Abstract
Objective: Systemic features influence disease prognosis and choice of treatment in primary Sjögren’s syndrome (pSS). Our aim was to investigate the prevalence of pulmonary involvement in pSS patients and to classify patients according to the pulmonary domain of the EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI).
Methods: This retrospective cohort study included consecutive pSS patients, fulfilling American–European Consensus Group/American College of Rheumatology classification criteria, who visited the Department of Rheumatology and Clinical Immunology, University Medical Center Groningen, in 2015. Data on pulmonary complaints and pulmonary tests were obtained from electronic patient records. Pulmonary involvement was recorded if therapy was needed or follow-up was recommended, and when it was possibly or assumed to be related to pSS instead of coincidental factors.
Results: Of the 262 included pSS patients, 88 (34%) had pulmonary complaints, mostly cough or dyspnoea on exertion. Pulmonary diagnostics were performed in 225 patients (86%). Pulmonary involvement was present and assumed to be related to pSS in 25 patients (10%) and possibly related to pSS in 14 (5%). Interstitial lung disease (ILD, n = 15), especially non-specific interstitial pneumonia (n = 7), was present most commonly. In total, 16 patients (6%) were scored as low (n = 4), moderate (n = 11), or high activity (n = 1) on the ESSDAI pulmonary domain.
Conclusion: In this cross-sectional study in daily clinical practice, pulmonary involvement was present in 10–15% of pSS patients, of which ILD was most common. Of all pSS patients, 6% were scored as active on the pulmonary domain of the ESSDAI.
Primary Sjögren’s syndrome (pSS) is a common systemic autoimmune disease characterized by lymphocytic infiltration of the exocrine glands. Besides sicca symptoms, many patients have extraglandular involvement, e.g. arthritis, cutaneous vasculitis, nephritis, and/or pulmonary involvement.
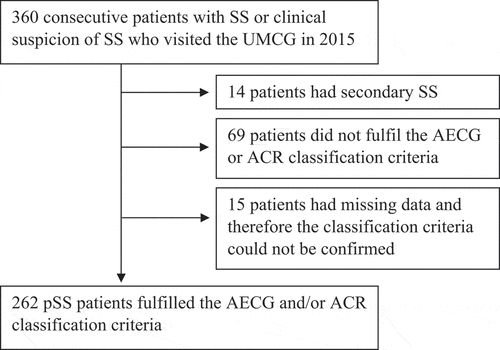
Figure 1. Flowchart of primary Sjögren’s syndrome (pSS) patients included in the analyses. SS, Sjögren’s syndrome; UMCG, University Medical Center Groningen.
In previous studies, the estimated prevalence of lung involvement in pSS has varied greatly, ranging from 9% to 75%, mostly depending on differences in the imaging modality used and criteria of pulmonary involvement (Citation1–Citation4). When pulmonary involvement is defined as the presence of pulmonary symptoms with an abnormal pulmonary function test (PFT) or radiograph, the prevalence of pulmonary involvement is reported to be 9– 24% (Citation1, Citation4, Citation5).
Most pulmonary manifestations in patients with pSS can be categorized as airway abnormalities, interstitial pneumonias, or lymphoproliferative disorders (Citation2). Pleural effusion and pulmonary hypertension also occur in pSS, although these are far less common in pSS than in other connective tissue diseases, such as scleroderma (Citation6–Citation9). As the patterns of pulmonary lesions are often mixed in pSS, some pulmonary manifestations are difficult to classify (Citation10, Citation11). Abnormal findings in the lungs of patients with pSS are predominant in the subpleural areas and lower lobes, as is typically seen in connective tissue disease (Citation12–Citation17).
The European League Against Rheumatism (EULAR) Sjögren’s Syndrome Disease Activity Index (ESSDAI) has been developed to measure systemic disease activity in various organs (Citation5, Citation18, Citation19). In the ESSDAI, pulmonary involvement is defined as the presence of pulmonary symptoms (persistent cough and/or dyspnoea) and abnormalities in pulmonary diagnostic tests such as PFTs and/or high-resolution computed tomography (HRCT).
