Abstract
Objective: To describe the social status and health-related quality of life of patients with psoriatic arthritis mutilans (PAM) in the Nordic countries.
Method: Patients with at least one mutilated joint confirmed by radiology were studied. Disease activity involving joints and skin, physician-assessed disease activity, and patient’s education and work status were recorded. Data from the 36-item Short Form Health Survey, Health Assessment Questionnaire and Dermatology Life Quality Index questionnaire were gathered and correlated with disease duration, pain, and general well-being (visual analogue scale). The controls were 58 Swedish patients with long-standing psoriatic arthritis sine PAM.
Results: Sixty-seven patients were included. Patients with PAM had a protracted disease history (33 ± 14 years) and disease onset at a relatively early age (30 ± 12 years). Overall inflammatory activity at inclusion was mild to moderate. The mean number of mutilated joints was 8.2 and gross deformity was found in 16% of patients. Forty per cent were treated with biological and 32% with conventional synthetic disease-modifying anti-rheumatic drugs. Forty-two per cent had retired early or were on sick leave. Impaired functional capacity with little or no ability to perform self-care or everyday tasks was reported by 21% of the patients. Patients between 45 and 60 years of age reported the most impaired quality of life in comparison to the control group.
Conclusion: PAM seriously affects social functioning. Whether early recognition of PAM and new forms of therapy can improve disease outcome and quality of life remains to be studied.
Psoriatic arthritis (PsA) is the most common arthritic condition associated with psoriasis (Citation1). The true prevalence of PsA in the general population is not fully clear, but is reported to be 0.13–0.25% in the Nordic countries (Citation2–Citation5). By applying classification criteria for psoriatic arthritis [ClASsification criteria for Psoriatic ARthritis (CASPAR) study] (Citation6), the prevalence of PsA in psoriasis patients was estimated to be 13.8% (Citation1), while other studies report a higher prevalence of 27–30% (Citation7–Citation9). Joint and muscle symptoms have been self-reported by up to one-third of psoriatic patients (Citation10).
PsA can present in a variety of forms, both in individual patients and between patients (Citation2). Initially, Moll and Wright described five major forms of PsA, with psoriatic arthritis mutilans (PAM) being the most severe and rarest phenotype (Citation11). Subsequent categorization of PsA suggested subdivision into two main groups, with or without axial involvement (Citation12), where coexisting polyarthritis predicted an unfavourable outcome (Citation13). The most deforming variant of PsA is PAM, with a prevalence of about 5% according to Moll and Wright (Citation11), even though this phenotype seems rare nowadays (Citation14). Nevertheless, the prevalence of PAM in one cohort of established PsA was reported to reach 21% (Citation15). In an early Swedish PsA cohort (n = 334) with a 10 year follow-up, none of the patients developed PAM (Citation16, personal communication T Husmark, 2014). In our recent epidemiological study in the Nordic population, the prevalence of PAM was found to be only 3.9 per million inhabitants (Citation14). Occasionally, arthritis mutilans can also be associated with other severe arthritic diseases, e.g. rheumatoid arthritis (Citation17). As there are no established classification criteria to define PAM, the Group for Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) has taken the initiative to develop a consensus definition of PAM (Citation18).
We describe here the phenotype of Nordic patients with PAM, and focus on the effects on daily life and social activities in comparison with patients with long-standing PsA but without mutilating disease.
Method
We have previously reported inclusion and exclusion criteria for the Nordic PAM Study, as well as demographics and disease settings in 59 patients with PAM (Citation14). The objectives of the present study are to report the quality of life of PAM sufferers in the Nordic countries, and to describe effects of the disease on functional capacity and social life. We have added eight new patients to the 59 previously reported; four from Denmark, three from Sweden, and one from Norway. Thus, the present study included 67 PAM patients.
A group of 58 patients with PsA sine PAM, with long-standing disease, using data from a 5 year follow-up, served as controls (Citation19).
Clinical examination and questionnaires
All patients underwent detailed dermatological and rheumatological clinical evaluation as previously described (Citation14). In addition, the patients answered three standardized questionnaires: the modified Health Assessment Questionnaire (mHAQ or HAQ) (Citation20, Citation21), the Health-related quality of life 36-item Short Form Health Survey (SF-36) (Citation22, Citation23), and the Dermatology Life Quality Index (DLQI) questionnaire (Citation24, Citation25). Self-reported demographic data on education, work, and marital status were also registered.
