Abstract
Objective: To study chronic widespread pain (CWP) over time in patients with spondyloarthritis (SpA), and to identify risk factors for development and persistence of CWP.
Methods: In this cohort study with baseline and 2.5 year follow-up postal surveys, patients with ankylosing spondylitis (AS) and undifferentiated spondyloarthritis (USpA) (47% women) answered questions regarding pain, and were categorized as no chronic pain (NCP), chronic regional pain (CRP), or CWP. For each risk factor candidate (disease duration, body mass index, smoking, and patient-reported outcome measures), logistic regression analyses with CWP as the main outcome were performed separately, together with a basic model including age, gender, and SpA subgroup.
Results: Altogether, 644 patients could be categorized at both time-points, yielding similar prevalence estimates at baseline and follow-up, although 38% transitioned between pain groups. Risk factors (odds ratio; 95% confidence interval) for development of CWP included more pain regions (1.36; 1.20‒1.53), higher pain intensity (1.35; 1.20‒1.52), worse fatigue (1.25; 1.13‒1.38), and worse global health (1.35; 1.19‒1.54). Persistent CWP was reported by 72%. In addition to factors predicting development of CWP, higher age (1.02; 1.00‒1.04), female gender (1.82; 1.06‒3.10), and anxiety (1.07; 1.00–1.14) also predicted persistence.
Conclusion: The prevalence of CWP remained high over time, but with individual transitions between the pain groups. The development and persistence of CWP were predicted by more pain and worse health, with the addition of female gender and higher age for persistent CWP. Special attention and treatment alternatives for patients with SpA and concomitant CWP are essential in the clinic.
Chronic pain is a multifactorial and common disorder, and an important contributor to disability (Citation1). It is identified through having long-lasting pain and can be divided according to the distribution of painful sites throughout the body into chronic regional pain (CRP) and chronic widespread pain (CWP) (Citation2). CWP is also a prerequisite for fibromyalgia (FM) (Citation2), a condition that has been proposed to represent the most extreme end of the CWP continuum (Citation3). In the general population, prevalence estimates of CWP are high (11–15%), and it is more frequently reported by women and older individuals (Citation4–Citation6). CWP has been associated with factors such as fatigue, depression, anxiety, poor sleep, reduction in overall health, and the ability to work (Citation7–Citation10). In patients with FM, compared to those with CWP who did not fulfil the criteria for FM, associations with more severe symptoms have been reported (Citation11).
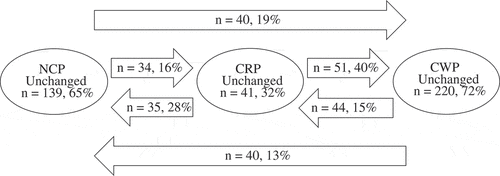
Figure 1. Transition of patients to and from the pain groups between 2009 and 2011. NCP, no chronic pain; CRP, chronic regional pain; CWP, chronic widespread pain.
Ankylosing spondylitis (AS) and undifferentiated spondyloarthritis (USpA) are two subgroups of spondyloarthritis (SpA), which is a family of chronic rheumatic diseases. Chronic back pain due to inflammation in the axial skeleton is a prominent and early feature, and the diseases share several other clinical characteristics including peripheral arthritis, enthesitis, and extra-articular features. AS is the most well-studied and typical disease in the SpA family, with structural axial damage in the sacroiliac joints and the spine, while USpA is a less defined subgroup (Citation12, Citation13). The diagnosis is difficult, and a delay between symptom onset and a clear diagnosis is still common for many SpA patients (Citation14–Citation16), and has, in AS, been associated with worse outcomes (Citation17, Citation18). Patients with SpA frequently report having a diminished quality of life, and factors such as stiffness, pain, and fatigue are important contributors (Citation19, Citation20).
