Abstract
Objective: Intra-articular glucocorticoid (IAGC) injection treatment is an easy and effective way to treat the signs and symptoms of arthritis, but there is limited knowledge on the adequate dosing for different joints. The aim of this study was to compare the outcome between two common doses of intra-articular triamcinolone hexacetonide (THA) for knee synovitis using the relapse rate during 6 months.
Methods: A total of 159 adult patients with rheumatoid arthritis (RA) or psoriatic arthritis (PsA) and active knee synovitis were randomized to IAGC injection with 20 mg or 40 mg THA. Participants were blinded to the treatment dose. The primary endpoint was relapse of arthritis. When symptoms from the treated joint recurred and signs of arthritis could be confirmed on a subsequent clinical examination, a relapse was recorded and the duration of response survival was calculated. At the end of the observation period, patients without relapse were telephoned to verify the persistence of the good treatment response.
Results: The proportion of relapse after 6 months was equal in the 20 mg and 40 mg THA treatment arms (30% vs 32%, respectively, p = 0.822), and no significant differences were found in the subgroups with RA and PsA patients.
Conclusion: As no difference in outcome was found between the compared doses, the lower 20 mg THA dose should be preferred in IAGC treatment for knee synovitis in chronic polyarthritis. This may also reduce pharmaceutical costs and metabolic side effects.
EudraCT number: 2014-000993-20, Clinical Trials.gov identifier: NCT02437461
Intra-articular glucocorticoid (IAGC) injection treatment is an effective method to rapidly reduce joint pain and swelling in arthritis disorders. The injection procedure is easy to learn and the clinical response is mostly excellent, and IAGCs have therefore been used in the treatment of rheumatic diseases for decades. Serious side effects of IAGC injection are rare and it has become a cornerstone in the therapy of rheumatoid arthritis (RA) and psoriatic arthritis (PsA). Despite modern effective treatment options with biological drugs, IAGCs are still very frequently used, and play an important role in the treat-to-target strategy for recent-onset RA (Citation1).
However, despite this long experience, the injection routines vary worldwide and are based more on local traditions than on evidence-based medicine. One question that is still under debate concerns the dosing of IAGC for knee synovitis.
Triamcinolone hexacetonide (THA) is one of the most used preparations, and studies have shown superior efficacy in the treatment of knee synovitis for IAGC with THA over methylprednisolone or triamcinolone acetonide (TA) (Citation2). However, in the literature the normal dosing of THA varies between 20 mg and 80 mg in RA patients (Citation3, Citation4), and when the present study was started no dose-finding studies had been published. The dosing has been based on the individual opinion of each treating doctor or the local traditions. In our clinics, we normally use 20 mg for knee injections, but in cases with early relapse or no response, we try 40 mg for the repeated injections. To improve treatment outcome, we additionally aspirate as much synovial fluid as possible (Citation5) and recommend strict postinjection rest (Citation6).
However, the outcome of IAGC treatment is dependent not only on the anti-inflammatory effects of the chosen preparation, synovial fluid aspiration, and postinjection routines, but also on the accuracy of the injection placement (Citation7). Furthermore, patient-related factors, such as the degree of joint destruction and the level of vascular endothelial growth factor in the synovial fluid, seem to be important (Citation3).
Several studies have shown that IAGCs influence the endogenous hormone production in the hypothalamic–pituitary–adrenal (HPA) axis, and reduced serum levels of adrenocorticotropin and cortisol have been found for a couple of weeks after a single joint injection (Citation8). In addition, serum levels of sex steroid hormones, such as testosterone, oestrogen, and dehydroepiandrosterone, are reduced (Citation9). However, the duration of hormone suppression seems to be highly individual. For arthritis patients with diabetes mellitus treated with IAGC, a transient elevation of blood glucose levels is anticipated (Citation10).
The present single-blinded randomized controlled trial (RCT) was investigator initiated. The objective was to compare the clinical treatment outcome of IAGC injection, using the relapse rate over 6 months, for the two most commonly used doses of THA for knee synovitis (20 mg and 40 mg), to find the optimal dose to inject in order to reduce the risk of inadequate treatment, as well as overtreatment of the inflamed joint, and minimize the ensuing endocrine and metabolic side effects.
