Abstract
Objective: The objective of this study was to investigate lower extremity function in early rheumatoid arthritis (RA) and assess its relation to other disease parameters.
Methods: An inception cohort (recruited in 1995–2005) of patients with early RA was followed according to a structured protocol. Lower extremity function was investigated at inclusion and after 1, 2, and 5 years using the Index of Muscle Function (IMF; total score 0–40). Self-reported disability was estimated using the Health Assessment Questionnaire (HAQ). The same rheumatologist assessed patients for swollen joints and joint tenderness.
Results: In total, 106 patients were included. Lower extremity function improved from baseline to the 1 year visit [IMF total median 10, interquartile range (IQR) 4–16 vs 7, IQR 3–12; p = 0.01]. This was followed by a decline in lower extremity function. Throughout the study, there were significant correlations between IMF and HAQ scores (r = 0.38–0.58; p < 0.001 at all time-points). Patients with knee and/or ankle synovitis at inclusion had significantly higher IMF scores than those without such joint involvement, with similar associations for joint tenderness. In multivariate linear regression analysis, ankle synovitis was significantly associated with higher IMF scores (β = 2.91, 95% confidence interval 0.28–5.54), whereas there was no such association for metatarsophalangeal (MTP) arthritis.
Conclusion: Lower extremity function in early RA improved during the first year, followed by a gradual decline. Ankle involvement had a greater impact than MTP involvement on lower extremity function. This highlights the importance of treating large-joint disease in RA.
Rheumatoid arthritis (RA) is a chronic inflammatory joint disease, which is common in most populations. The estimated prevalence of RA in southern Sweden is 0.7% (Citation1). The disease is characterized by polyarthritis involving the hands and feet, with often extensive joint pain and stiffness, leading to impairment such as limited joint mobility and decreased muscle strength (Citation2). The risk of disability, defined as having at least some self-reported difficulties in most activities of daily living, has been found to be more than seven-fold higher compared to a general population (Citation3).
Arthritis in the feet is common in RA. As many as 80% of people with established RA report foot problems (Citation4). Walking disability is more often caused by foot involvement than by knee- or hip-related problems (Citation4, Citation5). In a recent interview study of 59 patients with early RA, over two-thirds of the patients had foot-related disability at work, in the household, and during leisure time, despite access to effective medication and multi-professional interventions based on their individual needs (Citation6). Despite this, assessment of the feet and ankles is not included in the clinical examination that is the basis for calculating the Disease Activity Score based on 28 joints (DAS28) (Citation7). Because of this, there may be a risk of neglecting the feet and ankles in the assessment of patients with RA (Citation6, Citation8).
Good lower extremity function has, together with low pain and high physical activity, been shown to be important for the perception of good health in patients with RA (Citation9). However, the course of disability related to arthritis in the lower extremities has not been extensively investigated. In one of the few such studies, where patients with RA were followed for 8 years after diagnosis, it was shown that functional impairment in the lower extremities, assessed using the instrument Signals of Functional Impairment (SOFI) (Citation10), predicts future general disability. SOFI scores improved 1 year after diagnosis, followed by a gradual worsening, and 8 years after diagnosis the average scores were worse than at inclusion (Citation11).
The Index of Muscle Function (IMF) is based on 13 movements performed by the patient, all testing the function of the lower extremities. Different aspects of lower extremity function, such as general functional ability, muscle strength, muscle endurance, and balance/coordination, are included (Citation12). There is a rationale for using multi-dimensional tests, such as the IMF, in this context. In a study focusing on functional assessment of the lower extremities in patients with RA, it was concluded that both functional and postural tests should be used when evaluating lower extremity function (Citation13).
Disability related to arthritis in the lower extremities thus seems to have a major impact in many patients, but has not been extensively studied. The objective of this study was to investigate lower extremity function in early RA over the first 5 years, and to assess its relation to other disease parameters. In particular, we aimed to study the relation between involvement of individual joints and IMF at the time of diagnosis.
Method
Patients
An inception cohort of patients with early RA (symptom duration ≤ 12 months), recruited in 1995–2005, was investigated (Citation14). The patients were diagnosed with RA by a rheumatologist and fulfilled the 1987 American College of Rheumatology classification criteria for RA (Citation15). The study included individuals from a defined area, the city of Malmö, Sweden (population 260 000 in 2000). Patients were recruited from the rheumatology outpatient clinic of Malmö University Hospital, which was the only hospital serving the city, and from the four rheumatologists in private practice in Malmö. All patients gave their written informed consent to participate, and the study was approved by the Regional Ethical Review Board for southern Sweden (Lund, Sweden; LU 410-94, LU 311-02).