Systemic features, such as pulmonary involvement, clearly mark the prognosis of pSS and can influence the choice of treatment. A retrospective study showed a significant decrease in physical functioning in patients with pulmonary involvement compared to patients without pulmonary involvement which influences the quality of life (Citation20). Previous studies have shown that the prevalence of pulmonary involvement is probably considerable, but the rates are very different in the various studies. Therefore, the purpose of this study was to further investigate the prevalence and types of pulmonary involvement in pSS patients and to classify the manifestations according to the pulmonary domain of the ESSDAI.
Method
Study population
The study population was selected from the Diagnosis Treatment Combination (DTC) list of all consecutive patients who visited the Sjögren’s syndrome expertise centre of the University Medical Center Groningen (UMCG) in 2015. Inclusion criteria were a clinical diagnosis of pSS and fulfilment of the American–European Consensus Group (AECG) (Citation21) and/or American College of Rheumatology (ACR) (Citation22) classification criteria for pSS. Data were obtained retrospectively from electronic patient records.
Pulmonary involvement
Primary study parameters were the number and type of pulmonary complaints, the number of pulmonary tests performed, and the outcomes of these tests. The following pulmonary complaints were recorded: persistent cough, dyspnoea during exercise, dyspnoea at rest, and recurrent lower respiratory infections. Pulmonary tests were performed only when there was a clinical suspicion of lung involvement, and included conventional chest radiography, computed tomography (CT), PFTs, and pulmonary biopsy (n = 6). The most recently performed tests were recorded. Some radiological tests (five chest radiographs and seven CTs) were performed in and imported from other hospitals.
Pulmonary involvement was recorded when imaging of the lungs, PFTs, or pulmonary biopsies showed abnormalities and both of the following conditions applied: (i) therapy was needed and/or follow-up was recommended; and (ii) there was an assumed or possible relation with pSS. The investigator (AH) defined pulmonary manifestations as ‘assumed to be related to pSS’ when the pulmonary condition was known to occur in association with pSS, such as interstitial lung disease (ILD), and other causes could be excluded, such as drug-induced ILD. In addition, pulmonary manifestations were defined as ‘assumed to be related to pSS’ when the treating pulmonologist or rheumatologist, who has the overall clinical picture, interpreted the pulmonary manifestations as assumed to be related to pSS. Pulmonary manifestations were defined as ‘possibly related to pSS’ when it was not evident whether pulmonary manifestations were caused by pSS or by coincidental factors. In uncertain cases, the association with pSS was determined by an experienced pulmonologist (GDN). Pulmonary manifestations were assumed not to be related to pSS when, for example, chronic obstructive pulmonary disease (COPD) was present in current or previous smokers. COPD was noted as possibly related to pSS when it was present in non-smokers.
Active pulmonary involvement was classified according to the pulmonary domain of the ESSDAI (Citation18, Citation19).
Other assessments
Secondary study parameters included demographic and clinical data (gender, age, time since diagnosis of pSS, and duration of symptoms of pSS), smoking status, EULAR Sjögren’s Syndrome Patient Reported Index (ESSPRI), ESSDAI, parotid or labial salivary gland focus score, laboratory parameters [anti-Sjögren’s syndrome antigen A and B (anti-SSA, anti-SSB), antinuclear antibodies (ANAs), immunoglobulin G (IgG), rheumatoid factor (RF), complement C3, complement C4, cryoglobulin, and paraprotein] and current use of drugs to treat pulmonary conditions and systemic drugs with potential influence on the lungs.
Statistical analysis
Statistical analysis was performed using SPSS Statistics for Windows, version 23 (IBM Corp., Armonk, NY, USA). Results were expressed as number of patients (%), mean ± sd, or median [interquartile range (IQR)] for categorical, normally distributed, and non-normally distributed data, respectively. To compare differences between patients with and without pulmonary involvement, we used the chi-squared and Fisher’s exact tests for categorical data, and the independent samples t-test and Mann–Whitney U-test for continuous data. A p-value < 0.05 was considered statistically significant.
