Abstract
Objective: Low molecular mass hyaluronan causes inflammatory processes and can act as a pro-inflammatory cytokine in skin and other sites of activity in psoriatic arthritis (PsA). This study investigated whether the molecular mass distribution of hyaluronan (HA) in skin and the quantity of circulating HA are related to the clinical inflammatory picture in PsA with active disease and to the effect of treatment with anti-tumour necrosis factor-α (anti-TNF-α) adalimumab.
Methods: Twenty patients with TNF-α-naïve active polyarticular PsA were included in this prospective clinical trial of treatment with 40 mg s.c. adalimumab according to standard procedure. Clinical activity, patients’ assessments, and skin biopsies were captured at inclusion and at the 12 week follow-up. Ten healthy individuals were recruited for comparison of HA analyses. Histochemistry of skin inflammation, serum HA, and molecular mass of HA were determined.
Results: Overall improvements in clinical parameters were observed. Eight of 18 patients reached minimum disease activity after 12 weeks and disease activity was significantly reduced (p < 0.0001). Patients with elevated serum HA values were significantly older, had later onset of arthritis and more deformed joints, still had swollen joints after treatment, and had more circulating inflammatory biomarkers. More severe disease pathology showed a wide spectrum of high-molecular-mass HA accompanied by low mass HA. The treatment appears partly to normalize the HA mass distribution.
Conclusion: HA concentration and mass seem to be two possible factors in the inflammatory pathology of PsA acting as biomarkers for disease severity, resistance to treatment, and worse outcome.
Psoriatic arthritis (PsA) is classified according to the ClASsification criteria for Psoriatic ARthritis (CASPAR) (Citation1), where inflammation at the entheseal sites appears to be a pathological event connecting skin with joint disease (Citation2). There is no direct relationship between joint inflammation and the extent of skin affected by psoriasis. Patients with PsA usually exhibit low-grade skin inflammation (Citation3). However, in a previous study, we showed that patients with PsA can exhibit inflammatory changes in both lesional and non-lesional skin (Citation4). Moreover, in psoriatic patients with neither joint complaints nor disease, about half have shown entheseal inflammation at the Achilles tendon insertion.
Hyaluronan (HA), a high-molecular-mass polysaccharide, is ubiquitously present in various tissues and body fluids. HA has been recognized in receptor-mediated cellular events (Citation5). HA digested by hyaluronidase activity or oxygen free radicals down to low-molecular-mass forms (2 × 103 Da) (Citation5, Citation6) has distinct immune-regulatory properties. High-molecular-mass hyaluronan (HMHA) is anti-inflammatory while low-molecular-mass hyaluronan (LMHA) can, for example, activate the Toll-like receptors and initiate an immune response, further leading to autoimmune diseases. The release of cytokines, chemokines, reactive oxygen species, and degradative enzymes, e.g. interleukin-1β (IL-1β), tumour necrosis factor-α (TNF-α), and matrix metalloproteinases (MMPs), leads to further upregulation of hyaluronan synthetases (HAS) 1–3 and hyaluronidases, which degrade the newly synthesized HMHA to small fragments (Citation7–Citation9). In inflammatory diseases, elevated levels of HA have been found where HA is covalently bound to inter-α-inhibitor heavy chains (HCs). In synovial fluid from rheumatoid arthritis and osteoarthritis patients, HA-HC complexes were correlated with disease severity (Citation10, Citation11). The inflammatory function of HA is dependent on its size, concentration, environment, and binding partners. However, some earlier studies have failed to show the inflammatory properties of LMHA (Citation12, Citation13). In our earlier study of PsA, inflammation in skin of differing classification groups revealed a redistribution of HA in involved and non-involved psoriatic skin with epidermal imbalance between HA and its receptor CD44 in untreated patients with polyarticular disease (Citation4).
The aim of this study is to investigate whether LMHA is of importance for the inflammatory processes in PsA. We investigated whether the molecular mass distribution of HA in skin and the quantity of circulating HA are related to the clinical and histological inflammatory picture in PsA with active disease and the effect of treatment with anti-TNF-α adalimumab.