Patients were managed according to usual care, with no prespecified protocol for pharmacotherapy or rehabilitation. The patients were included before the current practice of treat-to-target (Citation16) was implemented, and before early treatment with biological disease-modifying anti-rheumatic drugs (bDMARDs) came into widespread use. In a structured follow-up programme, all patients were examined by the same rheumatologist. Visits were scheduled at 1, 2, and 5 years after inclusion. Treatment was assessed as previously described (Citation14). In brief, information on current treatment with disease-modifying anti-rheumatic drugs (DMARDs) and corticosteroids was obtained through a structured interview at each visit. Data on treatment with bDMARDs at any time during the study period was obtained through linkage to a regional biologics register (Citation17). Most patients were started on a DMARD at the time of diagnosis. The use of methotrexate as a first choice increased gradually during the study period. A limited number of patients with severe, refractory disease were treated with bDMARDs after their introduction in 1999.
Lower extremity function
The IMF (Citation12) was used to assess a subset of consecutive patients (recruited between October 1996 and August 2000) within the cohort. IMF is an instrument based on a battery of tests for assessing muscle function of the lower extremities in patients with RA, and includes 13 different performance tests. The scores are added for the total IMF (IMF total). IMF is divided into four areas: pretest of general ability (two tests), muscle strength (four tests), muscular endurance (three tests), and balance/coordination (four tests). The patient’s performance in the various tests is assessed by a physiotherapist on a three-point scale (0 = no difficulty in performance, 1 = some difficulty, 2 = severe difficulty or unable to do the test). The maximum score for the total IMF is 40, and the maximum scores for the subscales are 6 (pretest of general ability), 14 (muscle strength), 10 (muscular endurance), and 10 (balance/coordination). The pretests are designed to exclude patients who score 2 on any of the pretests (general walking ability or the ability to touch the knee with the other heel). Such patients should not be tested further. The original IMF does not specify how function in such patients should be quantified. In this study, they were assigned the maximum IMF total score of 40.
The IMF has been tested for reliability (Citation12, Citation18), validity (Citation12, Citation18, Citation19), and sensitivity to change (Citation19). [For a description of the instrument, see ref. (Citation12).] The IMF is recommended by the Swedish Association for Physiotherapists for the assessment of lower extremity function. Although it is used in clinical practice, it has not been used extensively in clinical research studies in rheumatology.
Disability related to the lower extremities
To estimate disability based on self-reported activity limitations, we used the Health Assessment Questionnaire (HAQ) (Citation20) [validated Swedish version (Citation21)]. This questionnaire consists of 20 activity questions divided into eight domains: Dressing and grooming, Arising, Eating, Walking, Hygiene, Reach, Grip, and Other usual activities, and is used to calculate the HAQ disability index (HAQ-DI; range 0–3). To more specifically address disability of the lower extremities, we calculated a subscore, HAQ-DI lower extremities (HAQ-DI-LE) (Citation18, Citation19). One way of doing this is to use the 10 questions that cover activities that are mainly dependent on function of the lower extremities. This method has been used previously in studies of the muscle function of the lower extremities in patients with RA and osteoarthritis, respectively, and when comparing dynamic versus static training of lower extremities in patients with RA (Citation18, Citation19).
Lower extremity joint involvement
Swollen joints and joint tenderness (Ritchie’s index) (Citation22) were assessed according to a structured protocol. All patients were examined by the same rheumatologist. Disease activity scores were calculated, i.e. the DAS28, based on 28-joint swollen and tender joint counts, the erythrocyte sedimentation rate (ESR), and visual analogue scale (VAS) for the patient’s global assessment of disease activity (VAS global) (Citation7), and the original Disease Activity Score (DAS), based on the 44-joint swollen joint count, Ritchie’s index for joint tenderness, ESR, and VAS global (Citation23). The swollen joint count for the lower extremities [knee, ankle, and metatarsophalangeal (MTP) joints] (range 0–14) and Ritchie’s index for the corresponding joints (range 0–18), were calculated.
There was no blinding to previous test results on standard clinical parameters. IMF scores were not available to the rheumatologist.
