types in the muscle contraction group was significantly decreased compared to that of the immobilization group.
Figure 3. Quantification of type I and type III collagen. The fluorescence intensity of the perimysium and endomysium of type I collagen (A, perimysium; B, endomysium) and type III collagen (D, perimysium; E, endomysium). The fluorescence-intensity levels of types I and III collagen in the perimysium (A,D) and endomysium (B,E) in the immobilization (IM) and muscle contraction (MC) groups were significantly increased compared to those of the control group, whereas the intensity level in the MC group was significantly decreased compared to that of the IM group. The mRNA expression levels of type I collagen (C) and type III collagen (F) in real-time RT-PCR. The expressions of type I and type III collagen mRNA in the IM group were significantly increased compared to the control group values. In the MC group, the mRNA expression level of both types of collagen was significantly decreased compared to those of the IM group. Data are mean ± SD; *p < 0.05.
Expression levels of types I and III collagen mRNA
The expression level of type I collagen mRNA in the immobilization group was significantly increased compared to that in the control group (). In the muscle contraction group, however, the mRNA level was significantly decreased compared to that in the immobilization group. The expression of type III collagen mRNA in the muscle contraction group was significantly decreased compared to that of the immobilization group, whereas no significant difference was observed between the control and muscle contraction groups ().
Expression levels of HIF-1α, TGF-β1, and α-SMA mRNA
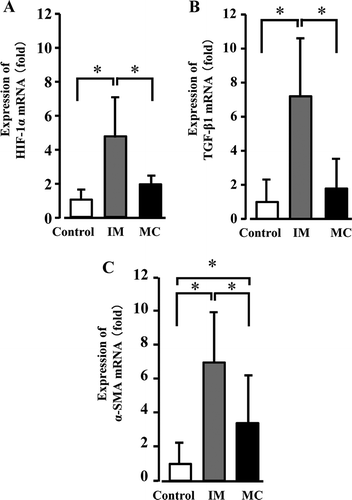
The expression levels of the fibrosis-related genes were detected by a real-time RT-PCR. The expression levels of HIF-1α and TGF-β1 mRNA in the immobilization group were significantly increased compared to those of the control and muscle contraction groups. In contrast, no significant difference was observed between the control and muscle contraction groups (,). The expression levels of α-SMA in the immobilization and muscle contraction groups were significantly increased compared to the control group values. However, in the muscle contraction group, the expression levels of α-SMA were significantly decreased compared to the immobilization group’s levels ().
Figure 4. The expression levels of HIF-1α, TGF-β1, and α-SMA mRNA. The mRNA expression levels of HIF-1α (A) and TGF-β1 (B) in the immobilization (IM) group were significantly increased compared to those in the control and muscle contraction (MC) groups. There were no significant differences between the control and MC groups. The mRNA expression levels of α-SMA (C) in the IM and MC groups were significantly increased compared to the control group values. However, the expression level of mRNA in the MC group was decreased significantly compared to that of the IM group. Data are mean ± SD; *p < 0.05.
Discussion
Our findings demonstrated that the ROM of ankle joint dorsiflexion in the immobilization group was decreased, which is similar to the results of previous studies (Citation7,Citation13). We speculate that muscle contracture occurred and progressed in the soleus muscle of the immobilized rats. In contrast, the ROM in the muscle contraction group was significantly higher than that in the immobilization group, indicating that cyclic muscle twitch contraction caused by NMES decreased the immobilization-induced muscle contracture. The gain in ROM with NMES in the muscle contraction group in this study was approximately 14°, which is small considering the extent of the contracture.
In previous studies, joint contracture progression was inhibited by approximately 50° with stretching (Citation14,Citation15). In contrast, Okita et al. reported that therapeutic ultrasound (frequency, 1 MHz; intensity, 1.0 W/cm2) for the triceps surae muscle during 4 weeks of immobilization significantly inhibited joint contracture deterioration by approximately 15° (Citation16), which is similar to our present result. We attribute these differences between stretching and therapeutic ultrasounds and NMES with a cast to the presence or absence of joint motion. Joint movement occurs when the skeletal muscle is stretched, which affects not only muscle but skin and joint capsule. These tissues are known as limiting factors for immobilization-induced joint contracture (Citation7,Citation17,Citation18); however, there is no joint movement when therapeutic ultrasound and NMES with cast were used. Thus, these therapeutic approaches could not have a significant effect on the skin and joint capsule. We thus believe that the effect of NMES with a cast on immobilization-induced joint contracture in the present study was smaller compared to that of the stretching in the previous studies.