Today, pain in inflammatory chronic arthritis is seen in a wider context than previously, and may also include different biopsychosocial considerations (Citation21, Citation22). In addition to having continuous peripheral inflammatory input that can cause increased sensitivity in peripheral nociceptors, some individuals may also develop a central sensitization, resulting in hyperalgesia and allodynia (Citation23, Citation24). This could partly explain the high prevalence of CWP (34%) reported in patients with rheumatoid arthritis (RA) (Citation25), and our own previous findings were that almost half (47%) of the patients with AS and USpA reported coexisting CWP (Citation26). According to previous research, increasing pain intensity and spread of pain in multiple body regions, together with psychological and social factors, can result in CWP (Citation27–Citation29). In the general population, the risk factors for transitioning from CRP to CWP have been proposed to include female gender, higher age, a family history of pain, psychological distress, and more sites of pain at baseline (Citation27, Citation28). In patients with SpA, however, chronic pain is inadequately identified, and to our knowledge there is little information on the development of chronic pain over time. Thus, our aim was to study how CWP develops over time in patients with SpA, and to identify possible risk factors that would predict the development and persistence of CWP.
Method
Design
This was a prospective longitudinal cohort study, with a baseline postal survey in 2009 and a 2.5 year follow-up survey in 2011, involving patients with a clinical diagnosis of AS and USpA.
Ethics
The study was approved by the Regional Ethical Review Board of Lund University, Sweden (Dnr 301/2007, 406/2008, 2011/547, 2013/128). Informed written consent was obtained from all patients, in compliance with the Declaration of Helsinki.
Study population
The patients were identified through the Skåne Health Care Register (SHCR), during the period 2003‒2007 (the SpAScania cohort). In the SHCR, all healthcare visits for inpatients and outpatients are registered using unique identification numbers, and the register includes information about the healthcare provider, the date of the visit, and the diagnosis codes according to the International Classification of Diseases and Related Health Problems, 10th Revision (ICD-10). Details of the SHCR have previously been published elsewhere (Citation30, Citation31).
In the SpAScania cohort, a diagnosis of SpA was required, given by either a rheumatologist or an internist once, or by any other physician in primary or secondary care on two separate occasions, to ensure higher specificity of the SpA diagnosis. The diagnostic codes for SpA in the SHCR have previously been validated (98% accuracy) (Citation30). Patients who were 18 years and older with ICD-10 codes of SpA [AS, psoriatic arthritis, arthritis associated with inflammatory bowel disease, and USpA] were identified through the healthcare register. A baseline questionnaire was sent in 2009 (n = 3711) and a second questionnaire in 2011 (Citation32). The 2009 questionnaire was answered by 2162 patients (58%). In the present study, only patients with a diagnosis (ICD-10) corresponding to AS (M45) or USpA (M46.0, M46.1, M46.8, M46.9) were included. A previous comparison of non-responders and responders to the SpAScania questionnaire (Citation32) showed that patients with AS were more likely to respond, and that higher age predicted a higher response in both men and women, except for women with AS, who responded less with age.
Chronic pain
The questionnaires consisted of commonly used, well-validated self-reported outcome instruments, including questions regarding duration of pain, distribution of pain, and intensity of pain. Pain was regarded as chronic if it had been persistent or recurrent for more than 3 months in the previous 12 months, and the distribution of pain was categorized using a mannequin with 18 predefined body regions (Citation4). The patients were asked to indicate the location of pain by markings in the mannequin, and each body region was described with explanatory names. According to the 1990 American College of Rheumatology (ACR) criteria for FM (Citation2), CWP was considered to be present if pain was indicated (i) in both sides of the body (left/right), (ii) above and below the waist, and (iii) in the axial regions of the mannequin (the cervical spine, anterior chest, thoracic spine, and lower back). CRP was considered to be present when the criteria for chronic pain were met, but not those for the CWP condition, and patients were regarded as having no chronic pain (NCP) if they answered ‘no’ to the question for defining chronic pain. Pain intensity was assessed with a numeric rating scale (NRS), ranging from 0 (no pain) to 10 (worst possible pain).