Method
Study design
This is a prospective, open-label, multi-centre study of HA molecular mass distribution in TNF-α-naïve PsA patients with active disease, first at inclusion and during 12 weeks’ treatment with adalimumab (IMM-12-0074). Five investigators assessed, according to protocol, patients with PsA at centres in Sweden: the Departments of Rheumatology, Uppsala University Hospital, Västerås Hospital, Östersund Hospital, University Hospital Malmö, and Umeå University.
Ethical statement
The study was approved by the regional ethical committee in Uppsala, Sweden (Dnr 2013/204) and by the Swedish Medical Products Agency (EudraCT nr 2012-004940-31). All individuals who took part in this study provided written informed consent. The study was conducted in accordance with the ethical standards of the Helsinki Declaration, 1975.
Participants
Twenty patients with TNF-α-naïve polyarticular PsA were included. All patients were at least 18 years old and fulfilled the diagnosis of PsA according to CASPAR (Citation1), with high inflammatory activity, defined as fulfilling the criteria for treatment with anti-TNF-α according to the Guidelines of the Swedish Association for Rheumatology: (i) high activity with polyarthritis (four or more swollen joints) or dachtylitis and increased erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP); and (ii) treatment failure of non-steroidal anti-inflammatory drugs (NSAIDs)/local steroids and one disease-modifying anti-rheumatic drug (DMARD) (methotrexate, sulphasalazine, leflunomide, gold salts, cyclosporine).
Patients had to be rheumatoid factor and anti-citrullinated protein/peptide antibody negative, have a psoriatic lesion with inflammation that could be biopsied, and have no other skin or inflammatory joint disease. Patients were allowed, and maintained, a stable dose of DMARD for at least 4 weeks prior to inclusion, and/or a stable oral dose of NSAIDs and corticosteroids for at least 2 weeks prior to inclusion.
Patient procedures and assessment
Treatment was initiated with adalimumab, 40 mg s.c. every second week according to standard procedure. The patients received instructions for self-injection, and the first injection of adalimumab was given by a nurse. At 2 and 6 week follow-up, the participating patients met a study nurse. Patients estimated skin disease activity using the Self-Assessment Psoriasis Area and Severity Index (SAPASI) (Citation14) and on a visual analogue scale (VAS skin), along with scales for pain (VAS pain) and overall disease activity (VAS overall) at inclusion, and at 2, 6 , and 12 week follow-up. The Health Assessment Questionnaire (HAQ) was used at inclusion and at the 12 week follow-up, for estimates of function.
Study procedure
At inclusion and the 12 week follow-up, or early exit, the following assessments were carried out: vital signs, 66/68 joint counts, deformity joint count, entheseal index, Psoriasis Area and Severity Index (PASI) (Citation15), physician’s overall assessment on a VAS, and blood sampling; skin biopsies (3 mm) were taken, two from affected and two from non-affected skin; and adverse events were recorded according to good clinical practice (GCP) guidelines.
At follow-up at 2 and 6 weeks, blood sampling was carried out and adverse events were recorded using GCP guidelines.
Activity scores
Minimum disease activity (MDA) according to Coates et al (Citation16) and the Disease Activity index for PSoriatic Arthritis (DAPSA) were used (Citation17, Citation18). Enthesitic activity was scored according to the Leeds index (Citation19). Skin disease activity before and after 12 weeks of treatment was estimated by the investigators using PASI (Citation15) and correlated to SAPASI (Citation14). American College of Rheumatology 20%/50%/70% response (ACR20/50/70) adapted for PsA (Citation20) was assessed to evaluate response to treatment.
Healthy individuals
Ten healthy individuals, four men (mean age 55, range 30–74 years) and six women (mean age 56, range 44–65 years), were included in the study as references. They gave a health declaration and were not allowed to take any medication. Blood was sampled and two skin biopsies (3 mm in diameter) were taken from the gluteal region.
HA molecular mass analysis
Owing to the extensive demand for skin material in molecular mass analysis, four out of 20 patients and eight healthy individuals were chosen for complete analyses. Two patients with very high initial disease activity and two with moderate high disease activity were selected. Skin samples were dried and homogenized. Proteins and nucleic acids were digested with proteinase K (Sigma-Aldrich, St Louis, MO, USA) and benzonase nuclease (Sigma-Aldrich) on two consecutive days. At the end of each day, chloroform was added to each sample and the extracted aqueous phase was dialysed against 0.1 M NaCl using Amicon Ultra 3K concentration units (Millipore, Billerica, MA, USA) followed by overnight precipitation in 99% ethanol.