Statistics
Changes in the IMF total score and subtest scores between visits were analysed using the Wilcoxon signed rank test. To assess possible underestimation of the IMF due to missing data related to disability, we performed two sensitivity analyses. In the first, patients with missing data were presumed to be unable to perform the test, and therefore assigned an IMF total score of 40. In the second, in which disability was presumed to be unchanged, the last IMF observation was carried forward. The relative underestimation of the median IMF total score was calculated.
IMF scores in those with versus without current synovitis of individual joints were compared using the Mann–Whitney test. Multivariate linear regression analysis, adjusted for age and gender, was used to further explore the relation between lower extremity synovitis of individual joints and IMF at baseline. Normality of distribution for the residuals for each model was examined using the Shapiro–Wilk test. The relation between joint tenderness and baseline IMF was assessed in separate models. To assess the potential synergistic effect of multiple joint involvement, interactions between involvement of different joints were tested, with IMF total as the dependent variable. Correlations between disease parameters were assessed using Spearman’s rank test.
Results
Patients
In total, 106 patients with early RA [median symptom duration 8 months, interquartile range (IQR) 6–11] were included (). The majority of the patients were treated with DMARDs from inclusion, with increasing use of methotrexate during the follow-up (). Treatment with a bDMARD was started before the 5 year follow-up in 7.5% of the patients (n = 8).
Table 1. Characteristics of the early rheumatoid arthritis cohort, by follow-up visit.
Lower extremity function
Data on IMF total were available for 100, 89, and 67 patients at the 1, 2, and 5 year visits (). The number of patients who, based on the pretest, were excluded from the other tests and thereby given the maximum IMF total score of 40, was one at inclusion, three at year 1, six at year 2, and three at year 5. Lower extremity function improved from baseline to the 1 year visit (). This was followed by a decline in lower extremity function, in particular between the 2 year and 5 year visits (). The change in IMF total for those with data available at 2 and 5 years was mainly due to worsening in test results for muscle strength and for balance/coordination ().
Table 2. Changes in lower extremity function [Index of Muscle Function (IMF) subscores] over time.
Figure 1. Changes in lower extremity function [Index of Muscle Function (IMF) total] between visits, in patients with data available at both time-points. *p = 0.01 for IMF at inclusion and at 1 year; **p = 0.009 for IMF at 1 year and at 2 years; ***p = 0.001 for IMF at 2 years and at 5 years. Boxes indicate medians and quartiles; whiskers indicate 95% highest and lowest within non-outlier range.
![Figure 1. Changes in lower extremity function [Index of Muscle Function (IMF) total] between visits, in patients with data available at both time-points. *p = 0.01 for IMF at inclusion and at 1 year; **p = 0.009 for IMF at 1 year and at 2 years; ***p = 0.001 for IMF at 2 years and at 5 years. Boxes indicate medians and quartiles; whiskers indicate 95% highest and lowest within non-outlier range.](/cms/asset/d118c9fb-0706-4bc7-a28d-8fb50e08b73a/irhe_a_1579859_f0001_oc.jpg)
Correlation of IMF with disability and other disease parameters
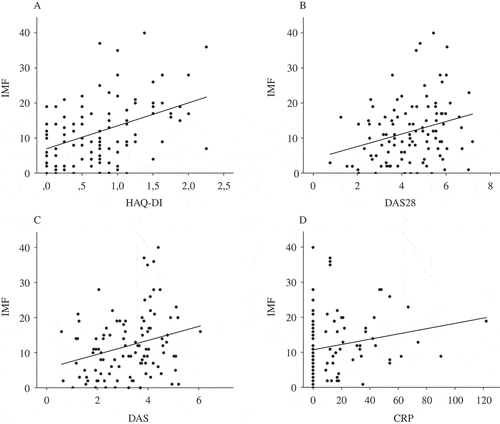
At baseline, IMF total correlated with HAQ-DI (r = 0.40; p < 0.001), whereas there were weaker correlations with DAS28, DAS, and C-reactive protein (CRP) (). IMF total also correlated with HAQ-DI LE at baseline (Supplementary figure S1). There were consistent moderate correlations for IMF total with HAQ-DI and HAQ-DI-LE at all time-points, whereas the correlations with CRP, DAS, and DAS28 were weaker, and at the 2 year visit not statistically significant (Supplementary table S1).
Figure 2. Correlations between Index of Muscle Function (IMF) total and disease parameters at inclusion (Spearman’s rank test). (A) Correlation between IMF total and Health Assessment Questionnaire disability index (HAQ-DI) (r = 0.46; p < 0.001). (B) Correlation between IMF total and 28-joint Disease Activity Score (DAS28) (r = 0.28; p = 0.004). (C) Correlation between IMF total and Disease Activity Score (DAS) (r = 0.28; p = 0.004). (D) Correlation between IMF total and C-reactive protein (CRP) (r = 0.24; p = 0.02).