Possible predictors
Information regarding age and gender was collected from the SHCR. Disease duration (years), fatigue [NRS; ranging from 0 (no fatigue) to 10 (worst possible fatigue)], global health [NRS; ranging from 0 (good health) to 10 (worst possible health)], smoking habits (ever smoker/never smoker), and body mass index (BMI; normal weight = 18.5–24.9, overweight = 25–29.9, obesity ≥ 30 kg/m2) (Citation33) were self-reported information from the questionnaires. In addition, various validated patient-reported outcome measures were included in the study. The Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) (Citation34) and the Bath Ankylosing Spondylitis Functional Index (BASFI) (Citation35) are two disease-specific instruments for measuring disease activity and physical function in patients with SpA. The BASDAI consists of six questions regarding fatigue, pain, tenderness, and stiffness, and the BASFI consists of 10 questions regarding physical function. The total scores for the indices range between 0 (best) and 10 (worst). The generic EuroQol 5 Dimensions (EQ-5D) (Citation36) is an instrument for measuring a person’s health status and consists of five questions (dimensions) regarding mobility, self-care, usual activity, pain/discomfort, and anxiety/depression. It has a summary score ranging from 0 (no health) to 1 (full health), and in this study we used the UK tariff for calculating the EQ-5D. Self-efficacy was measured by the Arthritis Self-Efficacy Scale (ASES) (Citation37), an instrument developed for measurement of perceived self-efficacy in people with arthritis. ASES includes subscales for handling pain (five items) and for handling other symptoms (six items), and ranges from 10 to 100 (with higher scores representing better self-efficacy). To assess psychological status, the Hospital Anxiety and Depression Scale (HADS) (Citation38) was used. The instrument consists of two subscales with seven questions for anxiety and seven questions for depression, and it ranges from 0 (no symptoms) to 21 (severe symptoms).
Statistical analysis
All analyses were performed using SPSS software version 23 for Windows (IBM Corp., Armonk, NY, USA). Patient characteristics were described with mean ± sd, and differences between groups were analysed with Student’s t-test. Statistical comparisons of prevalence estimates for self-reported pain between 2009 and follow-up were performed with two-sided chi-squared tests. To study predictors for the development and persistence of CWP between 2009 and 2011, logistic regression analyses with odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. Correlation analyses of potential risk factors, such as disease duration, BMI, smoking, pain regions, pain intensity, fatigue, general health, EQ-5D, BASDAI, BASFI, ASES–Pain/Symptoms, and HADS anxiety/depression, showed that some variables were highly correlated. Thus, we used a basic model with age, gender, and SpA subgroup together with separate logistic regression models for each of the independent variables. For each of the variables, logistic regression models were computed with simple contrast to a reference group.
Results
Characteristics
A total of 940 patients diagnosed as having AS or USpA responded to the 2009 questionnaire. Of those, 712 (76%) also responded to the follow-up in 2011, with 433 diagnosed with AS (38% women) and 279 with USpA (60% women). Patients with USpA were significantly younger and had shorter disease duration than patients with AS; they also reported having a higher number of pain regions than patients with AS, both at baseline in 2009 (mean ± sd 5.2 ± 4.8 vs 4.4 ± 4.5) and at follow-up 2.5 years later (5.4 ± 4.8 vs 4.6 ± 4.7). Self-reported use of synthetic disease-modifying anti-rheumatic drugs (sDMARDs) and prednisolone was similar between the two subgroups, but more patients with AS than USpA reported current use of biological disease-modifying anti-rheumatic drugs (bDMARDs) at baseline (19.4% vs 14.7%; p = 0.007) and follow-up (21.9% vs 16.5%; p = 0.017). No differences regarding self-reported sDMARDs, bDMARDs, or prednisolone were found between women and men. The patient characteristics for each subgroup (AS and USpA), and differences between women and men in 2009 and 2011, are presented in and .
Table 1. Patient characteristics and differences for the subgroups ankylosing spondylitis (AS) (n = 433) and undifferentiated spondyloarthritis (USpA) (n = 279) in 2009 and 2011.