Samples were then loaded on anion-exchange mini spin columns (Thermo Fisher Scientific, Waltham, MA, USA) and centrifuged to wash out sulphated glycosaminoglycans and remaining non-HA contaminants, based on NaCl binding. Finally, to remove salt the sample was dialysed against 20 mM ammonium acetate (pH 8.0) in Amicon Ultra 3K concentration units.
HA molecular analysis was undertaken using a gas-phase electrophoretic mobility macromolecule analysis (GEMMA) machine (TSI Corp., Shoreview, MN, USA) (Citation21). The molecule diameter analysed in the GEMMA was converted to molecular mass by analysing HA standards ranging from 30 kDa to 2500 kDa (Hyalose, Oklahoma City, OK, USA). In this study, we based the mass ranges of LMHA, HMHA, and very high-molecular-mass hyaluronan (vHMHA) on the observed HA peaks. LMHA was defined as a mass up to 50 kDa. vHMHA was defined as exceeding approximately 10 MDa (). The area under the curve corresponds to the number of molecules in the GEMMA analysis.
Measurement of HA concentration in serum
Serum HA levels were measured with an HA-binding protein-based enzyme-linked immunosorbent assay (ELISA)-like concentration measurement kit (HA Test Kit; Corgenix, Broomfield, CO, USA), according to the manufacturer’s instructions, run in duplicate; a coefficient of variation (CV) < 10% was considered acceptable. Absorbance was measured on a Thermo Multiskan Ascent (Thermo Fisher Scientific, MA, USA) and plotted by polynomial regression, against the concentration of the standard curve.
Fixation, embedding, and sectioning of skin biopsies
The skin biopsies were frozen immediately and stored at – 80°C until further processing. Prior to paraffin embedding, tissues were fixed in 4% buffered formaldehyde for 48 h at ambient temperature. Paraffin sections (4 µm) were then mounted on Superfrost Plus Slides (Thermo Fisher Scientific, USA) and dried overnight at 37°C.
Immunohistochemistry of CD44
Tissue sections were stained for CD44 expression using an automated Ventana Benchmark staining machine (Ventana Medical Systems, Tucson, AZ, USA). Citrate buffer at pH 8.0 for 8 min at 94°C and ethylenediaminetetraacetic acid (EDTA) buffer for 4 min at 100°C were applied for antigen retrieval. Thereafter, the primary antibody CD44 monoclonal immunoglobulin G2α diluted 1/100 (MA5-13890; Thermo Fisher Scientific, USA) was applied manually with subsequent incubation for 32 min. The sections were counterstained in the Ventana Benchmark staining machine with hematoxylin and bluing reagent.
Histochemistry of HA
HA was stained using a biotinylated HA binding protein, isolated from bovine nasal cartilage, according to a previously described protocol (Citation22). Control slides were pre-incubated with Streptomyces hyaluronidase 50 units/mL (Sigma, St Louis, MO, USA), a selective HA-digesting enzyme.
Histochemical evaluation
Three of the authors (UL, AE-L, and UH) independently evaluated the histological slides and consensus was then reached. Staining intensity was scored using an arbitrary grading scale designed to semi-quantify the HA and CD44 staining intensities graded 0 (no staining), 1 (faint), 2 (medium), and 3 (intense staining).
Chemical analysis
ESR was analysed by a modified Westergren method, and CRP levels were determined with standard turbidimetry methods using high-sensitivity CRP reagents.
Matrix metalloproteinase-3 (MMP-3) (DY513), MMP-9 (DY911), endostatin (DY1098), osteopontin (OPN) (DY1433), tumour necrosis factor receptor-1 (TNFR-1) (DY225), TNFR-2 (DY726), cathepsin S (DY1183), tissue inhibitor of metalloproteinases-1 (TIMP-1) (DY970), T-Cell Immunoglobulin Mucin (TIM-1) (DY1750), vascular cell adhesion molecule-1 (VCAM-1) (DY809), intercellular adhesion molecule-1 (ICAM-1) (DY720), IL-8 (DY208), E-selectin (DY724), and myostatin (DGDF80) were analysed with commercial sandwich ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. The assays were calibrated against highly purified recombinant human peptides. The total CV was approximately 6%.
