Abstract
Purpose: A clinical feature of myxofibrosarcoma is local recurrence, but knowledge about distant metastasis is sparse. We evaluated the tendency of clinical and histological features of metastasis in myxofibrosarcoma patients.
Methods: Fifty-eight patients with myxofibrosarcoma were treated in our hospitals, and a total of 16 consecutive patients with distant metastases were included in this retrospective study (9 males and 7 females, with a mean age of 77 years). Because there was no patient complicated by both lung and lymph node metastases, we compared the age, sex, tumor size and location, French Federation of Cancer Centers Sarcoma Group (FNCLCC) grade, American Joint Committee on Cancer (AJCC) stage, and times of the first metastasis from the initial examination between the lung and lymph node groups. In addition, we examined factors affecting the prognosis.
Results: The median follow-up period was 42.9 months (range 8–142). Eleven of 16 patients developed pulmonary metastases. The sites of extra pulmonary metastases were the lymph nodes in 5 patients, bone in 1, subcutaneous in 1, intramuscular in 1, and peritoneum in 1. The median time for patients to develop distant metastases was 17.4 months (range 0–59). The time until the onset of the first metastasis in the lung metastasis group was significantly shorter than in the lymph node group (p < 0.05). Also, the survival rate in the lymph node metastasis group was better than in the lung metastasis group (p < 0.05).
Conclusions: Not only lung metastasis but also lymph node metastasis occurs frequently in myxofibrosarcoma patients. Myxofibrosarcoma with lung metastasis is more aggressive than the type with lymph node metastasis.
Keywords:
Introduction
Myxofibrosarcoma is one of the most common soft-tissue sarcomas found in the extremities of older adults and accounts for approximately 20% of all soft-tissue sarcomas (Citation1). A clinical feature of myxofibrosarcoma is local recurrence. Complete surgical resection is the standard treatment, but negative margins are difficult to obtain because myxofibrosarcoma has an unusual infiltrative growth pattern along fascial planes (Citation2,Citation3). Therefore, the outcomes are characterized by a high local recurrence rate, from 15% to 57%, leading to poor overall survival (Citation4–11). Some factors of local recurrence, such as resection with positive or close margins, bone and joint involvement, and unplanned resection, have been examined in other studies (Citation4–6,Citation10,Citation11). However, there has been no systematic report on metastasis despite the many reports on local recurrence.
The aim of this study was to analyze the tendency of clinical and histological variables of metastasis in myxofibrosarcoma patients. In addition, we compared the clinical features and prognosis between patients with pulmonary metastasis and lymph node metastasis.
Patients and methods
Subjects
Fifty-eight patients with myxofibrosarcoma were treated in our two hospitals between 1992 and 2014 (28 males and 30 females, with a mean age of 70 years). In these patients, a total of 16 consecutive patients (27.6%) had distant metastases, and they were included in this retrospective study. Information on the patients including age, sex, and anatomical location and size of the tumor, is presented in . The stage of the primary tumor was determined according to the staging system of the American Joint Committee on Cancer (AJCC), 6th edition (Citation12). The specimens were assigned to the French Federation of Cancer Centers Sarcoma Group (FNCLCC) classification. This classification is based on the mitotic index, necrosis extension, and histological differentiation of the tumor (Citation13). In the absence of any events, the date of the last follow-up was considered as an end-point. In our series, there was no patient complicated by both lung and lymph node metastases, and we compared the age, sex, tumor size and location, FNCLCC grade, AJCC stage, and time until the first metastasis after the initial examination between the lung and lymph node groups. In addition, we examined factors affecting the prognosis. The study was approved by the Institutional Review Board for Clinical Research at our university, and informed consent was obtained from all patients enrolled in the study.
Table 1. Clinical information of 16 patients with myxofibrosarcoma.
Statistical analysis
All values are expressed as the mean ± standard deviation (SD). Student’s t test, the Welch t test, and chi-square test were used to compare the items between the two groups. The curve for overall survival was drawn according to the Kaplan–Meier method, and differences were analyzed by applying the generalized Wilcoxon test. Probability (p) values less than 0.05 were considered significant.