Several previous studies reported that skeletal muscle fibrosis is one of the main pathological changes of extensive muscle decline in immobilization-induced muscle contracture (Citation6,Citation7,Citation19,Citation20). Jozsa et al. (Citation19) reported that 3 weeks of immobilization markedly increased the amount of intramuscular connective tissue and the immunoreactivity of types I and III collagen in the endomysium of rat soleus muscles. These collagen types are the main components of the perimysium and endomysium and are believed to be extensive contributors to skeletal muscle extensibility (Citation21,Citation22).
The increase in the passive muscle stiffness with aging was closely correlated with the increased connective tissue in the endomysium and perimysium as measured in a quantitative histological analysis (Citation23). Our previous investigation revealed that immobilization-induced skeletal muscle fibrosis in the endomysium increased in a time-dependent manner until 4 weeks of immobilization (Citation7). In the present study, a semiquantitative analysis using immunofluorescence staining indicated that the immobilization-induced accumulation of types I and III collagen in the perimysium and endomysium was reduced by cyclic muscle twitch contraction.
This finding is comparable to the results reported by Qin et al. (Citation24), in a study in which it appeared that the contractile activity caused by electric stimulation significantly prevented the increase in intramuscular connective tissue accumulation that is typically induced by immobilization in the shortened position. In addition, our analysis of types I and III collagen mRNA with a real-time RT-PCR showed that the cyclic muscle twitch contraction caused by NMES inhibited the overexpression of the respective collagen genes. These results indicated that cyclic muscle twitch contraction reduces immobilization-induced muscle fibrosis, which may be associated with the inhibition of the progression of muscle contracture. Interestingly, our muscle fiber CSA results showed that the intensity of cyclic muscle twitch contraction produced by NMES could not decrease the disuse-induced soleus muscle fiber atrophy. These results suggest that the level of immobilized-induced muscle fiber atrophy does not affect muscle fibrosis development, at least during the 4 weeks of immobilization. Thus, the inhibitory effect of cyclic muscle twitch contraction produced by NMES on immobilization-induced muscle fibrosis is not a result of inhibiting muscle atrophy. Our molecular biological analysis showed that the increase in TGF-β1, HIF-1α, and α-SMA mRNA levels induced by immobilization was inhibited by cyclic muscle twitch contraction generated by NMES. We have suggested that the upregulation of TGF-β1 induced the overexpression of types I and III collagen by increasing myofibroblast levels after 1 week of the immobilization of the soleus muscle (Citation7). It is well-known that TGF-β1 is a multifunctional growth factor that promotes the production of collagen, which is necessary for normal wound healing (Citation25). However, TGF-β1 overexpression can lead to pathologic tissue fibrosis through the upregulation of α-SMA — a cytoskeletal protein found in myofibroblasts (Citation26) — and type I collagen expression levels (Citation27).
Moderate treadmill exercise in mice attenuated the TGF-β1 protein expression and collagen deposition in the gastrocnemius-soleus muscle induced by a high-fat diet (Citation28). Blaauboer et al. (Citation9) demonstrated that stretching stimulation for human lung fibroblasts inhibited TGF-β1 mRNA expression. Together, the above-mentioned findings may indicate that TGF-β1 mRNA and protein expressions are regulated by mechanical stimulation. Based on the findings of the previous reports and our earlier study, we propose that the decline of TGF-β1 mRNA expression in the muscle contraction group may be associated with the mechanical stimulation of cyclic muscle twitch contraction provoked by NMES, leading to a decrease in skeletal muscle fibrosis with excessive deposition of types I and III collagen after 4 weeks of immobilization . Hypoxia and the consequent marked upregulation of HIF-1α is a well-known promoter of the differentiation of fibroblasts to myofibroblasts as well as a promoter of TGF-β1 in active fibrotic sites, e.g., hepatitis, burn scars, and Dupuytren’s contracture (Citation29). We also reported that the hypoxic condition of the skeletal muscle induced by immobilization promoted the increase in myofibroblasts, which facilitated muscle fibrosis (Citation7). In human subjects, Altintas et al. demonstrated that immobilization of the forearm by splinting for 72 h reduced skin microcirculation (Citation30). We thus speculate that in addition to the mechanical stimulation to the immobilized muscle, an increase in the blood flow in the immobilized muscle is needed to inhibit muscle fibrosis.