Association between lower extremity involvement and IMF at inclusion
Synovitis of at least one knee joint, ankle joint, or MTP joint was present in 14%, 35%, and 43% of the patients, respectively, at inclusion. Forty-six patients (43%) had synovitis of at least one knee and/or ankle joint, whereas 37 (35%) had no synovitis in the lower extremities (). Those with knee and/or ankle synovitis had significantly higher IMF total scores than those without such joint involvement (median 13.5, IQR 7.8–19 vs median 9, IQR 3–16.8; p = 0.03) (). There were similar trends at 1, 2, and 5 years of follow-up, although the differences did not reach significance at these time-points (Supplementary table S2). Ankle synovitis also tended to be associated with higher IMF scores, whereas there were no such associations for MTP synovitis (, Supplementary table S2). In linear regression models with IMF total as the outcome variable, there were no significant interactions between ankle synovitis and knee synovitis (p = 0.98), ankle synovitis and MTP synovitis (p = 0.92), or knee synovitis and MTP synovitis (p = 0.58). In multivariate analysis, adjusted for age and gender, ankle synovitis was significantly associated with higher IMF total scores at inclusion [β = 2.91, 95% confidence interval (CI) 0.28–5.54] (). In this model, 44% of the variation in IMF total was explained. Residuals for all linear regression models were normally distributed.
Table 3. Lower extremity function at inclusion in the early rheumatoid arthritis cohort; by joint involvement (synovitis or joint tenderness at clinical examination).
Table 4. Relation between synovitis, age, gender, and Index of Muscle Function (IMF) total score at inclusion in the early rheumatoid arthritis cohort, by linear regression.
Similar patterns were observed for associations between joint tenderness and lower extremity function at inclusion (). Patients with ankle tenderness (median 13, IQR 8–20 vs median 9, IQR 3–17; p = 0.04) and those with knee and/or ankle tenderness (median 13, IQR 8–20 vs median 9, IQR 3–16; p = 0.02) had significantly higher IMF scores than those without such joint involvement. There were such patterns also at the 5 year follow-up, but no major differences in IMF total between those with versus those without joint tenderness at 1 and 2 years (Supplementary table S3). In age- and gender-adjusted multivariate analysis, there was a trend towards an association between ankle tenderness and IMF total score at inclusion (β = 2.23, 95% CI −0.80 to 5.25) (Supplementary table S4).
Discussion
In this study of patients with early RA, there was an improvement in the function of the lower extremities 1 year after inclusion, followed by a gradual worsening. These findings are in line with a previous study, in which evaluation was based on SOFI (Citation11).
From inclusion and throughout the whole study there were strong correlations between IMF total, HAQ-DI, and HAQ-DI-LE, suggesting that lower extremity function has a major influence on general disability in early RA. Correlations with disease activity parameters (DAS, DAS28, and CRP) were weaker and inconsistent. Häkkinen et al showed that muscle strength, which is one of the items assessed in IMF, has a strong impact on HAQ-DI, in addition to that of disease activity and pain (Citation24), which is in line with our findings.
The lack of association between IMF total and MTP joint involvement may be due to the fact that muscle function is more directly related to other parts of the lower extremities. On the other hand, balance/coordination also contributes to the IMF, and muscle function may be reduced secondary to pain and inactivity related to MTP involvement.
A previous study of RA patients with complaints from their feet explored associations between clinical signs and symptoms, structural damage, and function, as well as the impact of disease duration and the contribution of the individual foot segments (Citation25). Pain and swelling of the ankle were shown to contribute more to disability than forefoot involvement (Citation25). Patients with RA and deformity in the ankle/rear foot have been found to have greater foot-related disability than patients with isolated deformity in the forefoot (Citation26). In this study, we found that knee and ankle synovitis had a greater impact on lower extremity function in early RA compared to synovitis of the MTP joints. Ankle tenderness was also associated with worse lower extremity function.
Knee and ankle involvement represent only four out of a total of 28 and 44 joints assessed in the DAS28 and the DAS, respectively. This may explain the modest correlation between IMF and DAS or DAS28.