Results
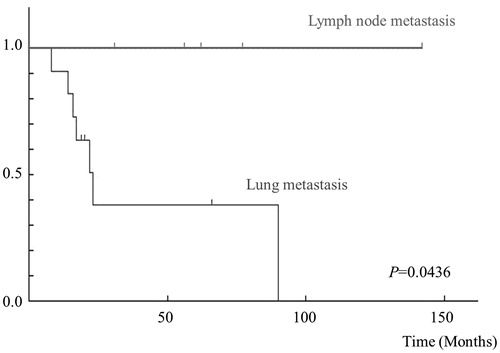
In the 16 patients with distant metastases, there were 9 males and 7 females, with a mean age of 77 years (range 46–89). The median follow-up period was 42.9 months (range 8–142). Five tumors were located in the upper extremities, 6 in the lower extremities, and 5 in the trunk. The mean radiological size of the tumors was 8.2 cm (range 1.5–22). A high FNCLCC grade (2 or 3) was observed in 9 (56%) patients, and a high AJCC stage (3 or 4) was observed in 4 (25%) patients. Surgery for the primary tumor was conducted in 13 patients, and an adequate tumor-free margin was obtained in 10 (76.9%). Three patients refused surgical treatment. Radiotherapy for the primary tumor was used for 8 (50%) patients. Eleven of the 16 metastasis patients (68.8% of the metastasis patients, and 17.2% of all 58 myxofibrosarcoma patients) developed pulmonary metastases. The sites of extra-pulmonary metastases were the lymph nodes in 5 of the 16 patients (31.3% of the metastasis patients, and 8.6% of all patients), bone in 1 patient, subcutaneous in 1 patient, intramuscular in 1 patient, and peritoneum in 1 patient. The median time for patients to develop distant metastases was 17.4 months (range 0–59). Treatments for metastases were surgery for 4 patients, chemotherapy for 4 patients, and radiotherapy for 5 patients. The outcome for the 16 patients was that there was no evidence of disease (NED) in 6 patients, 3 patients were alive with disease (AWD), and 7 patients died of their disease (DOD) (). In univariate analysis, the time until the first metastasis after the initial examination in the lung metastasis group (10.3 ± 5.0 months) was significantly shorter than in the lymph node group (32.8 ± 17.7 months) (p < 0.05) (). Moreover, the 5-year survival rate of the lung metastasis group was 25%, while that of the lymph node group was 100% (p < 0.05) ().
Table 2. Univariate logistic regression analysis between the two groups.
Discussion
Various outcomes regarding distant metastasis in myxofibrosarcoma patients have been reported with rates of metastasis from 9.5% to 23.6% (Citation4–11). In our study, the incidence of metastasis was quite high (27.6%). The lung has generally been thought to be the organ most frequently involved in the metastasis of soft tissue sarcoma, and certainly the lung was the most frequently involved organ in our study as well. However, lymph node metastasis was also relatively frequent in this study (8.6% of all patients). Although lymph node metastasis generally occurs in cancer, it is rare in sarcoma, affecting from 3% to 4% of all patients (Citation14–16). A much higher propensity for lymphatic spread has been described for certain histological subtypes, including clear cell sarcoma (25% to 50%), epithelioid sarcoma (20% to 29%), synovial sarcoma (10% to 19%), and rhabdomyosarcoma (8% to 15%) (Citation17–19). There have been few reports mentioning lymph node metastasis of myxofibrosarcoma. A review of several reports revealed an incidence of only 3% (Citation4,Citation8,Citation11). As the numbers of lymph node metastasis cases in each report are very small, detailed analyses of lymph node metastasis are also very rare. In the current series of a study involving our two institutions, we found that the incidence of lymph node metastasis was higher than that in previous reports.
Because there was no patient suffering from both lung and lymph node metastases in our series, we compared evaluation items between the two groups. The time until the first metastasis after the initial examination was significantly different between the two groups, and the onset of lymph node metastasis was later than that of lung metastasis. There have been few reports on metastasis onset. Sanfilippo et al. showed that the average time until metastasis onset including all organs was 11 months (Citation4), and Haglund et al. stated that the average time until metastasis onset not including lymph nodes was 21 months (Citation8). In the present study, the average time until the first metastasis onset was 32.8 months and was delayed compared with previous reports. However, this difference in the timing of onset between lung and lymph node metastases was not obvious. It is therefore necessary to conduct further studies to clarify this.
Distant metastasis-free survival of myxofibrosarcoma patients was reported to be associated with mitotic activity and the margin status (Citation20). Another study demonstrated an association between the presence of necrosis within the whole tumor and the development of distant metastasis (Citation21). Sanfilippo et al. suggested that the histological grade of the tumor also predicted the risk of metastasis (Citation4). In the current study, we failed to show a significant difference of such factors between lung and lymph node metastases. However, the survival rate of the lymph node metastasis group was better than that of the lung metastasis group, although only two of five lymph node metastases were surgically treated.
A limitation of this study was the small number of myxofibrosarcoma patients with distant metastasis. We could not analyze our data by multivariate analysis because of the small numbers, although various factors might lead to a bias. Myxofibrosarcoma is a relatively rare soft tissue sarcoma, and distant metastasis is even rarer. We need to perform further detailed studies with a larger number of patients with distant metastasis of myxofibrosarcoma.