Statistics
All levels of measurements are expressed as means and standard deviations. Comparisons of measured levels were performed with non-parametric methods using independent samples (Kruskal–Wallis test with a significance level of 0.05) or related samples (Wilcoxon signed rank test). Correlations between parameters were assessed by Pearson correlation (two-tailed).
Results
Twenty patients, 10 men (mean age 55.0 ± 10.4 years) and 10 women (mean age 56.2 ± 13.7 years), were included, of whom 18 completed the 12 week procedure. Two patients who left the trial early experienced a lack of improvement. The patients’ demographics and disease characteristics are given in .
Table 1. Baseline characteristics and outcome of 20 polyarthritic active psoriatic arthritis (PsA) patients included in the prospective study with 12 weeks’ treatment with adalimumab and at follow-up.
There were overall significant improvements in clinical parameters. Eight out of 18 patients reached MDA after 12 weeks. DAPSA improved significantly (p < 0.000). At the 12 week follow-up, ACR70 was reached in seven patients, ACR50 in four patients, and ACR20 in four patients. PASI 100 was reached in five patients, PASI 90 in one patient, and PASI 75 in three patients. There was a significant correlation between PASI and SAPASI at inclusion (p = 0.000, r = 0.826) and at follow-up (p = 0.000, r = 0.833).
ESR and CRP normalized in the whole group and likewise there were significant reductions in MMP-3, MMP-9, TNFR-1, and ICAM (, Supplementary table S1). In contrast, myostatin increased significantly (p = 0.006) (), possibly indicating increased muscle activity following treatment.
HA concentration in serum
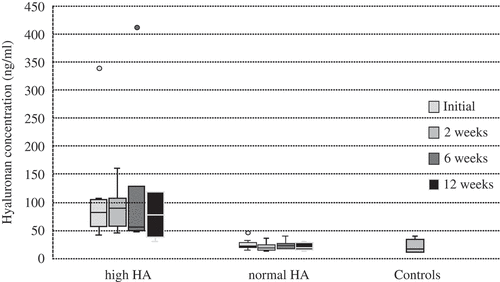
Overall, mean serum HA did not change over the duration of treatment (57.0 ± 73.3 ng/mL at inclusion, 46.7 ± 42.2 after 2 weeks, 61.9 ± 90.0 after 6 weeks, and 46.6 ± 38.7 at 12 weeks). The healthy individuals displayed a mean HA concentration of 21.7 ± 11.4 ng/mL. We found two separate groups of PsA patients, eight with elevated levels of HA and 11 with HA within the reference range, and the groups differed significantly at all four time-points ( and ). There was an equal gender distribution, and 60% reached MDA in both groups. The patients with abundant HA in serum were characterized by significantly older age and late onset of arthritic disease, and had significantly more deformed joints. They had more retained swollen joints and significantly higher overall disease activity was detected after 12 weeks of treatment. In general, patients with high levels of HA had significantly more circulating TNFR after 12 weeks. Likewise, OPN levels were significantly higher at inclusion and after 2 weeks. HA serum levels were significantly correlated with ESR, endostatin, MMP-9, and HAQ at inclusion; and with endostatin, MMP-3, SAPASI, and PASI at 2 week follow-up. The number of deformed joints was correlated with HA at 0, 2, and 6 weeks.
Table 2. Comparison of biomarkers and physical conditions in psoriatic arthritis (PsA) patients with normal and high levels of hyaluronan (HA).
Table 3. Pearson correlation (PC) of hyaluronan (HA) with clinical parameters and biomarkers over time in psoriatic arthritis patients with high levels of HA in serum.
