Abstract
Objective: To evaluate in a longitudinal study the influence of airway hyperresponsiveness (AHR) on lung function in patients with primary Sjögren’s syndrome (pSS).
Methods: Lung function was studied over an eight-year period in 15 patients who fulfilled the Copenhagen criteria for primary Sjögren’s syndrome and who were covered in our earlier published study on AHR in patients with Sjögren’s syndrome. Standard spirometry and measurements of lung volumes, diffusing capacity (DLCO), and AHR to methacholine were performed.
Results: A significant decline over time was found in total lung capacity (TLC), vital capacity (VC), forced vital capacity (FVC), functional residual capacity (FRC), and expiratory midflows (FEF50). A sign of small airway obstruction (decrease in FEF50) at entry correlated with VC at follow-up (r = .8, P < .003), and the individual change in FEF50 during the observation period correlated with the individual change in VC (r = .6, P < .05). Six patients had increased AHR, and three of them had decreased DLCO. Six of the patients progressively reduced DLCO over time, and five of them had spirometric signs of increased small airway obstruction.
Conclusions: During this eight-year follow-up we observed that one-third of the patients with pSS developed a significant reduction in lung function. Our findings suggest that small airways obstruction and AHR are associated with reduction of VC and development of impaired DLCO as a sign of interstitial lung disease in this group of patients.
Introduction
Primary Sjögren’s syndrome (pSS) is a chronic autoimmune inflammatory disease that mainly affects exocrine glands of the mucous membranes (Citation1). In addition, a wide spectrum of extraglandular symptoms may occur, e.g. from the respiratory system (Citation2–6). A significant number of patients with pSS have symptoms of xerotracheitis, characterized by a chronic, dry, non-productive cough and dyspnoea. These symptoms have been attributed to dryness in the large airways caused by dysfunction in the tracheal glands due to lymphocyte infiltration (Citation7).
These xerotracheitis symptoms are similar to the symptoms of airway hyperresponsiveness (AHR). In fact, previous studies have demonstrated that the majority of patients with pSS suffer from hyperresponsive airways (Citation8,Citation9). Furthermore, airflow obstruction has been reported in up to 12% of this group of patients (Citation10). It is important to distinguish between bronchial asthma and respiratory symptoms due to pSS, as the Sjögren’s patients may run the risk of developing interstitial pneumonitis and small airway disease, with irreversible lung dysfunction as a possible end result (Citation4).
Few longitudinal studies have been undertaken concerning lung function in patients with pSS (Citation4,Citation11,Citation12), and none of these studies have focused on AHR. Therefore, we have studied whether signs of peripheral or central airflow limitation and AHR are related to changes in lung function over time in this eight-year follow-up study of a group of patients with Sjögren’s syndrome.
Patients and methods
We have previously reported AHR in a group of patients with pSS (Citation8). Now, eight years later, we have been able to re-evaluate 15 of the 21 previously studied patients. Of the six patients that did not participate at follow-up study, three died, two of myocardial infarction and one of drug intoxication; two were unable to participate for geographical reasons; and one was classified as having systemic lupus erythematosus. Thus, 14 women and 1 man, with a mean age of 60 years (range 46–78) at follow-up, were included in this study. Their disease duration at baseline was 9–15 years (mean 13), with the diagnosis based on the Copenhagen criteria as in the previous study (Citation13). However, all patients who participated at follow-up fulfilled also the classification criteria from 2002 (Citation14).
Four out of 15 patients (26%) had glandular symptoms only, whereas 11 patients (73%) had extraglandular manifestations: Raynaud’s phenomenon (n = 9), non-erosive arthritis (n = 5), sun sensitivity, pancreatic insufficiency, Waldenström’s macroglobulinemia, and one patient had developed non-Hodgkin’s lymphoma. Eleven patients (73%) had pulmonary symptoms: dry cough (n = 7), asthma-like symptoms (n = 2), and exertional dyspnoea (n = 3). All but one were non-smokers, and none had a history of allergy disorders. Ten patients had a positive ANA, and five had SSA or SSB.
At baseline six patients were treated with glucocorticoids (mean dose: prednisolone 5 mg/day), and three of them were also treated with azathioprine and two with chloroquine. At the follow-up an additional patient was treated with glucocorticoids in combination with chloroquine. None of the subjects was medicated with inhaled corticosteroids.
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee at the Medical Faculty at Uppsala University. Informed consent was obtained prior to the study.