In conclusion, the present study shows that not only lung metastasis but also lymph node metastasis occurs frequently in patients with myxofibrosarcoma. In addition, aggressive treatment for lymph node metastasis led to a satisfactory management of such patients. Further, detailed studies on the pathogenesis of remote metastasis of myxofibrosarcoma are highly desirable.
Disclosure statement
The authors report no conflicts of interest.
References
- Mentzel T, Hogendoorn PCW, Huang HY. Myxofibrosarcoma. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, editors. WHO classification of tumours of soft tissue and bone. Lyon: IARC Press; 2013. p. 93–4.
- Waters B, Panicek DM, Lefkowitz RA, Antonescu CR, Healey JH, Athanasian EA, et al. Low-grade myxofibrosarcoma: CT and MRI patterns in recurrent disease. AJR Am J Roentgenol. 2007;188:193–8.
- Manoso MW, Pratt J, Healey JH, Boland PJ, Athanasian EA. Infiltrative MRI pattern and incomplete initial surgery compromise local control of myxofibrosarcoma. Clin Orthop Relat Res. 2006;450:89–94.
- Sanfilippo R, Miceli R, Grosso F, Fiore M, Puma E, Pennacchioli E, et al. Myxofibrosarcoma: prognostic factors and survival in a series of patients treated at a single institution. Ann Surg Oncol. 2011;18:720–5.
- Kaya M, Wada T, Nagoya S, Yamashita T. Bone and/or joint attachment is a risk factor for local recurrence of myxofibrosarcoma. J Orthop Sci. 2011;16:413–17.
- Dewan V, Darbyshire A, Sumathi V, Jeys L, Grimer R. Prognostic and survival factors in myxofibrosarcomas. Sarcoma. 2012;2012:830879.
- Mutter RW, Singer S, Zhang Z, Brennan MF, Alektiar KM. The enigma of myxofibrosarcoma of the extremity. Cancer. 2012;118:518–27.
- Haglund KE, Raut CP, Nascimento AF, Wang Q, George S, Baldini EH. Recurrence patterns and survival for patients with intermediate- and high-grade myxofibrosarcoma. Int J Radiat Oncol Biol Phys. 2012;82:361–7.
- Riouallon G, Larousserie F, Pluot E, Anract P. Superficial myxofibrosarcoma: assessment of recurrence risk according to the surgical margin following resection. A series of 21 patients. Orthop Traumatol Surg Res. 2013;99:473–7.
- Look Hong NJ, Hornicek FJ, Raskin KA, Yoon SS, Szymonifka J, Yeap B, et al. Prognostic factors and outcomes of patients with myxofibrosarcoma. Ann Surg Oncol. 2013;20:80–6.
- Kikuta K, Kubota D, Yoshida A, Suzuki Y, Morioka H, Toyama Y, et al. An analysis of factors related to recurrence of myxofibrosarcoma. Jpn J Clin Oncol. 2013;43:1093–104.
- Green F, Page D, Fleming I, Fritz A, Balch C, Haller D, et al. AJCC cancer staging handbook. 6th ed. New York: Springer; 2002.
- Coindre JM, Terrier P, Bui NB, Bonichon F, Collin F, Le Doussal V, et al. Prognostic factors in adult patients with locally controlled soft tissue sarcoma. A study of 546 patients from the French Federation of Cancer Centers Sarcoma Group. J Clin Oncol. 1996;14:869–77.
- Riad S, Griffin AM, Liberman B, Blackstein ME, Catton CN, Kandel RA, et al. Lymph node metastasis in soft tissue sarcoma in an extremity. Clin Orthop Relat Res. 2004;426:129–34.
- Behranwala KA, A’Hern R, Omar AM, Thomas JM. Prognosis of lymph node metastasis in soft tissue sarcoma. Ann Surg Oncol. 2004;11:714–19.
- Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg. 1993;217:72–7.
- Weingrad DN, Rosenberg SA. Early lymphatic spread of osteogenic and soft-tissue sarcomas. Surgery. 1978;84:231–40.
- Mazeron JJ, Suit HD. Lymph nodes as sites of metastases from sarcomas of soft tissue. Cancer. 1987;60:1800–8.
- Skinner KA, Eilber FR. Soft tissue sarcoma nodal metastases: biologic significance and therapeutic considerations. Surg Oncol Clin N Am. 1996;5:121–7.
- Lin CN, Chou SC, Li CF, Tsai KB, Chen WC, Hsiung CY, et al. Prognostic factors of myxofibrosarcomas: implications of margin status, tumor necrosis, and mitotic rate on survival. J Surg Oncol. 2006;93:294–303.
- Huang HY, Lal P, Qin J, Brennan MF, Antonescu CR. Low-grade myxofibrosarcoma: a clinicopathologic analysis of 49 cases treated at a single institution with simultaneous assessment of the efficacy of 3-tier and 4-tier grading systems. Hum Pathol. 2004;35:612–21.