HA molecular mass analysis
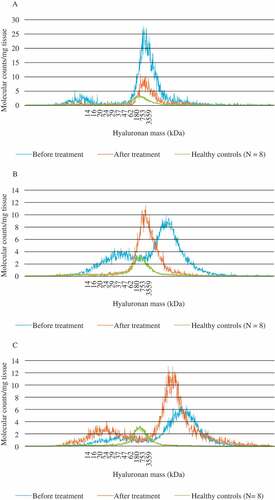
HA, isolated from skin biopsies taken from four patients before and after 12 weeks of adalimumab treatment, was compared with HA from skin biopsies from eight healthy individuals. The latter displayed a homogeneous molecular mass distribution with one peak ranging from 50 kDa to approximately 4 MDa. The patients were found to have inter-individual varying patterns. We report examples of three distinct variants of HA mass distribution in PsA (). Patient A, a 52-year-old male with moderate to high disease activity (DAPSA 11.9 and PASI 4.2) that improved after treatment, displayed both before and after treatment an HMHA peak in the same region as in the healthy controls and a small LMHA peak that disappeared after treatment. Before treatment, patient B, a 48-year-old male with high disease activity (DAPSA 37.5 and PASI 15.0), showed a peak of vHMHA, i.e. a larger mass than seen in healthy skin. Following treatment, his disease activity normalized (DAPSA 0.2 and PASI 0), and the skin contained normal HMHA. The LMHA seen before treatment also disappeared. Patient C, a 55-year-old male with high disease activity (DAPSA 23.7 and PASI 19.6) before treatment, displayed one prominent HMHA peak at inclusion, as well as a faint LMHA peak. After 12 weeks of treatment without improvement, there was a redistribution of molecular mass, with one high- and one low-mass peak, and thus not normalized. The fourth patient, a 54-year-old female with moderate to high disease activity (DAPSA 7.8 and PASI 2.2), improved after treatment and displayed the same pattern as patient A (not included in ). In all four examples we found an initial redistribution, with a prominent pathological high molecular mass of HA accompanied by low-mass peaks. The treatment seemed partly to normalize the mass distribution, except in patient C.
Figure 2. Hyaluronan (HA) mass distribution in skin biopsies of active psoriatic arthritis. HA was isolated from skin biopsies taken before and after 3 months of treatment with adalimumab, compared with skin biopsies from healthy controls. Examples are shown of three distinct variants of HA mass distribution in psoriatic arthritis. (A) Both before and after treatment, this patient showed a high-mass peak in the same region as in the healthy controls. A small low-mass peak disappeared after treatment. (B) Before treatment, this patient showed a peak of very high-mass HA, of larger mass than that seen in healthy skin. After treatment, the skin had a normal high-mass content of HA. Moreover, the low-mass HA seen before treatment disappeared afterwards. (C) This patient, like patient B, had both low-mass and very high-mass HA before treatment, which remained after treatment.
Histochemistry in skin biopsies
The results of histochemistry in skin biopsies are shown in and Supplementary table S2.
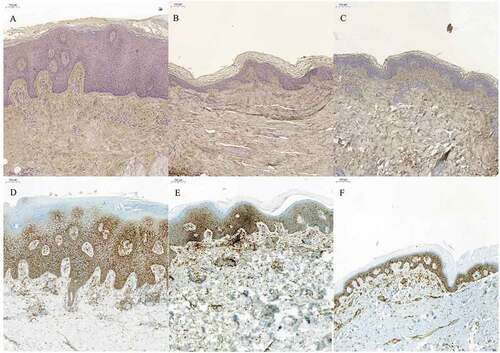
Figure 3. Hyaluronan (HA) and CD44 staining of skin biopsies of active psoriatic arthritis before and after 12 weeks of treatment with adalimumab. (A–C) HA staining (brown); (D–F) CD44 staining (brown). (A, D) Before treatment; (B, E) after 3 months’ treatment with adalimumab; (C, F) healthy skin. After treatment, the epidermal thickness had reduced to normal. There were no distinct differences in staining intensity in either HA or CD44 before or after treatment. HA stained in all skin layers.
Healthy individuals
Except for the basal membrane zone with intense staining, HA was faintly stained in the epidermis. The papillary dermis showed the most intense staining. In the reticular dermis the HA staining displayed a more irregular mesh-like staining pattern. CD44 staining was observed in stratum spinosum and stratum basale and also in the dermis, being especially intense around the blood vessels.
Affected skin in PsA prior to treatment
Faint HA staining was observed in the epidermis but there was very little staining in the basal membrane zone. More intense staining was seen in both the papillary and reticular dermis. CD44 was observed in the epidermis, with the exception of stratum corneum, and in the dermis.