A pulsed increase in muscle blood flow was confirmed during cyclic contraction (Citation10–Citation12). Our present results regarding HIF-1α mRNA levels may indicate that the cyclic muscle twitch contraction caused by NMES has a muscle pumping action, causing an increase in blood flow in addition to mechanical stimulation. Misra et al. demonstrated that under hypoxic conditions, the differentiation of fibroblasts to myofibroblasts was promoted along with the increased collagen expression (Citation31). Therefore, the cyclic muscle twitch contraction caused by NMES also has a positive effect by inhibiting the increase in myofibroblasts that followed types I and III collagen overexpression. This study has some limitations. First, the results are limited to an animal model and cannot be generalized to a human model. Second, we were unable to examine the time-course changes in the ROM or in ankle joint dorsiflexion, muscle fibrosis, and fibrosis-related molecules. Additionally, there was no analysis of the protein levels of TGF-β1 and HIF-1α. Further investigations are needed to clarify the inhibitory mechanism of cyclic muscle twitch contraction for immobilized-induced muscle fibrosis. Moreover, we did not confirm the effects on the skin, joint capsule, or ligaments. These soft tissues surrounding the joint also affect the restriction of the ROM. A measurement of muscle stiffness would clarify whether the limitation in the ROM can be attributed to muscle or to other soft tissue surrounding the joint. In conclusion, we examined the effect of cyclic muscle twitch contraction generated by NMES on immobilization-induced muscle contracture and skeletal muscle fibrosis. Our findings indicated that the restriction of the ROM after 4 weeks of immobilization was reduced by the cyclic muscle twitch contraction, which may contribute to the inhibition of skeletal muscle fibrosis by attenuating the upregulation of TGF-β1 and by circumvention of the hypoxic condition. NMES for immobilized skeletal muscle may be used as a therapeutic approach to treating immobilization-induced muscle contractures.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Funding
This work was supported by a JSPS KAKEN Grant-in-Aid for Young Scientists (B) (no. 24700539) from the Ministry of Education, Science, Sports and Culture (MEXT) between 2012 and 2014.
Additional information
Funding
References
- Gillies AR, Lieber RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 2011;44:318–331.
- Williams P, Kyberd P, Simpson H, Kenwright J, Goldspink G. The morphological basis of increased stiffness of rabbit tibialis anterior muscles during surgical limb-lengthening. J Anat 1998;193:131–138.
- Schleip R, Naylor IL, Ursu D, Melzer W, Zorn A, Wilke HJ, Lehmann-Horn F, Klingler W. Passive muscle stiffness may be influenced by active contractility of intramuscular connective tissue. Med Hypotheses 2006;66:66–71.
- Hibino I, Okita M, Inoue T, Banno Y, Hoso M. Effect of immobilization on insoluble collagen concentration and type I and type III collagen isoforms of rat soleus muscle. J Jpn Phys Ther Assoc 2008;11:1–6.
- Jozsa L, Thöring J, Järvinen M, Kannus P, Lehto M, Kvist M. Quantitative alterations in intramuscular connective tissue following immobilization: an experimental study in the rat calf muscles. Exp Mol Pathol. 1988;49:267–278.
- Williams PE, Goldspink G. Connective tissue changes in immobilised muscle. J Anat 1984;138:343–350.
- Honda Y, Sakamoto J, Nakano J, Kataoka H, Sasabe R, Goto K, Tanaka M, Origuchi T, Yoshimura T, Okita M. Upregulation of IL-1β/TGF-β1 and hypoxia relate to molecular mechanisms underlying immobilization-induced muscle contracture. Muscle Nerve 2015;52:419–427.
- Chen YH, Peng YL, Wang Y, Weng Y, Li T, Zhang Y, Chen ZB. TGF-β1-induced synthesis of collagen fibers in skeletal muscle-derived stem cells. J Huazhong Univ Sci Technolog Med Sci 33:238–243.
- Blaauboer ME, Smit TH, Hanemaaijer R, Stoop R, Everts V. Isometric mechanical stretch reduces myofibroblast differentiation of primary lung fibroblasts. Biochem Biophys Res Commun 2011;404:23–27.
- Laughlin MH. Skeletal muscle blood flow capacity: role of muscle pump in exercise hyperemia. Am J Physiol 1987;253:H993–H1004.
- Magder S. Venous mechanics of contracting gastrocnemius muscle and the muscle pump theory. J Appl Physiol 1985;79:1930–1935.