Table 2. Characteristics of women (n = 331) and men (n = 381) with spondyloarthritis, 2009 and 2011.
Chronic pain
In total, 670 of the 712 patients answered the pain duration and pain distribution questions in 2009. Of those, 644 also answered the pain questions in 2011, and could be categorized into three groups (NCP, CRP, and CWP) on both occasions. The patients who answered the pain questions in 2009 but not at follow-up (n = 26, 50% women) had a mean age of 51 years and a large percentage were diagnosed as AS (77%). The estimated prevalence of CRP was 19.7% in 2009 and 18.5% in 2011, and for CWP the prevalence was 46.7% in 2009 and 47.7% in 2011.
Transition of patients between the pain groups (NCP, CRP, and CWP)
Of those patients who reported having NCP in 2009, 35% developed chronic pain at follow-up, 16% of whom developed to CRP and 19% to CWP, and 40% of the patients who initially reported CRP in 2009 developed CWP at follow-up. Most (72%) of the patients who reported CWP in 2009 also reported having persistence of CWP at follow-up, but 15% had transitioned to CRP and 13% had transitioned to NCP ().
A higher proportion of patients with USpA than patients with AS (25% vs 15%), transitioned from NCP initially to CWP at follow-up, and more patients with USpA than those with AS reported having persistent CWP between 2009 and 2011 (77% vs 69%; p < 0.001). In addition, more women than men developed to CRP from NCP (24% vs 16%) and reported having persistent CWP (women 78% and men 66%) between 2009 and 2011 (p < 0.001). More women with USpA than women with AS developed to chronic pain from NCP (CRP 27% vs 9%; CWP 30% vs 19%; p < 0.001) (data not shown).
Risk factors for development and persistence of CWP
The results from the logistic regression analysis () showed that development of CWP from NCP or CRP at baseline was predicted by a higher number of pain regions, higher pain intensity, worse fatigue, worse global health, and worse health status (all p < 0.001). In addition, worse self-reported disease activity, physical function, self-efficacy for handling pain and symptoms, and depression predicted development to CWP (when controlling for age, gender, and SpA subgroup). Persistence of CWP, compared to improving from CWP to NCP or CRP (), was predicted by being older (OR 1.02; 95% CI 1.00–1.04) and female gender (OR 1.82; 95% CI 1.06–3.10) when controlling for SpA subgroup. In addition, the same factors that predicted development to CWP, and higher anxiety scores were also risk factors for persistent CWP. No associations were found between persistence of CWP and disease duration, BMI, or smoking.
Table 3. Results from the multiple logistic regression analysis with patients who had developed chronic widespread pain at follow-up (2011), compared to those who still reported no chronic pain or chronic regional pain.
Table 4. Results from the multiple logistic regression analysis with patients who reported having persistent chronic widespread pain at follow-up (2011), compared to those who had improved to no chronic pain or chronic regional pain.
Discussion
In this longitudinal study, the development of chronic pain was assessed over 2.5 years in a cohort of patients diagnosed as having AS or USpA. The prevalence rates for NCP, CRP, and CWP were fairly consistent between baseline and follow-up, but an individual variation was found and more than one-third of the patients transitioned between the pain groups. A similar pattern of risk factors for developing CWP and having persistent CWP was found, and this included a higher number of pain regions, higher pain intensity, worse fatigue, worse general health, worse health status, worse disease activity, lower physical function, lower self-efficacy for handling pain and symptoms, and higher depression scores at baseline. Moreover, persistent CWP was also predicted by older age, female gender, and anxiety; but not by SpA subgroup, disease duration, BMI, or smoking. Few studies have focused on the development of chronic pain over time in SpA patients, and to our knowledge this has been the first study to investigate risk factors for the development of CWP and the persistence of CWP in AS and USpA, making comparisons difficult.