Affected skin after treatment with TNF-α inhibitor adalimumab
Faint HA staining was seen in all skin layers except for the papillary and reticular dermis, where staining was intense. CD44 staining was similar to the CD44 staining pattern in pretreated patients.
Unaffected skin from patients with PsA
In spite of the PsA diagnosis and with macroscopically non-involved skin, the HA and CD44 staining patterns were similar to those described in skin biopsies from psoriatic lesions.
Comparison of morphology between pretreated and treated patients with TNF-α inhibitor
The epidermis in the pretreated patients was typically thickened, while in most of the treated patients it had regained its normal appearance.
Discussion
In the present study on active PsA, where treatment with a TNF inhibitor was intended, we focused on the role of HA in disease activity and elucidated the effect of therapy. We conducted a 12 week course of treatment with adalimumab, with marked improvement of signs and symptoms in PsA.
We found that an increased serum HA concentration in active PsA, compared with HA in healthy individuals, is associated with a larger number of deformed joints. Similarly, a high HA serum level was found to be associated with increased OPN concentration at all time-points, compared to patients with low HA levels. Moreover, the low and high mass of HA in skin was disparate, compared with healthy individuals. Finally, we found inflammatory activity with a redistribution of HA and CD44 in both involved and unaffected skin, before and after treatment, compared with healthy people.
Treatment with adalimumab, in PsA with high disease activity, is well documented both in research and in clinical practice (Citation23, Citation24). TNF inhibitors are the first line recommendation in Sweden (Citation25). It is also well known that PsA is a disease with variation within and between subgroups, as well as different expression of the disease over time (Citation26). We regarded the patients in this study to be representative of active polyarticular PsA. The number of patients included in this study could be a limitation of the study. However, there is a lack of patients in Sweden with high disease activity who have not already been treated with TNF inhibitors.
It is well known that there are two types of psoriasis, comprising patients with early onset and late onset, where early onset has higher disease activity and is genetically different from the late-onset type (Citation27). Likewise, in rheumatology practice, we experience different types of PsA; early-onset PsA seems to be more like spondylarthropathy, with more frequent occurrence of human leucocyte antigen B27 compared to late-onset disease. In this study, the group with a high level of serum HA was elderly and more resistant to treatment. So, possibly, the HA level could be an indicator of a separate PsA group.
Patients with normal HA serum levels experienced a better response to adalimumab treatment and had significantly fewer deformed joints. Levels of the biomarkers OPN and TNFR-1 were also lower in the group with normal HA concentrations. Joint deformation is partly due to bone remodelling or enthesitic calcification. OPN is involved in osteoblast/osteoclast activity, while both HA and OPN may be involved in the pathological process. In the high-HA group, TNFR-1 was high, both at the start and during treatment, possibly indicating a weaker effect of the TNF-inhibitor treatment. Both biological age and age at PsA onset differed significantly between the groups. Those who were older and had a later onset were found to have high HA concentrations. As HA serum concentration increases with age (Citation28), this could be the explanation. With late onset, joint deformation, increased OPN, and weakened effect of therapy, HA as such is an indicator of a separate mechanism of the disease type in elderly people. Cell culture studies have shown HA to increase OPN expression, and vice versa, which could explain higher levels of OPN in patients with high levels of HA (Citation29, Citation30). Moreover, when we adjusted the linear regression analysis for age, serum HA values still increased significantly in the group with high levels of HA, except at week 6 (data not shown).
Assessment of the HA mass distribution in skin showed marked differences in response to treatment. Both LMHA and vHMHA were normalized in some cases, whereas in one case LMHA and vHMHA remained after treatment. These observed changes in HA mass distribution corresponded with clinical findings. In addition, more HA molecules were found in skin biopsies from patients compared to healthy individuals. This is likely to be due to the inflammatory process in the skin. The initial presence of LMHA and vHMHA seems to be an indicator of more severe disease, in accordance with clinical disease activity. As we find in rheumatology practice, there are differences in the clinical picture and response to treatment in PsA. This is also obvious in our findings, with no effect on HA mass distribution after treatment in patient C. Either the treatment period was too short or there is a need for another type of treatment, such as IL-17 or IL-12/23 inhibitors.