Lung function measurements
All subjects underwent lung function measurements according to American Thoracic Society (ATS) standards (Citation15), which included measurements of total lung capacity (TLC), functional residual capacity (FRC), and residual volume (RV). Vital capacity (VC), forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), flow volume registrations with maximum expiratory flow (MEF), and flows measured at 50% (FEF50) and 25% (FEF25) of FVC were measured with a Masterlab body plethysmograph, and diffusion capacity for CO (DLCO) was measured using the Masterlab Transfer test (Eric Jaeger AB, Würtsburg, Germany). Lung function values are presented as a percentage of reference values according to gender, age, and body size (Citation16,Citation17), and values less than 80% of predicted value were considered below normal values.
Methacholine challenges
The methacholine test was modified from Hargreave’s method (Citation18). A hand-held DeVilbiss 646 nebulizer was used. After an initial test with saline, the patients were tested with double dilutions of methacholine, at 3-min intervals, starting with 1.2 mg/mL up to a maximum dose of 20 mg/mL. The subject inhaled for 2 min, actuating the nebulizer during each inhalation. The nebulizer was weighed before and after each concentration, and the consumed dose was calculated. The inhalation was discontinued when there was a fall in the FEV1 of 20% or more below the lowest post-saline value. Test results were expressed as the provocation dose that caused a fall in FEV1 of 20% (PD20). Degree of AHR was divided into categories based on the PD20 value: severe (<0.125 mg methacholine), moderate (0.125–1.29), mild (1.3–5.0), and slight (5.1–9.0).
Thirteen of 15 patients underwent the methacholine challenge test; one had a FEV1 of less than 1.0 L/min, and the other was not able to participate in this part of the study. Both these patients demonstrated AHR at baseline.
In addition to PD20, we calculated the least-squares slope for methacholine from the regression equation for the percentage decline in FEV1 on a cumulative dose of methacholine using all the measured points (Citation18).
Statistics
The paired t test was used to assess changes in lung function and airway responsiveness over time. In comparisons within a group, the Spearman rank correlation test was used. All calculations were done in StatView for Macintosh. A P value of <.05 was regarded as statistically significant.
Results
The individual data on FEF50, DLCO, and the response to methacholine (PD20) at baseline and at follow-up are presented in . The results for the whole group illustrate significant decreases in several lung volumes: TLC, VC, FVC, and FRC (). The patients also had a decrease (P < .05) in peripheral airflow (FEF50), whereas the measurements of central airway obstruction (MEF) did not change during the study period.
Table 1. Results of FEF50, DLCO, and methacholine challenge test at baseline and follow-up in 15 patients with primary Sjögren’s syndrome.
Table 2. Comparison of lung function tests in patients with primary Sjögren’s syndrome, values as a percentage of predicted values according to actual age and body size at baseline and at the eight-year follow-up (mean ± SD).
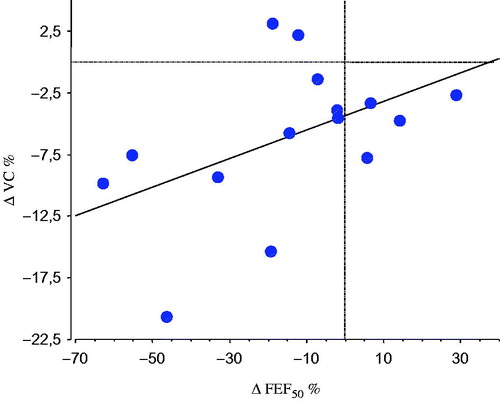
When evaluating the airway obstruction over time, we found that FEF50 at baseline was correlated with VC at follow-up (r = .8, P < .003), and there was also an association between the FEF50 at baseline and the change (Δ) in VC over time (). The individual change in FEF50 during the observation period correlated with the individual change in VC (r = .6, P < .05) ().
Figure 1. Linear correlation between individual percentage change (Δ) in VC at follow-up and FEF50 at baseline in 15 patients with primary Sjögren’s syndrome.
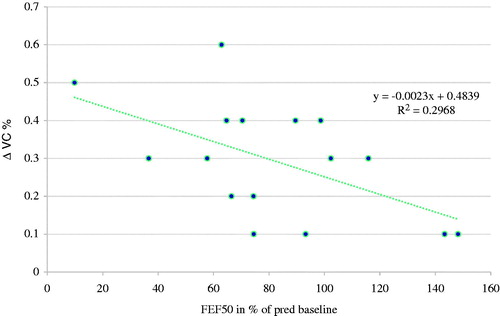
Figure 2. Linear correlation between the individual percentage change (Δ) in FEF50 and VC during the observation period in 15 patients with primary Sjögren’s syndrome (r = .6, P < .05).