The HAQ includes questions associated with assistance necessitated by functional incapacity, consisting of 20 items (Citation20, Citation21), and results in disability indices for eight categories: dressing and grooming, rising, eating, walking, hygiene, reach, grip strength, and extramural activities.
The SF-36 gives results on eight specific scales (Citation22, Citation23): physical functioning, physical role, bodily pain, general health, vitality, social interactivity, emotional role, and mental health.
The DLQI (Citation24, Citation25) comprises 10 questions concerning symptoms and feelings, daily activities, leisure, work, school, personal relationships, and treatment. Each question is answered by marking a tick box: ‘not at all’, ‘a little’, ‘a lot’, or ‘very much’, and is scored from 0 to 3. The scores are summed, giving a range from 0 (no impairment of quality of life) to 30 (maximum impairment).
Data analysis
All patient data were coded and analysed using the Microsoft Excel for Mac 2011 and GraphPad Prism version 6.0 statistical software packages. The χ2 and t-tests were used to compare dichotomous values and means, respectively. We calculated 95% confidence intervals (CIs) for prevalence rates using the binomial distribution. All reported p-values are based on two-tailed analysis.
To assess the association between PAM and disease expression, we performed logistic regression analysis using the independent variables skin/joint age at onset, skin/joint disease duration, minimum disease activity (MDA) score (0–7), and number of swollen joints and tender joints (grouped into 1–4 vs 0 and ≥ 5 vs 0). We estimated univariable models followed by four multivariable models. In the first model, we included skin disease age at onset and skin disease duration, and in the second model joint disease age at onset and joint disease duration. In the third model, we included the variables which had a significant association with PAM in model 1 or 2. Finally, we estimated a model including the significant variables in model 3, MDA score, and the number of swollen and tender joints.
To investigate the risk of a high HAQ score (HAQ > 1), univariable logistic regression models were estimated including the independent variables PAM, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), global assessment of disease activity visual analogue scale (VAS), assessment of pain VAS, skin/joint age at onset, skin/joint disease duration, MDA score, number of swollen joints, and number of tender joints. Furthermore, the variables significantly associated with high HAQ score in the univariate analyses were included in a multivariable model.
Ethical issues
Written informed consent was obtained from all study participants. The bioethics Committee and Data Protection Authorities in all four countries approved the study protocol.
Results
Demographics and disease activity
Demographic data and disease activity of 67 patients with PAM compared to 58 individuals in the reference group are presented in and . At inclusion, MDA was present in 11 (19%) of the reference patients compared with 14 (25%) of the PAM patients (difference not significant). Patients’ assessments of disease activity and of pain correlated significantly with the physician’s assessment of arthritis (r = 0.577, p < 0.0001; r = 0.560, p < 0.0001), while there was no correlation between skin and joint disease activity, by either patients’ or physicians’ estimates.
Table 1. Demographics of 67 patients with psoriatic arthritis mutilans (PAM) in comparison to 58 patients with psoriatic arthritis (PsA) sine PAM.
Table 2. Disease activity and quality of life in 67 patients with psoriatic arthritis mutilans (PAM) in comparison to 58 patients with psoriatic arthritis (PsA) sine PAM.
Forty-two per cent of patients had attended elementary school and 24% high school, and 34% had a college education (47% of the women and 19% of the men). Altogether, 48% of PAM patients were retired or on sick leave. Thirty-eight per cent lived in a house and received regular or occasional help, but the majority (61%) managed daily activities without additional assistance. Most of the patients (78%) had full or nearly full functional capacity, according to the physician’s assessment, while 21% had restricted functional capacity with scarcely any ability to perform self-care or everyday tasks. Only one patient was incapable of any self-care. There was no significant difference between men and women in this respect.
Quality of life
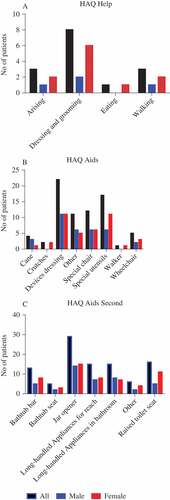
The overall estimated HAQ score was 0.98 ± 0.81, with no significant differences between countries or gender. Dressing, gripping, and opening tins and jars proved difficult and required help for several patients ().