LMHA is considered to induce a cytokine cascade by binding to toll-like receptors. TNF-α can induce tumour necrosis factor-stimulated gene-6 (TSG-6), which can subsequently mediate the transfer of the HC from inter-α inhibitors to HA. This forms an expanded extracellular matrix with HA cross-linked with HC, as has been observed in both rheumatoid arthritis and osteoarthritis (Citation31, Citation32).
The observed peak of vHMHA could consist of HA molecules linked by HCs. HA may protect HCs from degradation by proteinase in the HA isolation process. The treatment with a TNF-α inhibitor reduces the expression of TSG-6, decreasing the formation of HA–HC complexes. This mechanism could explain the disappearance of the vHMHA peak after 12 weeks of treatment in patient C.
Conclusion
Both HA concentration and molecular mass seem to be possible factors in the inflammatory pathology of PsA, acting as biomarkers for disease severity, resistance to treatment, and worse outcome. However, it is important to further investigate the mechanisms of the remodelled extracellular matrix in PsA and to clarify how TNF-α inhibitors act in the disease process.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Supplementary table S1. Biomarkers analysed in active psoriatic arthritis at inclusion, after 2 and 6 weeks and at 12 week follow-up after treatment with adalimumab.
Supplementary table S2. Staining intensity of hyaluronan and CD44 in sick and healthy skin.
Please note that the editors are not responsible for the content or functionality of any supplementary material supplied by the authors. Any queries should be directed to the corresponding author.
Supplemental Material
Download PDF (59 KB)Acknowledgements
We thank Gerd-Marie Alenius MD, PhD, Elke Theander MD, PhD, Christine Bengtsson MD, PhD, and Maria Zajaczkowska MD for cooperation and assessment of patients included in the study.
This clinical study was supported by the Swedish Research Council, the Swedish Research Council Formas, and the Torsten and Ragnar Söderberg Foundations, and AbbVie Sweden kindly supplied the TNF inhibitor adalimumab.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H, the CASPAR Study Group. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–73.
- Tau AL, Benjamin M, Toumi H, Grainger AJ, Tannes F, Emery P, et al. The relationship between the extensor tendon enthesis and the nail in distal interphalangeal joint disease in psoriatic arthritis - a high resolution MRI and histological study. J Rheumatol 2007;46:253–6.
- Elkayam O, Ophir J, Yaron M, Caspi D. Psoriatic arthritis: interrelationships between skin and joint manifestations related to onset, course and distribution. Clin Rheumatol 2000;19:301–5.
- Lindqvist U, Pihl-Lundin I, Engström-Laurent A. Dermal distribution of hyaluronan in psoriatic arthritis; coexistence of CD 44, MMP3 and MMP9. Acta Derm Venereol 2012;92:372–7.
- Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol Rev 2011;91:221–64.
- Stern R, Kogan G, Jedrzejas MJ, Soltes L. The many ways to cleve hyaluronan. Biotechnol Adv 2007;25:537–57.
- Laurent TC, Fraser JR. Hyaluronan. FASEB J 1992;6:2397–404.
- Laurent TC, Laurent UB, Fraser JR. Functions of hyaluronan. Ann Rheum Dis 1995;54:429–32.
- Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol 2006;6:823–35.
- Yingsung W, Zhuo L, Morgelin M, Yoneda M, Kida D, Watanabe H, et al. Molecular heterogeneity of the SHAP-hyaluronan complex. Isolation and characterization of the complex in synovial fluid from patients with rheumatoid arthritis. J Biol Chem 2003;278:32710–18.
- Mateos J, Lourido L, Fernandez-Puente P, Calamia V, Fernandez-Lopez C, Oreiro N, et al. Differential protein profiling of synovial fluid from rheumatoid arthritis and osteoarthritis patients using LC-MALDITOF/TOF. J Proteomics 2012;75:2869–78.
- Baeva LF, Lyle DB, Rios M, Langone JJ, Lightfoote MM. Different molecular weight hyaluronic acid effects on human macrophage interleukin 1β production. J Biomed Mater Res A 2014;102:305–14.