Seven of 13 (54%) patients with pSS were hyperresponsive to methacholine at baseline, while 6 (46%) were hyperresponsive at follow-up. In the patients with AHR at follow-up, the decrease in DLCO was related to the dose–response slope for methacholine (r = .9, P < .05). Six (46%) patients had increased AHR over time, and three of them had a reduction in DLCO. Two patients who were AHR-positive to methacholine in the previous evaluation did not demonstrate increased responsiveness to methacholine at follow-up. One of these two patients received no medical treatment, whereas the other started treatment with glucocorticosteroids and hydroxychloroquine during the study period. One patient developed AHR during the study period.
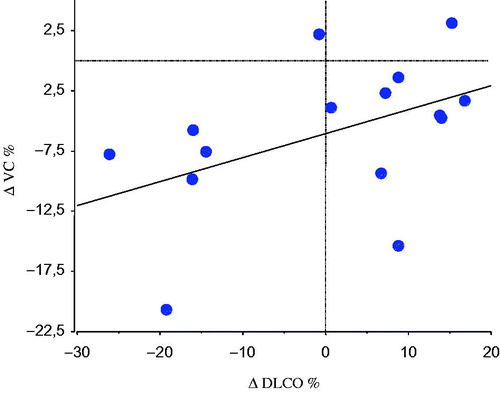
The DLCO did not decline in the group during the study period, but three patients who had decreased DLCO at baseline and had AHR progressed in terms of DLCO impairment. Individual changes in DLCO correlated with individual changes in VC (r = .6, P < .05) (). Moreover, we observed that five of six patients whose DLCO decreased progressively during the period also had progression of peripheral airflow limitation. DLCO corrected for alveolar volume did not change, so the decrease in DLCO could not be explained by decreased alveolar volume.
Figure 3. Linear correlation between the individual percentage change (Δ) in DLCO and VC during the observation period in 15 patients with primary Sjögren’s syndrome (r = .6, P < .05).
The pulmonary compliance (Cst) was significantly lower at follow-up compared to baseline (P < .05).
Discussion
In the present eight-year follow-up study of patients with primary Sjögren’s syndrome (pSS), signs of increased airflow limitation were found during the observation period. Furthermore, the degree of FEF50 at entry and during the observation period was significantly related to the decrease in VC over time. These findings suggest that small airway disease may be an important pathophysiological mechanism in lung involvement in patients with pSS.
The few longitudinal studies which have examined the progression of lung involvement in pSS have shown a minimal deterioration in respiratory function over time (Citation11,Citation12). However, a reduction in the end-expiratory flow in pSS may not always be a permanent sign, but may vary over time. Evidence of obstructive lung disease has been demonstrated by spirometry findings in 20%–50% of patients with pSS (Citation3,Citation10), and HRCT studies have reported bronchiolectasis and bronchiolar and bronchial thickening (Citation19).
AHR is a characteristic finding in bronchial asthma and has been related to bronchial inflammation and oedema of the airway walls (Citation20). Inhaled glucocorticoids decrease AHR in bronchial asthma (Citation21), but seem to have little significant effect on AHR in pSS (Citation22), a finding that further supports the conclusion that there are differences in underlying mechanisms for bronchoconstriction in these diseases. Furthermore, it has been demonstrated using different provocation agents that patients with pSS display different bronchial responsiveness profiles compared to patients with asthma who often show positive response to more than one type of provocation, while patients with pSS more often are only positive to methacholine provocations (Citation23). It is possible that the AHR in pSS may be attributed to an inflammatory process located in the smaller airways. In our study the airway hyperresponsiveness in patients with pSS was associated with a decline in DLCO, a finding which favours the hypothesis that inflammation in the small airways may underlie AHR. The dryness of the bronchial mucosa in pSS may also make the epithelium more vulnerable to damage and probably induces a hyperosmolar stage, thereby predisposing to airway remodelling and AHR. Increased concentration of nitric oxide in the expired air of patients with pSS further provides evidence of a chronic inflammatory process in the airway epithelium in patients with pSS (Citation24).
The clinical significance of decrease in pulmonary compliance in the present study is unknown. However, increased age of the study subjects at follow-up may possibly play a role. In contrast, increased pulmonary compliance has been associated with pulmonary fibrosis, which we did not see in our study group.