Figure 1. Need for (A) daily help and (B, C) aids for 67 patients with psoriatic arthritis mutilans. HAQ, Health Assessment Questionnaire.
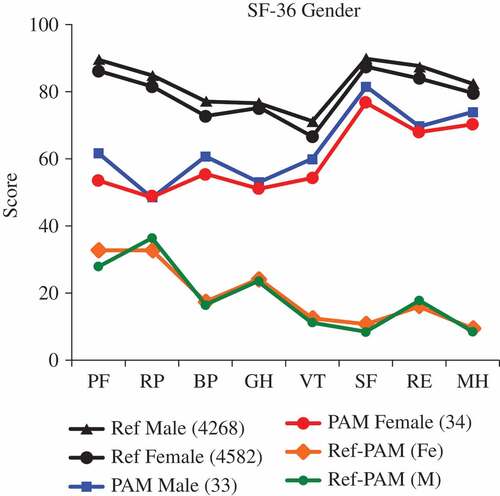
Four out of eight SF-36 subscales, i.e. physical function, physical role, bodily pain, and general health, were somewhat impaired compared to those of a healthy adult reference population (). Physical function and physical role were most impaired in 45–60-year-olds, of both genders, and thus deteriorated with advancing age (data not shown).
Figure 2. Absolute values of the 36-item Short Form Health Survey (SF-36) in 67 patients with psoriatic arthritis mutilans (PAM) (blue and red lines), compared with a general healthy population (black lines), separated by gender and differences between the absolute values for a general population and for PAM (green and orange lines). PF, physical function; RP, physical role; BP, bodily pain; GH, general health; VT, vitality; SF, social interactivity; RE, emotional role; MH, mental health.
Regarding the skin disease, which was mild in the majority of patients, only a minor deterioration in DLQI was recorded, with no gender difference ().
Comparison between quality of life and disease activity
Functional capacity, measured as HAQ score, correlated with ESR (r = 0.558, p < 0.0001), Disease Activity Score based on 28-joint count (DAS28)-CRP (r = 0.566, p = 0.0004), and Disease Activity in Psoriatic Arthritis (DAPSA) (r = 0.445, p = 0.0051). The HAQ score was independent of treatment with anti-tumour necrosis factor-α (anti-TNF-α) treatment.
The mental component summary and physical component summary on the SF-36 scale were correlated with the total number of deformed joints (r = −0.521, p = 0.0028; r = −0.362, p = 0.0455). The physical component summary was also correlated with ESR (r = −0.424, p = 0.0011). Patients treated with anti-TNF-α had a worse physical role (p = 0.05) and poorer mental health (p = 0.05) on the SF-36 scale compared to untreated PAM-patients.
Psoriatic arthritis mutilans versus reference group
Patients with long-standing PsA, chosen as the reference for PAM, and matched for age and gender, revealed significantly different demographic data for the onset and duration of PsA and psoriasis (). Patients with PAM had fewer swollen joints (2.0 ± 3.4 vs 3.6 ± 5.0, p = 0.043) but significantly more tender joints (5.3 ± 9.1 vs 2.6 ± 3.8 p = 0.032) compared to the reference group. Also, functional capacity, measured as HAQ score, was deteriorated compared to the reference group (0.98 ± 0.81 vs 0.56 ± 0.45, p = 0.001).
In logistic regression models including duration and age at onset, only duration was significantly associated with PAM (). Furthermore, in a model including duration of both skin and joint disease, only duration of joint disease was significantly associated with PAM (OR 1.14, 95% CI 1.07–1.21; p < 0.001). MDA, and the number of swollen and tender joints did not influence the association between the duration of joint disease (adjusted OR 1.19, 95% CI 1.11–1.28; p < 0.001) and PAM, although a high number (≥ 5 vs 0) of tender joints was significantly associated with PAM (adjusted OR 6.16, 95% CI 1.21–31.29; p = 0.028).
Table 3. Logistic regression of psoriatic arthritis mutilans patients versus a reference group of psoriatic arthritis (PsA).