The finding that more than 30% of the patients transitioned between the pain groups during the study period is in line with previous studies investigating risk factors for development and persistence of CWP in the general population (Citation27, Citation39). This transition could possibly reflect a variability within the CWP spectrum, with fluctuating severity of pain and influences of different stressors. It may also reflect the fluctuating nature of rheumatic disease, described as flares in AS (Citation40). We also found that 40% of the patients with CRP at baseline had developed CWP at follow-up. This incidence is almost twice as high as in reports from the general population (Citation41). Moreover, subjects with severe back and neck pain have been reported to have an almost five-fold risk of developing CWP after 6 years (Citation28). In that study, the authors hypothesized that patients with regional spinal pain may be more likely to develop CWP compared to patients with peripheral pain syndromes, and that this could be an indicator of a sensitization to painful stimulation (Citation42). In our cohort, 80% of the SpA patients with CRP reported pain in any of the axial regions of the pain mannequin. A large proportion (72%) of the SpA patients reported having persistent CWP in our study, and this is in accordance with some previous studies in the general population (Citation27, Citation39, Citation43, Citation44), where CWP persisted in about 50% or more of the study population.
We found that more pain regions, higher pain intensity, and worse fatigue were possible risk factors for development to CWP, and for persistence of CWP. Similar findings, with the number of painful regions being a predictor of development of CWP in RA (Citation25) and persistent CWP in the general population, have been reported previously (Citation27). Our results are also interesting in light of the results of a study investigating fatigue in AS (Citation45), in which the authors found a strong association between pain and fatigue, and that residual levels of pain and fatigue persisted after anti-inflammatory (anti-tumour necrosis factor) treatment. Also, in RA, persistent pain, despite effective anti-inflammatory therapy, has been reported in 10–30% of patients (Citation46, Citation47). In addition, a previous study of RA and chronic pain found that changes between DMARDs occurred more frequently in patients with chronic pain, and that more patients with CWP were treated with long-term corticosteroids, compared to those with no chronic pain (Citation25). In the same study, inflammatory parameters such as erythrocyte sedimentation rate and C-reactive protein, and swollen joint count were less associated with CWP compared to more pain-related disease activity variables (28-joint Disease Activity Score, tender joint count, patient global assessment, and Health Assessment Questionnaire). As previously highlighted by Yunus (Citation48), in patients with a chronic disease such as SpA who report having increasing pain and fatigue, recognition and thorough evaluation are very important before increasing medicines such as biologics or corticosteroids.
Patients with worse health status, worse disease activity, worse physical function, less self-efficacy in handling pain and symptoms, and higher anxiety and depression scores had a higher risk of development to CWP (although this only approached significance for anxiety) and of having persistent CWP in this study. These results are in line with findings in the general population, where development and persistence of CWP were found to be predicted by both physical and psychological factors (Citation49). Persistent pain, regardless of type of pain, has previously been reported to affect pain-processing areas in the brain, with consequences for cognitive and emotional factors (Citation50).
In the present study, older age and female gender were associated with persistent CWP, but not with development to CWP from NCP initially or CRP initially. These results are both in accordance with, and conflict with studies in the general population, in which age and female gender were either identified or rejected as possible risk factors for transitioning to CWP from CRP initially (Citation27, Citation28), or in which age and male gender were found to be protective factors for persistent CWP (Citation43). The differences in study populations, designs, and the classification criteria used for CWP (the ACR definition or the Manchester definition) may account for the conflicting results. It is well known that more women than men report having CWP, as was also found in our study, where female gender was a predictor of persistent CWP but not of development of CWP; this finding may partly explain the female predominance in prevalence estimates of CWP.
In addition, neither smoking nor BMI predicted development to CWP or persistent CWP in the present study. These results are in conflict with a study in the general population, where both previous smoking and obesity predicted persistent CWP (Citation43). We also included SpA subgroup (AS and USpA) in the basic model for each logistic regression analysis, but it was not identified as a risk factor. This may be explained by the fact that patients with AS, which typically represents one end of the SpA spectrum, and those with USpA, which is a more variable disease with a blend of SpA features at the other end of the spectrum (Citation13), often report having similar disease burden. In the present study, our intention was to study CWP as this is a requirement for the FM diagnosis, the more severe part of the CWP spectrum, and therefore important to evaluate. In addition to CWP, a diagnosis of FM requires a physical examination.