- Olsson M, Bremer L, Aulin C, Harris HE. Fragmented hyaluronan has no alarmin function assessed in arthritis synovial fibroblast and chondrocyte cultures. Innate Immun 2018;24:131–41.
- Feldman SR, Fleischer AB Jr, Reboussin DM, Rapp SR, Exum ML, Clark AR, et al. The self-administered psoriasis area and severity index is valid and reliable. J Invest Dermatol 1996;106:183–6.
- Fredriksson T, Pettersson U. Severe psoriasis - oral therapy with a new retinoid. Dermatologica 1978;157:238–44.
- Coates LC, Fransen J, Helliwell PS. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis 2010;69:48–53.
- Machado PM. Measurements, composite scores and the art of ‘cutting-off’. Ann Rheum Dis 2016;75:787–90.
- Schoels M, Aletaha D, Alasti F, Smolen J. Disease activity in psoriatic arthritis (PsA): defining remission and treatment success using the DAPSA score. Ann Rheum Dis 2016;75:811–18.
- Healy PS, Helliwell PS. Measuring clinical enthesitis in psoriatic arthritis: assessment of existing measures and development of an instrument specific to psoriatic arthritis. Arthritis Rheum 2008;59:686–91.
- Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995;38:727–35.
- Malm L, Hellman U, Larsson G. Size determination of hyaluronan using a Gas-Phase Electrophoretic Mobility Molecular Analysis. Glycobiology 2012;22:7–11.
- Hellström M, Johansson B, Engström-Laurent A. Hyaluronan and its receptor CD44 in the heart of newborn and adult rats. Anat Rec A Discov Mol Cell Evol Biol 2006;288:587–92.
- Mease PJ, Gladman DD, Ritchlin CT, Ruderman EM, Steinfeld SD, Chog EHS, et al. Adalimumab Effectiveness in Psoriatic Arthritis Trial Study Group. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum 2005;52:3279–89.
- van Kuijk AWR, Gerlag DM, Vos K, Wolbink G, de Groot M, de Rie MA, et al. A prospective, randomised, placebo-controlled study to identyify biomarkers associated with active treatment on synovial tissue. Ann Rheum Dis 2009;68:1303–9.
- SRF. Swedish Society for Rheumatology. Guidelines for drug treatment in axial spondylarthropathies and psoriatic arthritis 2018 (http://svenskreumatologi.se/wp-content/uploads/2018/03/riktlinjer-focc88r-lacc88kemedelsbehandling-ax-spa-och-psa_20180227.pdf). Accessed 27 February 2018.
- Theander E, Husmark T, Alenius G-M, Larsson PT, Teleman A, Geijer M, et al. Early psoriatic arthritis: short symptom duration, male gender and preserved physical functioning at presentation predict favourable outcome at 5-year follow-up. Results from the Swedish Early Psoriatic Arthritis Register (SwePsA). Ann Rheum Dis 2014;73:407–13.
- Henseler T, Christophers EJ. Psoriasis of early and late onset: characterization of two types of psoriasis vulgaris. Am Acad Dermatol 1985;13:450–6.
- Lindqvist U, Laurent TC. Serum hyaluronan and aminoterminal propeptide of type III procollagen. Variation with age. Scand J Clin Lab Invest 1992;52:613–21.
- Zhang FJ, Gao SG, Cheng L, Tian J, Xu WS, Luo W, et al. The effect of hyaluronic acid on osteopontin and CD44mRNA of fibroblast-like synoviocytes in patients with osteoarthritis of the knee. Rheumatol Int 2013;33:79–83.
- Cook AC, Chambers AF, Turley EA, Tuck AB. Osteopontin induction of hyaluronan synthase 2 expression promotes breast cancer malignancy. J Biol Chem 2006;281:24381–9.
- Bayliss MT, Howat SL, Dudhia J, Murphy JM, Barry FP, Edwards JC, et al. Up-regulation and differential expression of the hyaluronan-binding protein TSG-6 in cartilage and synovium in rheumatoid arthritis and osteoarthritis. Osteoarthritis Cartilage 2001;9:42–8.
- Chou CH, Attarian DE, Wisniewski HG, Band PA, Kraus VB. TSG-6 - a double-edged sword for osteoarthritis (OA). Osteoarthritis Cartilage 2018;26:245–54.