HAQ score was significantly associated with PAM, global assessment of disease activity VAS, assessment of pain VAS, disease duration of arthritis, and ESR in the univariate analysis (). In the multivariable model, including the four significant predictors, only PAM (OR 6.99 95% CI 1.63–29.92; p < 0.009) and disease activity VAS remained significantly associated with HAQ score (OR 1.05, 95% CI 1.01–1.09; p = 0.026).
Table 4. Logistic regression HAQ_D50 = 1 if Health Assessment Questionnaire (HAQ) score > 1: reference group = 0 and psoriatic arthritis mutilans (PAM) = 1.
Discussion
The main purpose of the present study was to describe the expression of PAM and the effects of the disease on health-related quality of life. At study inclusion, a mean disease duration of three decades was present and thus we have been studying the effects of a long disease history.
PAM patients in the present study had a long duration of PsA and were young at disease onset, with a mean age of 30 years, compared with our reference group and a Swedish cohort of individuals with early PsA, the SwePsA, with a mean age of 47 years at onset (Citation16). In the SwePsA, the patients were followed prospectively and a current subanalysis of data shows that the mean age at onset of polyarthritic PsA disease was 43 years for men and 52 years for women (personal communication T Husmark, 2014). So far, no PAM has been identified in this cohort, which has a mean follow-up of 10 years at the time of writing. In a literature review of 38 studies on PsA comprising 18 768 patients, the mean age at onset of PsA was 49 years (Citation26). The age at onset of PAM is comparable to that of spondylarthropathies with axial involvement (Citation27). Moreover, spinal involvement has been associated with PAM, e.g. in the CASPAR study (Citation28). PsA patients with human leucocyte antigen (HLA) B*27, DQ B1*02, and killer-cell immunoglobulin-like receptor (KIR) gene 3DS1 run an increased risk of developing PAM (Citation29). Furthermore, patients with HLA B*27 have an earlier onset of disease and shorter interval between onset of psoriasis and PsA (Citation30). Thus, early age at onset, and long duration of disease, would appear to be an important predictor for the development of PAM, probably including disease activity as well as outcome, and seems to be closely related to other spondylarthropathies.
The age at onset of psoriasis usually ranges from childhood to adulthood, with three peaks: in early adolescence, at about 30 years, and at 50 years (Citation31). In the Stockholm Psoriasis cohort, recruiting individuals >15 years old within a year of disease onset, the mean age at onset was 41 years, but ranged from 15 to 84 years, with two distinct peaks: before and after 40 years of age (Citation32).
In the present PAM cohort, disease activity measures, except for tender joints, were low to moderate with no significant differences compared with the reference group. The effect of long-standing PAM disease and a substantial interindividual variation are possible confounders on our results. Having more than five tender joints is of importance. This is an expression of long-standing disease, not on an inflammatory basis, but rather as an indicator of mutilated or severely damaged joints.
The patients’ standard of education was similar to that of the general population in the Nordic countries, i.e. college (tertiary) level in about one-third of the patients (Citation33). Most of the college graduates were women. The loss of productivity was substantial for PAM patients. Forty per cent had retired early and another 10% were partly retired at inclusion in the study, compared with the general population in the Nordic countries, where a mean of 17% were pensioners (2008) in the age group 55–59 years (Citation33). Despite having a good level of education, the economic status of the patients must have been affected by the loss of lifetime income and by the increased costs of necessary regular help and of medical service and treatment.
There are no specific instruments to assess health-related quality of life in PAM. We have focused on the HAQ to assess the effect of impairment, by disease-caused deformity, on physical function. An important finding was that the odds of having an HAQ score > 1 was higher in the PAM group than in the reference group (PAM: OR 6.99, 95% CI 1.63–29.92; p = 0.009). However, the mean assessed HAQ score was not higher than estimates of HAQ score in early untreated PsA or rheumatoid arthritis (Citation34). Earlier studies have shown that the burden of PsA, estimated as actively inflamed joints and the number of clinically deformed joints, is positively related to the HAQ score, but decreases with the duration of the arthritic condition (Citation35). As the reference group had a significantly shorter disease duration than the PAM group, it could be surmised that the real difference in function between PAM and the reference group may be even more pronounced than observed herein. Rodriguez-Moreno et al reported reduced functional capacity in PAM, estimated by HAQ, and functional status according to American College of Rheumatology criteria, being affected primarily by disease duration and distal interphalangeal involvement (Citation36).