The strengths of this study include the longitudinal data and the relatively large study population. The pain measures are validated, well-known, and standardized self-reported measures that are often used in clinical practice. There are also some limitations to consider in the study, one of which is that the relatively low response rate may have introduced bias and affected the generalizability, as in other questionnaire-based surveys (Citation4, Citation43). There is also a need for more formal guidelines in the setting of SpA diagnoses in the clinic. In this study, the patients were included on the basis of the ICD-10 codes for AS or USpA, and not the more recent Assessment of Spondylo-Arthritis international Society (ASAS) classification criteria for axSpA. The clinical visits were patient driven, and the ICD-10 diagnosis was a result of the individual physician’s judgement, which may have caused misclassification. However, we found few differences between the AS and USpA groups, and SpA diagnosis did not predict either development to or persistence of CWP. Symptoms such as chronic back pain, stiffness, and fatigue are common in both SpA and CWP. In the present study, the prevalence rates of CWP were based on the common ACR definition of CWP. With this definition, a wider spectrum with cases of less severe CWP may also have been included, in comparison to the case if the more stringent Manchester definition had been used. The study lacked information on medication for treating pain, anxiety, or depression, and the information available on anti-inflammatory medications as described in and was self-reported data regarding sDMARDs, bDMARDs, and prednisolone.
Conclusion
The development and persistence of concomitant CWP in SpA is complex. We found that the prevalence of CWP remained high over time and that the risk factors for development of CWP and persistence of CWP were similar, and included pain, fatigue, and different aspects of health. Since CWP develops and persists more often in patients with SpA, special attention to risk factors and pain development is essential in the clinic, including knowledge regarding treatment strategies for both the inflammatory- and the non-inflammatory-driven pain. Further research on concomitant CWP in SpA is needed to better understand the complex interactions driving the spread and persistence of CWP.
Acknowledgements
We thank the SpAScania group for assistance with data extraction.
This study was supported by grants from the Region Skåne, Sweden, and the Swedish Rheumatism Association. All funding was received as unrestricted grants.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012;380:2163–96.
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American college of rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum 1990;33:160–72.
- Croft P, Burt J, Schollum J, Thomas E, Macfarlane G, Silman A. More pain, more tender points: is fibromyalgia just one end of a continuous spectrum? Ann Rheum Dis 1996;55:482–5.
- Bergman S, Herrstrom P, Hogstrom K, Petersson IF, Svensson B, Jacobsson LT. Chronic musculoskeletal pain, prevalence rates, and sociodemographic associations in a Swedish population study. J Rheumatol 2001;28:1369–77.
- Croft P, Rigby AS, Boswell R, Schollum J, Silman A. The prevalence of chronic widespread pain in the general population. J Rheumatol 1993;20:710–13.
- Macfarlane GJ, McBeth J, Silman AJ. Widespread body pain and mortality: prospective population based study. BMJ 2001;323:662–5.
- Bergman S. Psychosocial aspects of chronic widespread pain and fibromyalgia. Disabil Rehabil 2005;27:675–83.
- Kamaleri Y, Natvig B, Ihlebaek CM, Benth JS, Bruusgaard D. Number of pain sites is associated with demographic, lifestyle, and health-related factors in the general population. Eur J Pain 2008;12:742–8.
- Miranda H, Kaila-Kangas L, Heliovaara M, Leino-Arjas P, Haukka E, Liira J, et al. Musculoskeletal pain at multiple sites and its effects on work ability in a general working population. Occup Environ Med 2010;67:449–55.