Airway disease in pSS appears to be produced both by glandular and non-glandular pathology. The main histological lesion in Sjögren’s syndrome consists of focal lymphocytic infiltrates of exocrine glands (Citation25). This may result in dysfunction of the airway glands, which may give rise to dry and irritating cough – the xerotracheitis symptoms. Extraglandular lymphocytic infiltration of the respiratory system has also been reported in pSS. Endobronchial biopsies have detected follicular lymphocytic bronchiolitis (Citation5,Citation26,Citation27), while bronchoalveolar lavage (BAL) studies reported subclinical alveolitis (Citation28,Citation29), characterized also by lymphocytes. In another study an increased number of alveolar neutrophils were associated with a reduction in DLCO, and abnormal findings on chest HRCTs were found (Citation30). However, our previous study did not show an increased number of inflammatory cells or pro-inflammatory cytokines in the BAL fluid from patients with pSS compared to healthy controls (Citation31). Controversial BAL findings have therefore been reported in pSS. However, several investigators have questioned the meaning of BAL fluid lymphocytosis, as it may not always represent alveolitis but rather reflect bronchial lymphocytic infiltration (Citation7).
Our study has several limitations. Firstly, it is a small study, and although these patients have been reported previously, this group is of interest in context of follow-up. Secondly, results from HRCT would have strengthened our results. Furthermore, our patient group is initially a consecutive series of patients from a specialist clinic. However, we were not able totally to control the care of the patients. We used FEF50 as an indicator of small airway obstruction, but FEF25–75 might have been more suitable. In spite of these limitations, the result of our study is of interest for those clinicians who are caring for patients with pSS who frequently have pulmonary symptoms.
In summary, the present study further supports that patients with pSS may develop AHR and signs of small airways disease. Spirometry, bronchial challenge tests, and DLCO measurements may be of value to identify patients at risk of developing lung disease associated with Sjögren’s syndrome. Further studies are needed on this issue.
Acknowledgements
We thank Ulrike Spetz-Nyström, Monika Hall, and all the staff at the Department of Clinical Physiology, Uppsala University Hospital, for their technical assistance.
Disclosure statement
The authors report no conflicts of interest.
Funding
This work was supported by grants from the Swedish National Heart and Lung Fund.
References
- Moutsopoulos H, Chused T, Mann D, Klippel J, Fauci A, Frank M, et al. Sjögren’s syndrome (sicca syndrome): current issues. Ann Intern Med. 1980;92:212–26.
- Vitali C, Tavoni A, Viegi G, Begliomini E, Agnes A, Bombardier S. Lung involvement in Sjögren’s syndrome: a comparison between patients with primary and with secondary syndrome. Ann Rheum Dis. 1985;44:455–61.
- Lahdensuo A, Korpela M. Pulmonary findings in patients with primary Sjögren’s syndrome. Chest. 1995;108:316–19.
- Kelly C, Gardiner P, Pal B, Griffiths I. Lung function in primary Sjögren’s syndrome: a cross sectional and longitudinal study. Thorax. 1991;46:180–3.
- Gardiner P, Ward C, Allison A, Ashcroft T, Simpson W, Walters H, et al. Pleuropulmonary abnormalities in primary Sjögren’s syndrome. J. Rheumatol. 1993;20:831–7.
- Contantopoulos S, Papadimitriou C, Moutsopoulos H. Respiratory manifestations in primary Sjögren’s syndrome. Chest. 1985;88:226–9.
- Flament T, Bigot A, Chaigne B, Henique H, Diot E, Marchand-Adam S. Pulmonary manifestations of Sjögren’s syndrome. Eur Respir Rev. 2016;25:110–23.
- Gudbjörnsson B, Hedenström H, Stålenheim G, Hällgren R. Bronchial hyperresponiveness to methacholine in patients with primary Sjögren’s syndrome. Ann Rheum Dis. 1991;50:36–40.
- Potena A, La Corte R, Fabbri L, Papi A, Trotta F, Ciaccia A. Increased bronchial responsiveness in primary and secondary Sjogren’s syndrome. Eur Respir J. 1990;3:548–53.
- Newball H, Brahim S. Chronic obstructive airway disease in patients with primary Sjögren’s syndrome. Am Rev Resp Dis. 1977;115:295–304.
- Linstow M, Kriegbaum N, Backer V, Ulrik C, Oxholm P. A follow-up study of pulmonary function in patients with primary Sjögren’s syndrome. Rheumatol Int. 1990;10:47–9.