Another important finding was that the HAQ score was influenced by patients’ assessment of global disease activity. The level of function, estimated as HAQ score, was a direct assessment of patients’ ability to use their hands, with the need for help in dressing, gripping, and opening tins and jars, and these factors are predestined to affect patients’ global assessment of disease.
The SF-36, being a generic health assessment questionnaire, is not specific for PsA. SF-36 identifies mental as well as physical components. It is reportedly responsive to short-term changes, but not to disease progression. In PAM, SF-36 could further visualize the effect of the disease on patients’ function – and expectation of function – and thus of general health. The implication of reduced function was most prominent in the middle-aged group and declined with age.
Conclusion
In summary, we present a large series of patients from the Nordic PAM Study where the most pronounced attributes were duration of disease, number of tender joints, and reduced functional capacity measured by the HAQ. PAM causes loss of work ability and a need for help with everyday activities. The foremost outcome was the early retirement of patients suffering from PAM.
Acknowledgements
The members of the scientific committee (the authors) are grateful to NORDPSO, who organized and secured funding for the Nordic Psoriatic Arthritis Mutilans Study. We also thank Kerstin Bergh at the Karolinska Institutet and Mona-Lisa Wernroth at Uppsala Clinical Research Center, Uppsala University, for excellent statistical support, and Leena Paimela for valuable assistance in the early phase by preparing study protocols.
References
- Ibrahim G, Waxman R, Helliwell PS. The prevalence of psoriatic arthritis in people with psoriasis. Arthritis Rheum 2009;61:1373–8.
- Love TJ, Gudbjornsson B, Gudjonsson JE, Valdimarsson H. Psoriatic arthritis in Reykjavik, Iceland: prevalence, demographics, and disease course. J Rheumatol 2007;34:2082–8.
- Nossent JC, Gran JT. Epidemiological and clinical characteristics of psoriatic arthritis in northern Norway. Scand J Rheumatol 2009;38:251–5.
- Pedersen OBV, Svendsen AJ, Ejstrup L, Skytthe A, Junker P. The occurrence of psoriatic arthritis in Denmark. Ann Rheum Dis 2008;67:1422–6.
- Haglund E, Bremander AB, Petersson IF, Strömbeck B, Bergman S, Jacobsson LT, et al. Prevalence of spondyloarthritis and its subtypes in southern Sweden. Ann Rheum Dis 2011;70:943–8.
- Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–73.
- Haroon M, Kirby B, Fitzgerald O. High prevalence of psoriatic arthritis in patients with severe psoriasis with suboptimal performance of screening questionnaires. Ann Rheum Dis 2013;72:736–40.
- Walsh JA, Callis DK, Krueger GG, Clegg DO. Limitations in screening instruments for psoriatic arthritis: a comparison of instruments in patients with psoriasis. J Rheumatol 2013;40:287–93.
- Mease PJ, Gladman DD, Papp KA, Khraishi MM, Thaçi D, Behrens F, et al. Prevalence of rheumatologist-diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol 2013;69:729–35.
- Zachariae H, Zachariae R, Blomqvist K, Davidsson S, Molin L, Mörk C, et al. Quality of life and prevalence of arthritis reported by 5,795 members of the Nordic Psoriasis Associations: data from the Nordic quality of life study. Acta Derm Venereol 2002;82:108–13.
- Moll JM, Wright V. Psoriatic arthritis. Semin Arthritis Rheum 1973;3:55–78.
- Eder L, Gladman DD. Psoriatic arthritis: phenotypic variance and nosology. Curr Rheumatol Rep 2013;15:316–23.
- Gladman DD, Farewell VT, Nadeau C. Clinical indicators of progression in psoriatic arthritis: multivariate relative risk model. J Rheumatol 1995;22:675–9.
- Gudbjornsson B, Ejstrup L, Gran JT, Iversen L, Lindqvist U, Paimela L, et al. Psoriatic arthritis mutilans (PAM) in the Nordic countries: demographics and disease status. The Nordic PAM study. Scand J Rheumatol 2013;42:373–8.
- Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course and outcome. Ann Rheum Dis 2005;64:ii14–17.