- Davies KA, Macfarlane GJ, Nicholl BI, Dickens C, Morriss R, Ray D, et al. Restorative sleep predicts the resolution of chronic widespread pain: results from the EPIFUND study. Rheumatology (Oxford) 2008;47:1809–13.
- Coster L, Kendall S, Gerdle B, Henriksson C, Henriksson KG, Bengtsson A. Chronic widespread musculoskeletal pain - a comparison of those who meet criteria for fibromyalgia and those who do not. Eur J Pain 2008;12:600–10.
- Dougados M, van der Linden S, Juhlin R, Huitfeldt B, Amor B, Calin A, et al. The European spondylarthropathy study group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum 1991;34:1218–27.
- Burgos-Vargas R, Casasola-Vargas JC. From retrospective analysis of patients with undifferentiated spondyloarthritis (SpA) to analysis of prospective cohorts and detection of axial and peripheral SpA. J Rheumatol 2010;37:1091–5.
- Feldtkeller E, Khan MA, van der Heijde D, van der Linden S, Braun J. Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol Int 2003;23:61–6.
- Masson Behar V, Dougados M, Etcheto A, Kreis S, Fabre S, Hudry C, et al. Diagnostic delay in axial spondyloarthritis: A cross-sectional study of 432 patients. Joint Bone Spine 2017;84:467–71.
- Slobodin G, Reyhan I, Avshovich N, Balbir-Gurman A, Boulman N, Elias M, et al. Recently diagnosed axial spondyloarthritis: gender differences and factors related to delay in diagnosis. Clin Rheumatol 2011;30:1075–80.
- Aggarwal R, Malaviya AN. Diagnosis delay in patients with ankylosing spondylitis: factors and outcomes–an Indian perspective. Clin Rheumatol 2009;28:327–31.
- Ibn Yacoub Y, Amine B, Laatiris A, Bensabbah R, Hajjaj-Hassouni N. Relationship between diagnosis delay and disease features in Moroccan patients with ankylosing spondylitis. Rheumatol Int 2012;32:357–60.
- Ward MM. Health-related quality of life in ankylosing spondylitis: a survey of 175 patients. Arthritis Care Res 1999;12:247–55.
- Kiltz U, Baraliakos X, Karakostas P, Igelmann M, Kalthoff L, Klink C, et al. Do patients with non-radiographic axial spondylarthritis differ from patients with ankylosing spondylitis? Arthritis Care Res (Hoboken) 2012;64:1415–22.
- Fitzcharles MA, Almahrezi A, Shir Y. Pain: understanding and challenges for the rheumatologist. Arthritis Rheum 2005;52:3685–92.
- Clauw DJ, Witter J. Pain and rheumatology: thinking outside the joint. Arthritis Rheum 2009;60:321–4.
- Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011;152(Suppl 3):S2–15.
- Scholz J, Woolf CJ. Can we conquer pain? Nat Neurosci 2002;5:1062–7.
- Andersson ML, Svensson B, Bergman S. Chronic widespread pain in patients with rheumatoid arthritis and the relation between pain and disease activity measures over the first 5 years. J Rheumatol 2013;40:1977–85.
- Mogard E, Bremander A, Lindqvist E, Bergman S. Prevalence of chronic widespread pain in a population-based cohort of patients with spondyloarthritis – a cross-sectional study. BMC Rheumatol 2018;2:11.
- Bergman S, Herrstrom P, Jacobsson LT, Petersson IF. Chronic widespread pain: a three year followup of pain distribution and risk factors. J Rheumatol 2002;29:818–25.
- Kindler LL, Jones KD, Perrin N, Bennett RM. Risk factors predicting the development of widespread pain from chronic back or neck pain. J Pain 2010;11:1320–8.
- Gupta A, Silman AJ, Ray D, Morriss R, Dickens C, MacFarlane GJ, et al. The role of psychosocial factors in predicting the onset of chronic widespread pain: results from a prospective population-based study. Rheumatology (Oxford) 2007;46:666–71.