- Davidson B, Kelly C, Griffiths I. Ten year follow up of pulmonary function in patients with primary Sjogren’s syndrome. Ann Rheum Dis. 2000;59:709–12.
- Manthorpe R, Oxholm P, Prause J, Schiodt M. The Copenhagen criteria for Sjögren’s syndrome. Scand J Rheumatol. 1986;61(suppl):19–21.
- Goules AV, Tzioufas AG, Moutsopoulos HM. Classification criteria of Sjögren’s syndrome. J Autoimmun. 2014;48–9:42–5.
- American Thoracic Society. Standardization of spirometry 1994 update. Am J Respir Crit Care Med. 1995;152:1107–36.
- Hedenström H, Malmberg P, Agarwai K. Reference values for lung function tests in females: regression equations with smoking variables. Bull Eur Physiopathol Respir. 1985;21:551–7.
- Hedenström H, Malmberg P, Fridriksson H. Reference values for lung function tests in men: regression equations with smoking variables. Ups J Med Sci. 1986;91:299–310.
- Hargreave F, Ryan G, Thomson N, O’Byrne P, Latimer K, Juniper E. Bronchial responsiveness to histamine or methacholine in asthma: measurements and clinical significance. Eur J Resp Dis. 1982;63(suppl 121):79–88.
- Taouli B, Brauner MW, Mourey I, Lemouchi D, Grenier PA. Thin-section chest CT findings of primary Sjögren’s syndrome: correlation with pulmonary function. Eur Radiol. 2002;12:1504–11.
- Oddera S, Silvestri M, Balbo A, Jovovich B, Penna R, Crimi E, et al. Airway eosinophilic inflammation, epithelial damage, and bronchial hyperresponsiveness in patients with mild-moderate, stable asthma. Allergy. 1996;51:100–7.
- Lim S, Jatakanon A, John M, Gilbey T, O’Connor B, Chung K, et al. Effect of inhaled budesonide on lung function and airway inflammation. Am J Respir Crit Care Med. 1999;159:22–30.
- Stålenheim G, Gudbjörnsson B. Anti-inflammatory drugs do not alleviate bronchial hyperreactivity in Sjögren’s syndrome. Allergy. 1997;52:423–7.
- Lúdvíksdóttir D, Janson C, Björnsson E, Stålenheim G, Boman G, Hedenström D, et al. Different airway responsiveness profiles in atopic asthma, nonatopic asthma and Sjögren’s syndrome. BHR study group. Allergy. 2000;55:259–65.
- Lúdvíksdóttir D, Jansson C, Högman M, Gudbjörnsson B, Björnsson E, Valtýsdóttir ST, et al. Increased nitric oxide in expired air in patients with Sjögren’s syndrome. Eur Respir J. 1999;13:739–43.
- Moutsopoulos H. Sjögren’s syndrome: autoimmune epithelitis. Clin Immunol Immunopathol. 1994;72:162–5.
- Papiris S, Maniati M, Contantopoulos S, Roussos C, Moutsopoulos H, Skopouli F. Lung involvement in primary Sjögren’s syndrome is mainly related to the small airway disease. Ann Rheum Dis. 1999;58:61–4.
- Deheinzelin D, Capelozzi V, Kairalla R, Filho J, Saldiva P, de Carvalho C. Interstitial lung disease in primary Sjögren’s syndrome. Am J respir Crit Care Med. 1996;154:794–9.
- Hatron P, Wallaert B, Gosset D, Tonnel A, Voisin C. Subclinical lung inflammation in primary Sjögren’s syndrome: relationship between bronchoalveolar lavage cellular analysis findings and characteristics of the disease. Arthritis Rheum. 1987;30:1226–31.
- Dalavanga Y, Constantopoulos S, Galanopoulo V, Zerva L, Moutsopoulos H. Alveolitis correlates with clinical pulmonary involvement in primary Sjögren’s syndrome. Chest. 1991;99:1394–7.
- Salaffi F, Manganelli P, Carotti M, Baldelli S, Blasetti P, Subiaco S, et al. A longitudinal study of pulmonary involvement in primary Sjögren’s syndrome: relationship between alveolitis and subsequent lung changes on high-resolution computed tomography. Br J Rheumatol. 1998;37:263–9.
- Gudbjörnsson B, Hällgren R, Nettebladt O, Gustafsson R, Mattson A, af Geijerstam E, et al. Phenotypic and functional activation of alveolar macrophages, T-lymphocytes and NK cells in patients with systemic sclerosis and primary Sjögren’s syndrome. Ann Rheum Dis. 1994;53:574–9.