All patients gave their written informed consent. The protocol was approved by the Regional Ethical Review Board in Uppsala (Dnr: 2013/449), the Local Radiation Committees at the hospitals in Gävle and Falun, and the Medical Products Agency, Sweden. The study was performed in accordance with Good Clinical Practice and the Helsinki Declaration, and was monitored by Uppsala Clinical Research Center.
Method
At the outpatient rheumatology units at the hospitals in Gävle and Falun, Sweden, between April 2015 and May 2017, adult patients (age > 18 years) with chronic polyarthritis [RA (Citation11) or PsA (Citation12)] and clinical signs and symptoms of knee synovitis (joint swelling and tenderness) were invited to participate in this trial. Most patients were recruited at injection visits on demand, but some were also recruited at regular visits when clinical signs of knee synovitis were present. Those who had received IAGCs in this joint in the past 3 months, those with > 10 mg oral prednisolone treatment, those planning joint surgery in the knee, and those in functional class 4 according to Steinbrocker et al (Citation13) were excluded. For patients with synovitis in both knees during the study period, only the first IAGC treatment was included. Data on patient characteristics (age, gender, diagnosis, disease duration, and medical treatment) were collected and blood samples for erythrocyte sedimentation rate and C-reactive protein were drawn. The number of tender and swollen joints was counted and the 28-joint Disease Activity Score (DAS28) was calculated (Citation14). Disability was evaluated using the Swedish version of the Health Assessment Questionnaire (HAQ) (Citation15). Within 2 months, a radiographic examination of the knee was performed and joint destruction was graded from 0 to 5 (0 = no changes, 5 = severe changes) according to the Larsen–Dale score (Citation16) by an independent radiologist.
A randomization procedure was prepared by an independent research nurse using 200 sealed opaque envelopes containing information on the allocation dose (ratio 1:1). They were sorted in blocks of six (3 + 3). The envelopes in each block were carefully mixed, followed by a careful mixing of the blocks, which created the random allocation sequence used in the trial.
Syringes of 2 mL (with 1 or 2 mL THA) were used for all injections and were prepared out of the patient’s sight, behind the locker doors used for THA storage.
The knee was entered with a lateral technique using a 0.7 × 40 mm needle. After joint puncture, as much as possible of the synovial fluid was aspirated. According to the randomization, a dose of 20 mg or 40 mg intra-articular THA (Lederspan ® Meda, Solna, Sweden) was injected in the knee, with the patient blind to the treatment. The patient was recommended to rest at home for 24 h after the injection and was asked to contact the rheumatology department if there was no treatment response or when symptoms from the treated joint recurred. If so, the knee was examined again by an experienced rheumatologist and if clinical signs of synovitis were confirmed a relapse was recorded and the time from injection to relapse was calculated. All patients were followed with visits according to clinical routines and were observed for 6 months. Knees with mild swelling at the regular visits were not regarded as relapsed unless the patient required IAGC therapy for symptom relief. Patients without relapse received a telephone call at the end of the observation period to confirm a good treatment response and that no unknown events had occurred.
Statistical methods
Based on expectations of a 40% relapse rate after 6 months (Citation3), power calculations indicated that 160 patients were needed to show a difference of 25% between the groups with a power of 80%. The Student’s t-test or Mann–Whitney U-test was used when appropriate for comparisons between group characteristics at baseline. The chi-squared test was used when comparing differences in proportions. Survival of treatment effect was described with Kaplan–Meier curves and the log-rank test was used to compare the groups. The statistical calculations were carried out using the program IBM SPSS Statistics version 23 (IBM Corp., Armonk, NY, USA).
Results
In total, 161 patients were recruited to this study, but one of them was included twice, and consequently, for this patient the second injection was excluded. Another patient had too short an interval from the previous injection in the treated joint and was also excluded. In the remaining group of 159 patients, 102 patients had RA and 57 PsA. Patients with RA were significantly (p < 0.001) older than those with PsA (mean ± sd age: 65.3 ± 12.1 vs 53.8 ± 15.1 years), had more joint damage on radiographs (p < 0.05), and were more often using oral glucocorticoids (p < 0.01). Methotrexate was the most used synthetic disease-modifying anti-rheumatic drug (sDMARD) (56%) and tumour necrosis factor blockers were the most used biological disease-modifying anti-rheumatic drug (bDMARD) (26%) in both subgroups.