- Haglund E, Bremander AB, Petersson IF, Strombeck B, Bergman S, Jacobsson LT, et al. Prevalence of spondyloarthritis and its subtypes in southern Sweden. Ann Rheum Dis 2011;70:943–8.
- Englund M, Joud A, Geborek P, Felson DT, Jacobsson LT, Petersson IF. Prevalence and incidence of rheumatoid arthritis in southern Sweden 2008 and their relation to prescribed biologics. Rheumatology (Oxford) 2010;49:1563–9.
- Haglund E, Bergman S, Petersson IF, Jacobsson LT, Strombeck B, Bremander A. Differences in physical activity patterns in patients with spondylarthritis. Arthritis Care Res (Hoboken) 2012;64:1886–94.
- WHO. Physical status: the use and interpretation of anthropometry. World Health Organization 1995 (http://apps.who.int/iris/bitstream/handle/10665/37003/WHO_TRS_854.pdf). Accessed 3 April 2019.
- Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the bath ankylosing spondylitis disease activity index. J Rheumatol 1994;21:2286–91.
- Calin A, Garrett S, Whitelock H, Kennedy LG, O‘Hea J, Mallorie P, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the bath ankylosing spondylitis functional index. J Rheumatol 1994;21:2281–5.
- Hurst NP, Jobanputra P, Hunter M, Lambert M, Lochhead A, Brown H. Validity of Euroqol–a generic health status instrument–in patients with rheumatoid arthritis. Economic and health outcomes research group. Br J Rheumatol 1994;33:655–62.
- Lorig K, Chastain RL, Ung E, Shoor S, Holman HR. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis Rheum 1989;32:37–44.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70.
- McBeth J, Macfarlane GJ, Hunt IM, Silman AJ. Risk factors for persistent chronic widespread pain: a community-based study. Rheumatology (Oxford) 2001;40:95–101.
- Brophy S, Calin A. Definition of disease flare in ankylosing spondylitis: the patients‘ perspective. J Rheumatol 2002;29:954–8.
- Larsson B, Bjork J, Borsbo B, Gerdle B. A systematic review of risk factors associated with transitioning from regional musculoskeletal pain to chronic widespread pain. Eur J Pain 2012;16:1084–93.
- Flor H, Diers M, Birbaumer N. Peripheral and electrocortical responses to painful and non-painful stimulation in chronic pain patients, tension headache patients and healthy controls. Neurosci Lett 2004;361:147–50.
- Mundal I, Grawe RW, Bjorngaard JH, Linaker OM, Fors EA. Prevalence and long-term predictors of persistent chronic widespread pain in the general population in an 11-year prospective study: the HUNT study. BMC Musculoskelet Disord 2014;15:213.
- Papageorgiou AC, Silman AJ, Macfarlane GJ. Chronic widespread pain in the population: a seven year follow up study. Ann Rheum Dis 2002;61:1071–4.
- Brophy S, Davies H, Dennis MS, Cooksey R, Husain MJ, Irvine E, et al. Fatigue in ankylosing spondylitis: treatment should focus on pain management. Semin Arthritis Rheum 2013;42:361–7.
- Lee YC, Cui J, Lu B, Frits ML, Iannaccone CK, Shadick NA, et al. Pain persists in DAS28 rheumatoid arthritis remission but not in ACR/EULAR remission: a longitudinal observational study. Arthritis Res Ther 2011;13:R83.
- Altawil R, Saevarsdottir S, Wedren S, Alfredsson L, Klareskog L, Lampa J. Remaining pain in early rheumatoid arthritis patients treated with methotrexate. Arthritis Care Res (Hoboken) 2016;68:1061–8.
- Yunus MB. The prevalence of fibromyalgia in other chronic pain conditions. Pain Res Treat 2012;2012:584573.
- Bergman S, Jacobsson LT, Herrstrom P, Petersson IF. Health status as measured by SF-36 reflects changes and predicts outcome in chronic musculoskeletal pain: a 3-year follow up study in the general population. Pain 2004;108:115–23.
- Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 2013;14:502–11.