Although recommended, the IMF has not been extensively used in clinical research studies in rheumatology. The outcome measure instrument includes a pretest, and the instructions are not to let the patient continue if he or she scores a 2 (= severe difficulty or unable to do the test on any of the tasks included) in the pretest. In the present study, we gave such patients the maximum total IMF score of 40, to enable statistical analyses of all patients. We cannot exclude that some patients who failed the pretest would have been able to perform some of the tasks in the subtests. Other potential limitations are the lack of a healthy control group in the present study, and of reference values in the literature. Therefore, the degree of disability related to the lower extremities can only be compared within the RA cohort, and the difference from the general population cannot be estimated.
There was a loss of IMF data of just over 30% at the 5 year follow-up. If the loss of IMF data was partly due to disability (inability to perform the test), this would lead to an underestimation of the worsening of lower extremity function. Based on the sensitivity analyses, this could have a major impact at 2 and 5 years.
The IMF assessments were performed by different observers. However, a standardized procedure was used by physiotherapists at our unit during the entire study period. A good interobserver reliability has been demonstrated for the IMF (Citation12).
The difficulties in clinical assessment of synovitis in the MTP joints are well known (Citation27). However, in this study, there was a similar impact on lower extremity function for swollen and tender joints. We also had all patients examined by the same rheumatologist, which ensures a uniform assessment.
The patients were included in this study just before or shortly after the introduction of bDMARDs for the treatment of RA, and only a small proportion was treated with biologics before the 5 year follow-up. The results of this study may not apply to patients treated according to a treat-to-target strategy (Citation16), including ready access to bDMARDs.
Strengths of this study include the structured longitudinal follow-up of an inception cohort from a defined catchment area. Therefore, selection bias is not a major issue in this study, and the results could be generalized to patients with early RA seen in clinical practice. Furthermore, the assessment of swollen joints and joint tenderness was performed by the same rheumatologist according to a structured protocol throughout the study.
Conclusion
Lower extremity function in early RA improved during the first year, possibly owing to the effects of treatment and rehabilitation. Then there was a gradual decline, which, based on the changes in IMF subscores, may be explained by worsening in muscle strength and balance/coordination. The lower extremity function has a major impact on general disability in early RA. In this study, knee and ankle involvement had a greater impact on lower extremity function compared to MTP joint involvement, which was probably related to differences in influence on muscle function. The results highlight the importance of focusing on these larger joints in the medical treatment and rehabilitation of patients with early RA.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supplementary material. Results of sensitivity analyses.
Supplementary table S1. Correlations between lower extremity function and other disease parameters.
Supplementary table S2. Lower extremity function in early RA; by joint swelling. Index of Muscle Function; median (IQR).
Supplementary table S3. Lower extremity function in early RA; by joint tenderness. Index of Muscle Function; median (IQR).
Supplementary table S4. Relation between joint tenderness, age, sex, and IMF total score at inclusion in the early RA cohort.
Supplementary figure S1. Correlation between IMF total and HAQ-DI-LE at inclusion.
Please note that the editors are not responsible for the content or functionality of any supplementary material supplied by the authors. Any queries should be directed to the corresponding author.
Supplemental Material
Download PDF (193.7 KB)Acknowledgements
In remembrance of Christina Book, MD, PhD, who initiated this project and performed a major part of the data collection. She passed away before the preparation of this manuscript.
This study was supported by the Swedish Research Council [2015-02228], the Swedish Rheumatism Association [R-664091], and Lund University [ALFSKANE-446501].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Englund M, Joud A, Geborek P, Felson DT, Jacobsson LT, Petersson IF. Prevalence and incidence of rheumatoid arthritis in southern Sweden 2008 and their relation to prescribed biologics. Rheumatology (Oxford) 2010;49:1563–9.
- Stenstrom CH, Minor MA. Evidence for the benefit of aerobic and strengthening exercise in rheumatoid arthritis. Arthritis Rheum 2003;49:428–34.
- Sokka T, Krishnan E, Häkkinen A, Hannonen P. Functional disability in rheumatoid arthritis patients compared with a community population in Finland. Arthritis Rheum 2003;48:59–63.
- Grondal L, Tengstrand B, Nordmark B, Wretenberg P, Stark A. The foot: still the most important reason for walking incapacity in rheumatoid arthritis: distribution of symptomatic joints in 1,000 RA patients. Acta Orthop 2008;79:257–61.
- van der Leeden M, Steultjens MP, Ursum J, Dahmen R, Roorda LD, Schaardenburg DV, et al. Prevalence and course of forefoot impairments and walking disability in the first eight years of rheumatoid arthritis. Arthritis Rheum 2008;59:1596–602.