Patient characteristics are presented in . At baseline, there were no statistical differences between patients injected with 20 mg versus 40 mg THA on any parameter. Successful synovial fluid aspiration, which indicates correct injection placement, was also equal in the two groups. Seven patients did not show up for the radiographic examination.
Table 1. Patient characteristics at baseline and outcome.
During the observation period, 9% of the patients changed their DMARD therapy and 6% changed their oral glucocorticoids. In total, 154 extra IAGC injections in other joints were given to 99 participants, in similar proportions in both treatment arms (p = 0.72).
Only one adverse event was reported. One month after IAGC treatment, a male RA patient had erysipelas in both legs, followed by an infection in the untreated knee, on which he had undergone surgery with joint replacement 6 years earlier. The patient was treated with intravenous antibiotics and arthroplastic exchange. He recovered completely. The joint infection was assessed to be caused by erysipelas, and not by the IAGC injection.
During the observation period, three participants had contact with our departments for pain in the treated knee without clinical signs of arthritis. Anserine bursitis was diagnosed in two cases and prepatellar bursitis in one case.
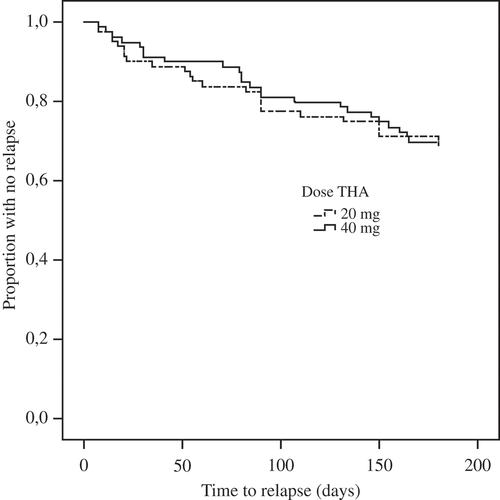
As shown in , there was no significant difference in relapse rate after 6 months between the group of patients injected with 20 mg THA and the group injected with 40 mg THA (p = 0.822). In addition, no significant differences were found in the RA and PsA subgroups (p = 0.282 and 0.134, respectively). The IAGC response survival is shown in –. There were no statistical differences between the treatment arms in any subgroup.
Figure 2. Treatment response survival in rheumatoid arthritis patients (n = 102). THA, triamcinolone hexacetonide.
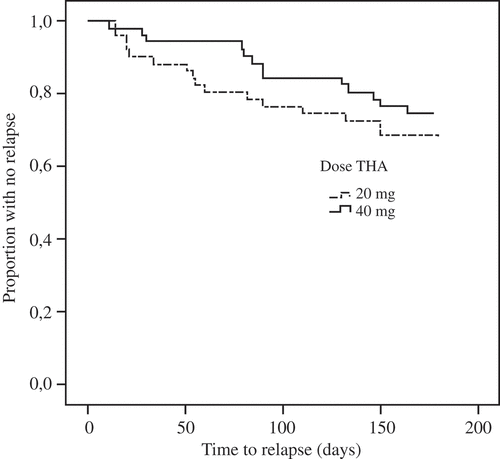
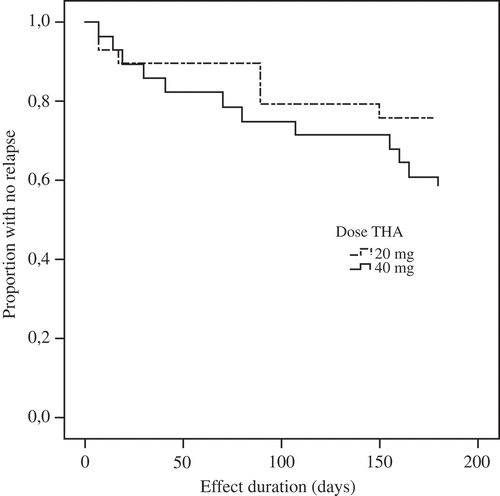
Figure 3. Treatment response survival in psoriatic arthritis patients (n = 57). THA, triamcinolone hexacetonide.
