Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease that mainly affects the joints. Many rheumatologists have recently correlated the pathogenesis of RA with the citrullination of self-proteins. Anti-citrullinated protein antibodies (ACPAs) are produced specifically in RA patients and play an important role as a diagnostic tool in RA. However, it is unclear and controversial whether ACPAs are pathogenic.
A 31-year-old woman with RA became pregnant in April 2014. She began treatment with prednisolone (PSL) 10 mg instead of a biological agent for her pregnancy. However, her 28-joint count Disease Activity Score based on the erythrocyte sedimentation rate (DAS-28ESR) increased from 3.19 in April to 5.71 in October. Therefore, treatment with certolizumab pegol (CZP), a tumour necrosis factor (TNF)-α inhibitor that is not actively transported across the placenta, was begun with the patient’s consent. After the CZP treatment, the DAS-28ESR decreased to 3.58 in January 2015, and symptoms of RA improved. In the same month the patient gave birth to a boy at 40 weeks’ gestation. He had developed normally without deformity during the pregnancy despite the mother’s worsening RA. At birth he was healthy (Apgar score 8/9) with a birthweight of 3022 g ().
Figure 1. Disease activity of the patient (mother) with rheumatoid arthritis (RA) and growth and development of her baby. (A) Disease Activity Score for 28 joints based on the erythrocyte sedimentation rate (DAS28-ESR) and C-reactive protein (CRP) levels of the patient. Prednisolone (PSL) dose is indicated above the plot. Arrows indicates subcutaneous (S.C.) injection of certolizumab pegol (CZP). (B) Body weight (BW) of the baby measured and calculated by ultrasound scan.
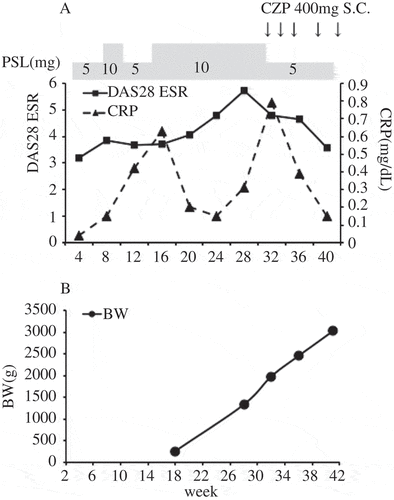
To evaluate whether the mother’s RA had affected her son, we collected samples of the mother’s serum on the day before delivery, samples of cord blood immediately after delivery, and the neonate’s serum at 3 days postpartum. Using these samples, we measured levels of ACPA, interleukin (IL)-6, and TNF-α. The ACPA levels in the mother’s serum, the cord blood, and the neonate’s serum were 358, 257, and 121 U/mL, respectively, indicating that ACPAs had passed through the placenta. Levels of IL-6 and TNF-α in the mother’s serum were 57.3 and 3.7 pg/mL, respectively, while those in the cord blood were 1.6 and 4.7 pg/mL, respectively. There was no marked increase in either IL-6 or TNF-α in the cord blood. One month after delivery, the infant’s weight was increasing normally (3948 g). His muscle tone and joint motion range were normal and no other problems were detected. At 1 year of age he was healthy and showed normal development.
Neonates of mothers with lupus erythematosus sometimes present neonatal lupus. Autoantibodies, for example anti-SS-A antibodies, are able to pass through the placenta and induce neonatal lupus (Citation1). By contrast, it is known empirically that neonates of mothers with RA do not develop ‘neonatal RA’. However, these findings have not been reported officially or demonstrated based on solid evidence. The potential role of ACPAs in the pathogenesis of RA is controversial. Although some patients have demonstrated ACPA production several years before RA onset, magnetic resonance imaging (MRI) findings of joint symptoms were not detected before RA onset (Citation2). Injection of monoclonal ACPA did not cause arthritis in healthy mice (Citation3). However, most ACPA-positive persons later developed RA and about 50% developed RA within 18 months (Citation4). Progression of bone erosion after onset of RA was more severe in ACPA-positive RA patients than ACPA-negative RA patients (Citation5). ACPAs stimulated macrophages and induced production of TNF-α (Citation6), and arthritis activity worsened following injection of monoclonal ACPAs into collagen-induced arthritis mice (Citation3). These research findings indicate that ACPAs might not cause arthritis directly but may exacerbate arthritis symptoms. However, we could not confirm this hypothesis because we could not ethically transfer ACPAs from RA patients to healthy persons and observe whether arthritis subsequently developed. Nonetheless, we showed that ACPAs of a mother with high-activity RA passed through the placenta and did not cause arthritis in her foetus, suggesting that ACPAs from patients with high-activity RA may not cause symptoms of RA in non-arthritic persons directly. Although the avidity and antigen specificity of ACPAs are also thought to correlate with RA pathogenicity (Citation7, Citation8), we suggest that this case was sufficient to evaluate the pathogenicity of ACPAs because of the patient’s RA onset with high activity.
Our data were based on only one case and we could not evaluate the relationship between the neonate’s serum ACPA levels and his long-term health. A larger number of cases and longer follow-up will clarify the pathogenesis of ACPAs.
Ethics committee approval (Authorization code: 11122-2) for this study was obtained from our hospital ethics committee (Medical Centre for Translational and Clinical Research).
Acknowledgements
This study received support for Scientific Research (C) from the Japan Society for the Promotion of Science (No. 25460495).
Additional information
Funding
References
- Buyon JP, Hiebert R, Copel J, Craft J, Friedman D, Katholi M, et al. Autoimmune-associated congenital heart block: demographics, mortality, morbidity and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol 1998;31:1658–66.
- van de Sande MG, de Hair MJ, van der Leij C, Klarenbeek PL, Bos WH, Smith MD, et al. Different stages of rheumatoid arthritis: features of the synovium in the preclinical phase. Ann Rheum Dis 2011;70:772–7.
- Kuhn KA, Kulik L, Tomooka B, Braschler KJ, Arend WP, Robinson WH, et al. Antibodies against citrullinated proteins enhance tissue injury in experimental autoimmune arthritis. J Clin Invest 2006;116:961–73.
- Szodoray P, Szabó Z, Kapitány A, Gyetvai A, Lakos G, Szántó S, et al. Anti-citrullinated protein/peptide autoantibodies in association with genetic and environmental factors as indicators of disease outcome in rheumatoid arthritis. Autoimmun Rev 2010;9:140–3.
- Courvoisier N, Dougados M, Cantagrel A, Goupille P, Meyer O, Sibilia J, et al. Prognostic factors of 10-year radiographic outcome in early rheumatoid arthritis: a prospective study. Arthritis Res Ther 2008;10:R106.
- Sokolove J, Zhao X, Chandra PE, Robinson WH. Immune complexes containing citrullinated fibrinogen costimulate macrophages via Toll-like receptor 4 and Fcγ receptor. Arthritis Rheum 2011;63:53–62.
- Suwannalai P, van de Stadt LA, Radner H, Steiner G, El-Gabalawy HS, Zijde CM, et al. Avidity maturation of anti-citrullinated protein antibodies in rheumatoid arthritis. Arthritis Rheum 2012;64:1323–8.
- Van Steendam K, Tilleman K, Deforce D. The relevance of citrullinated vimentin in the production of antibodies against citrullinated proteins and the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford) 2011;50:830–7.