- Theander E, Husmark T, Alenius G-M, Larsson PT, Teleman A, Geijer M, et al. Early psoriatic arthritis: short symptom duration, male gender and preserved physical functioning at presentation predict favourable outcome at 5-year follow-up. Results from the Swedish Early Psoriatic Arthritis Register (SwePsA). Ann Rheum Dis 2014;73:407–13.
- Yoshida M, Belt EA, Kaarela K, Kauppi MJ, Shimamura T. Prevalence of mutilans-like hand deformities in patients with seropositive rheumatoid arthritis. A prospective 20-year study. Scand J Rheumatol 1999;28:38–40.
- Haddad A, Chandran V. Arthritis mutilans. Curr Rheumatol Rep 2013;15:321–5.
- Lindqvist U, Rudsander A, Boström A, Nilsson B, Michaëlsson G. IgA antibodies to gliadin and coeliac disease in psoriatic arthritis. Rheumatology (Oxford) 2002;41:31–7.
- Fries JF, Spitz PW, Young DY. The dimensions of health outcomes: the health assessment questionnaire, disability and pain scales. J Rheumatol 1982;9:789–93.
- Daltroy LH, Larson MG, Roberts NW, Liang MH. A modification of the Health Assessment Questionnaire for the spondyloarthropathies. J Rheumatol 1990;17:946–50.
- Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83.
- Sullivan M, Karlsson J, Ware J Jr. The Swedish SF-36 Health Survey--I. Evaluation of data quality, scaling assumptions, reliability and construct validity across general populations in Sweden. Soc Sci Med 1995;41:1349–58.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI): a simple practical measure for routine clinical use. Clin Exp Dermatol 1994;19:210–16.
- Lewis V, Finlay AY. 10 years experience of the Dermatology Life Quality Index (DLQI). J Investig Dermatol Symp Proc 2004;9:169–80.
- Lee S, Mendelsohn A, Sarnes E. The burden of psoriatic arthritis: a literature review from a global health systems perspective. P T 2010;35:680–9.
- Feldtkeller E, Khan MA, Van Der Heijde D, Van Der Linden S, Braun J. Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol Int 2003;23:61–6.
- Chandran V, Gladman DD, Helliwell PS, Gudbjörnsson B. Arthritis mutilans: a report from the GRAPPA 2012 Annual Meeting. J Rheumatol 2013;40:1419–22.
- Chandran V, Thavaneswaran A, Pellett PJ, Gladman DD. The association between human leukocyte antigen and killer-cell immunoglobulin-like receptor gene variants and the development of arthritis mutilans in patients with psoriatic arthritis (abstract). Arthritis Rheum 2011;63( Suppl):S307.
- Queiro R, Tejón P, Alonso S, Coto P. Age at disease onset: a key factor for understanding psoriatic disease. Rheumatology (Oxford) 2014;53:1178–85.
- Swanbeck G, Inerot A, Martinsson T, Wahlström J, Enerbäck C, Enlund F. Age at onset and different types of psoriasis. Br J Dermatol 1995;133:768–73.
- Mallbris L, Larsson P, Bergqvist S, Vingård E, Granath F, Ståhle M. Psoriasis phenotype at disease onset: clinical characterization of 400 adult cases. J Invest Dermatol 2005;124:499–504.
- The Nordic countries in figures 2008 (www.norden.org/sv/fakta-om-norden/siffror-och-statistik). Accessed 2 January 2017.
- Lindqvist UR, Alenius GM, Husmark T, Theander E, Holmström G, Larsson PT. The Swedish early psoriatic arthritis register – 2-year follow-up: a comparison with early rheumatoid arthritis. J Rheumatol 2008;35:668–73.
- Husted JA, Tom BD, Farewell VT, Schentag CT, Gladman DD. A longitudinal study of the effect of disease activity and clinical damage on physical function over the course of psoriatic arthritis: does the effect change over time? Arthritis Rheum 2007;56:840–9.
- Rodriguez-Moreno J, Bonet M, Del Blanco Barnusell J, Castano C, Clavaquera T, Mateo Soria L, et al. Mutilating/resorptive arthritis. A study of 24 patients in a series of 360 patients with psoriatic arthritis. Rheumatol Clin 2013;9:38–41.