The relapse rate in patients with low disease activity (DAS28 < 3.2) did not differ from the relapse rates in those with moderate/high disease activity (p = 0.374), and no difference was found between patients with and those without oral glucocorticoid treatment (p = 0.357) in any treatment arm.
All patients without relapse could be reached by a telephone call after 6 months. At the time of this call, no patients needed more treatment for knee symptoms and, consequently, none was lost to follow-up.
Discussion
In this study, there was no significant difference in relapse rate during 6 months after IAGC treatment with 20 mg compared with 40 mg THA for knee synovitis in chronic polyarthritis disease.
The optimal dose in IAGC treatment has been an unexplored field for years and when this study was initiated we could not find any dose-finding studies for IAGC treatment. However, recently two RCTs were published and our results are in accordance with these smaller investigations (Citation17, Citation18).
Popma et al (Citation17) did not find any significant differences in joint function, swelling, pain, Likert burden scale, or synovitis activity when comparing 40 and 80 mg TA (which is an active metabolite to THA) for knee synovitis in a 12 week RCT on 97 patients with various arthritis diseases. Furthermore, in a 12 week double-blind RCT comparing 20 mg versus 40 mg THA for wrist synovitis in 60 RA patients, assessing pain, swelling, HAQ, and motion, Pereira et al (Citation18) found no differences between the groups. In support of these findings, Karuppiah and Dhaliwal (Citation19), in a retrospective non-RCT, compared joint pain after IAGC with 40 and 80 mg TA in the hip of 120 patients with osteoarthritis and found the lower dose to be as effective as the higher dose.
These studies, including the present investigation, indicate that there is no dose–response relationship in IAGC treatment for arthritis. The local anti-inflammatory treatment effect may reach a maximum plateau and a higher dose may only cause more side effects, such as increased and prolonged suppression of the HPA axis. Lower doses have not been adequately investigated and may, perhaps, be enough to produce a good response.
In this study, we have only included patients with RA and PsA. RA patients had significantly more joint destruction on radiographs, but as they were significantly older as well, this difference may possibly be explained by comorbidity with age-related osteoarthritis. We found no statistical differences in relapse rate between the treatment arms in any subgroup (, –).
Although the previously published investigations involved different joints in patients with different rheumatic diseases, the study findings are surprisingly homogeneous. Taken together, they suggest that many physicians all over the world may have used unnecessarily high doses for IAGC treatment for decades. Consequently, it is important to identify the optimal dose of IAGC therapy for each single joint type. Besides the inevitable endocrine and metabolic side effects, a higher dose is also associated with a larger volume of glucocorticoid solution and may therefore cause painful stretching of the joint capsule, especially in the smallest joints.
The relapse rate in this material was lower than expected. Consequently, a type II error cannot be excluded. However, to our knowledge, the present trial is the largest dose-finding RCT for IAGC therapy with the longest observation period so far, and the results are consistent with the findings in the previous, smaller studies.
A limitation of the present investigation may be the chosen outcome measure, defined by the recurrence of joint symptoms followed by a clinical examination of the knee. The patient delay for contact may vary, because the threshold for pain and discomfort is highly individual. However, this method is very relevant because it mimics the situation in everyday clinical practice and the findings can therefore directly be applied to most outpatient clinical settings. It is also simple and has been used already in studies evaluating IAGC (Citation3, Citation5).
Conclusion
The lower dose of 20 mg THA not only is as effective as the higher dose, but also may be associated with lower costs and fewer side effects, and should therefore be preferred in IAGC treatment for knee synovitis.
Acknowledgements
We thank Radu Lesanu for assessing the radiographs, Sara Gustavsson for statistical support, and Liselotte Sundgren for assistance with preparing the randomization procedure. We also thank Sven Tegmark, Abdalla Elkhalifa, Ann-Sofie Svennberg, and Ildiko Jonap in Gävle, and Jörgen Lysholm, Anna Svärd, Malin Hemberg, Emma Grenholm, Helena Hellström, and Elin Staffas in Falun, for helping us with patient recruitment.