- Sandberg ML, Sandberg MK, Dahl J. Blood flow changes in the trapezius muscle and overlying skin following transcutaneous electrical nerve stimulation. Phys Ther 2007;87:1047–1055.
- Okita M, Yoshimura T, Nakano J, Motomura M, Eguchi K. Effects of reduced joint mobility on sarcomere length, collagen fibril arrangement in the endomysium, and hyaluronan in rat soleus muscle. J Muscle Res Cell Motil 2004;25:159–166.
- Williams PE. Effect of intermittent stretch on immobilised muscle. Ann Rheum Dis 1988;47:1014–1016.
- Williams PE. Use of intermittent stretch in the prevention of serial sarcomere loss in immobilised muscle. Ann Rheum Dis 1990;49:316–317.
- Okita M, Nakano J, Kataoka H, Sakamoto J, Origuchi T, Yoshimura T. Effects of therapeutic ultrasound on joint mobility and collagen fibril arrangement in the endomysium of immobilized rat soleus muscle. Ultrasound Med Biol 2009;35:237–244.
- Chimoto E, Hagiwara Y, Ando A, Itoi E. Progression of an arthrogenic motion restriction after immobilization in a rat experimental knee model. Ups J Med Sci 2007;112:347–355.
- Trudel G, Uhthoff HK. Contractures secondary to immobility: is the restriction articular or muscular? An experimental longitudinal study in the rat knee. Arch Phys Med Rehabil 2000;81:6–13.
- Jozsa L, Thöring J, Järvinen M, Kannus P, Lehto M, Kvist M. Quantitative alterations in intramuscular connective tissue following immobilization: an experimental study in the rat calf muscles. Exp Mol Pathol 1988;49:267–278.
- Järvinen TA, Józsa L, Kannus P, Järvinen TL, Järvinen M. Organization and distribution of intramuscular connective tissue in normal and immobilized skeletal muscles. An immunohistochemical, polarization and scanning electron microscopic study. J Muscle Res Cell Motil 2002;23:245–254.
- Tabary JC, Tabary C, Tardieu C, Tardieu G, Goldspink G. Physiological and structural changes in the cat’s soleus muscle due to immobilization at different lengths by plaster casts. J Physiol 1972;224:231–244.
- Witzmann FA, Kim DH, Fitts RH. Hindlimb immobilization: length-tension and contractile properties of skeletal muscle. J Appl Physiol Respir Environ Exerc Physiol 1982;53:335–345.
- Alnaqeeb MA, Al Zaid NS, Goldspink G. Connective tissue changes and physical properties of developing and ageing skeletal muscle. J Anat 1984;139:677–689.
- Qin L, Appell HJ, Chan KM, Maffulli N. Electrical stimulation prevents immobilization atrophy in skeletal muscle of rabbits. Arch Phys Med Rehabil 1997;78:512–517.
- Quaglino D Jr, Nanney LB, Ditesheim JA, Davidson JM. Transforming growth factor-beta stimulates wound healing and modulates extracellular matrix gene expression in pig skin: incisional wound model. J Invest Dermatol 1991;97:34–42.
- Hu B, Wu Z, Phan SH. Smad3 mediates transforming growth factor-beta-induced alpha-smooth muscle actin expression. Am J Respir Cell Mol Biol 2003;29:397–404.
- Reed MJ, Vernon RB, Abrass IB, Sage EH. TGF-beta 1 induces the expression of type I collagen and SPARC, and enhances contraction of collagen gels, by fibroblasts from young and aged donors. J Cell Physiol 1994;158:169–179.
- Pincu Y, Linden MA, Zou K, Baynard T, Boppart MD. The effects of high fat diet and moderate exercise on TGFβ1 and collagen deposition in mouse skeletal muscle. Cytokine 2015;73:23–29.
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 2002;3:349–363.
- Altintas AA, Vogt PM, Altintas MA. Acute effects of splint immobilization of the forearm on in vivo microcirculation and histomorphology of the human skin. Microsc Res Tech 2014;77:99–103.
- Misra S, Fu AA, Misra KD, Shergill UM, Leof EB, Mukhopadhyay D. Hypoxia-induced phenotypic switch of fibroblasts to myofibroblasts through a matrix metalloproteinase 2/tissue inhibitor of metalloproteinase-mediated pathway: implications for venous neointimal hyperplasia in hemodialysis access. J Vasc Interv Radiol 2010;21:896–902.