- Björk M, Thyberg I, Valtersson E, Östlund G, Stenström B, Sverker A. Foot barriers in patients with early rheumatoid arthritis: an interview study among Swedish women and men. Arthritis Care Res (Hoboken) 2018;70:1348–54.
- Prevoo ML, van ‘t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8.
- Landewe R, van der Heijde D, van der Linden S, Boers M. Twenty-eight-joint counts invalidate the DAS28 remission definition owing to the omission of the lower extremity joints: a comparison with the original DAS remission. Ann Rheum Dis 2006;65:637–41.
- Eurenius E, Brodin N, Lindblad S, Opava CH. Predicting physical activity and general health perception among patients with rheumatoid arthritis. J Rheumatol 2007;34:10–15.
- Eberhardt KB, Svensson B, Mortiz U. Functional assessment of early rheumatoid arthritis. Br J Rheumatol 1988;27:364–71.
- Hallert E, Bjork M, Dahlstrom O, Skogh T, Thyberg I. Disease activity and disability in women and men with early rheumatoid arthritis (RA): an 8-year followup of a Swedish early RA project. Arthritis Care Res (Hoboken) 2012;64:1101–7.
- Ekdahl C, Englund A, Stenström C. Development and evaluation of the index of muscle function. Advances in Physiotherapy 1999;1:45–55.
- Warenczak A, Lisinski P, Huber J. Importance of the functional examination in lower extremities in patients with rheumatoid arthritis. Acta Bioeng Biomech 2014;16:103–10.
- Rydholm M, Book C, Wikstrom I, Jacobsson L, Turesson C. Course of grip force impairment in patients with early rheumatoid arthritis over the first five years after diagnosis. Arthritis Care Res (Hoboken) 2018;70:491–8.
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24.
- Smolen JS, Aletaha D, Bijlsma JW, Breedveld FC, Boumpas D, Burmester G, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis 2010;69:631–7.
- Geborek P, Nitelius E, Noltorp S, Petri H, Jacobsson L, Larsson L, et al. Population based studies of biological antirheumatic drug use in southern Sweden: comparison with pharmaceutical sales. Ann Rheum Dis 2005;64:1805–7.
- Ekdahl C, Andersson SI, Svensson B. Muscle function of the lower extremities in rheumatoid arthritis and osteoarthrosis. A descriptive study of patients in a primary health care district. J Clin Epidemiol 1989;42:947–54.
- Ekdahl C, Andersson SI, Moritz U, Svensson B. Dynamic versus static training in patients with rheumatoid arthritis. Scand J Rheumatol 1990;19:17–26.
- Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum 1980;23:137–45.
- Ekdahl C, Eberhardt K, Andersson SI, Svensson B. Assessing disability in patients with rheumatoid arthritis. Use of a Swedish version of the Stanford Health Assessment Questionnaire. Scand J Rheumatol 1988;17:263–71.
- Ritchie DA, Boyle JA, McInnes JM, Jasani MK, Dalakos TG, Grieveson P, et al. Evaluation of a simple articular index for joint tenderness in rheumatoid arthritis. Ann Rheum Dis 1969;28:196.
- van der Heijde DM, van ‘t Hof M, van Riel PL, van de Putte LB. Development of a disease activity score based on judgment in clinical practice by rheumatologists. J Rheumatol 1993;20:579–81.
- Häkkinen A, Kautiainen H, Hannonen P, Ylinen J, Mäkinen H, Sokka T. Muscle strength, pain, and disease activity explain individual subdimensions of the Health Assessment Questionnaire disability index, especially in women with rheumatoid arthritis. Ann Rheum Dis 2006;65:30–4.
- Baan H, Drossaers-Bakker W, Dubbeldam R, van de Laar M. We should not forget the foot: relations between signs and symptoms, damage, and function in rheumatoid arthritis. Clin Rheumatol 2011;30:1475–9.
- Turner DE, Woodburn J. Characterising the clinical and biomechanical features of severely deformed feet in rheumatoid arthritis. Gait Posture 2008;28:574–80.
- Riente L, Delle Sedie A, Scire CA, Filippucci E, Meenagh G, Iagnocco A, et al. Ultrasound imaging for the rheumatologist. XXXI. Sonographic assessment of the foot in patients with rheumatoid arthritis. Clin Exp Rheumatol 2011;29:1–5.