Grants were given by the Center for Research and Development, Uppsala University/Region of Gävleborg.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Hetland ML, Ostergaard M, Ejbjerg B, Jacobsen S, Stengaard-Pedersen K, Junker P, et al. Short- and long-term efficacy of intra-articular injections with betamethasone as part of a treat-to-target strategy in early rheumatoid arthritis: impact of joint area, repeated injections, MRI findings, anti-CCP, IgM-RF and CRP. Ann Rheum Dis 2012;71:851–6.
- Garg N, Perry L, Deodhar A. Intra-articular and soft tissue injections, a systematic review of relative efficacy of various corticosteroids. Clin Rheumatol 2014;33:1695–706.
- Weitoft T, Ronnelid J, Knight A, Lysholm J, Saxne T, Larsson A. Outcome predictors of intra-articular glucocorticoid treatment for knee synovitis in patients with rheumatoid arthritis - a prospective cohort study. Arthritis Res Ther 2014;16:R129.
- Lopes RV, Furtado RN, Parmigiani L, Rosenfeld A, Fernandes AR, Natour J. Accuracy of intra-articular injections in peripheral joints performed blindly in patients with rheumatoid arthritis. Rheumatology (Oxford) 2008;47:1792–4.
- Weitoft T, Uddenfeldt P. Importance of synovial fluid aspiration when injecting intra-articular corticosteroids. Ann Rheum Dis 2000;59:233–5.
- Chakravarty K, Pharoah PD, Scott DG. A randomized controlled study of post-injection rest following intra-articular steroid therapy for knee synovitis. Br J Rheumatol 1994;33:464–8.
- Jones A, Regan M, Ledingham J, Pattrick M, Manhire A, Doherty M. Importance of placement of intra-articular steroid injections. BMJ 1993;307:1329–30.
- Johnston PC, Lansang MC, Chatterjee S, Kennedy L. Intra-articular glucocorticoid injections and their effect on hypothalamic-pituitary-adrenal (HPA)-axis function. Endocrine 2015;48:410–16.
- Weitoft T, Larsson A, Ronnblom L. Serum levels of sex steroid hormones and matrix metalloproteinases after intra-articular glucocorticoid treatment in female patients with rheumatoid arthritis. Ann Rheum Dis 2008;67:422–4.
- Habib GS, Miari W. The effect of intra-articular triamcinolone preparations on blood glucose levels in diabetic patients: a controlled study. J Clin Rheumatol 2011;17:302–5.
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24.
- Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–73.
- Steinbrocker O, Traeger CH, Batterman RC. Therapeutic criteria in rheumatoid arthritis. J Am Med Assoc 1949;140:659–62.
- Prevoo ML, van ‘t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8.
- Ekdahl C, Eberhardt K, Andersson SI, Svensson B. Assessing disability in patients with rheumatoid arthritis. Use of a Swedish version of the Stanford Health Assessment Questionnaire. Scand J Rheumatol 1988;17:263–71.
- Larsen A, Dale K, Eek M. Radiographic evaluation of rheumatoid arthritis and related conditions by standard reference films. Acta Radiol Diagn 1977;18:481–91.
- Popma JW, Snel FW, Haagsma CJ, Brummelhuis-Visser P, Oldenhof HG, van der Palen J, et al. Comparison of 2 dosages of intraarticular triamcinolone for the treatment of knee arthritis: results of a 12-week randomized controlled clinical trial. J Rheumatol 2015;42:1865–8.
- Pereira DF, Natour J, Machado NP, Furtado RN. Effectiveness of intra-articular injection in wrist joints according to triamcinolone hexacetonide dose in rheumatoid arthritis: a randomized controlled double-blind study. Am J Phys Rehabil 2015;94:131–8.
- Karuppiah SV, Dhaliwal A. Does larger dose of steroid improve the outcome of intraarticular hip injection? J Autoimmune Dis Rheumatol 2014;2:5–7.