Results
Patient characteristics
In total, 360 patients with Sjögren’s syndrome (SS) or clinical suspicion of SS visited the UMCG in 2015. Of these patients, 14 patients had secondary SS, most commonly in combination with rheumatoid arthritis, 69 patients did not fulfil the AECG or ACR classification criteria, and 15 patients had missing data and therefore the classification could not be confirmed ().
Of the 262 included pSS patients, the mean age was 56 ± 15 years, and most patients were female (93%) and anti-SSA positive (93%). Median time since diagnosis was 6 years (IQR 2–11 years). Additional patient characteristics are shown in Supplementary table S1.
Pulmonary complaints
One or more pulmonary complaints were present in 88 patients (34%). Cough was reported most often; this was present in 70 pSS patients (27%). Dyspnoea on exertion was present in 42 patients (16%) and recurrent lower airway infections in 17 patients (7%). Dyspnoea at rest was not mentioned by any of the patients.
Pulmonary involvement related and possibly related to pSS
Pulmonary involvement was found in 25–39 pSS patients (10–15%). This range is composed of pulmonary manifestations assumed to be related to pSS (10%) and pulmonary manifestations possibly related to pSS (5%) (). Overall, ILD was most common, being present in 15 patients; in particular, non-specific interstitial pneumonia (NSIP) was present in seven patients, of whom four had fibrotic NSIP. Usual interstitial pneumonia (UIP) was not found in any of the patients (). A mucosa-associated lymphoid tissue (MALT) lymphoma in the lung was seen in two patients, who also had MALT lymphomas in other parts of the body: in the submandibular and parotid glands (both patients), in the conjunctiva of the right eye (one patient), and in lymph nodes in the neck (the other patient).
Table 1. Number and types of pulmonary manifestations which are possibly or assumed to be related to primary Sjögren’s syndrome (pSS).
Undefined features were seen in three patients. For one patient, these included dyspnoea during exercise and abnormal PFT with slightly reduced diffusing capacity of the lungs for carbon monoxide (DLCO) and restrictive abnormalities. Another patient had recurrent lower respiratory infections without bronchiectasis or bronchopathy, with a solid globular lesion of 7 mm and a second smaller lesion in the lung. The third patient had also a stationary solid globular nodule of 5 mm on CT.
An overview of all clinically relevant pulmonary manifestations, defined as not related, possibly related, or assumed to be related to pSS, is shown in .
Figure 2. Flowchart of pulmonary manifestations related and not related to primary Sjögren’s syndrome (pSS): all manifestations (26% of the pSS patients), not related to pSS (11%), possibly related to pSS (5%), and assumed to be related to pSS (10%). COPD, chronic obstructive pulmonary disease; ILD, interstitial lung disease; MALT, mucosa-associated lymphoid tissue.
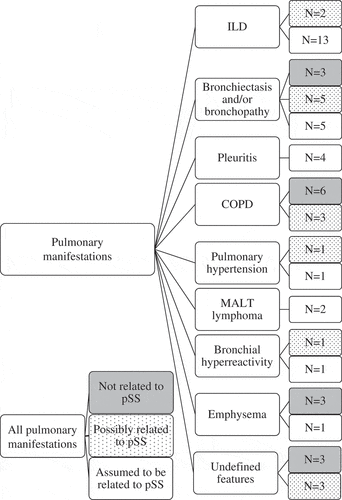
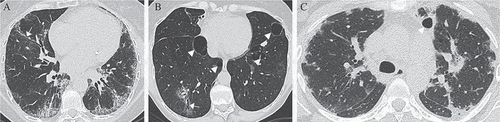
Figure 3. Types of interstitial pneumonia on high-resolution computed tomography (HRCT). (A) A primary Sjögren’s syndrome (pSS) patient with fibrotic non-specific interstitial pneumonia. HRCT shows reticular abnormalities with traction bronchiolectasis (white arrow), ground-glass opacity and lymphadenopathy. (B) A pSS patient with lymphocytic interstitial pneumonia. HRCT shows multiple air cysts (white arrowheads), ground-glass opacity, and traction bronchiolectasis (white arrow). (C) A pSS patient with organizing pneumonia. HRCT shows bilateral patchy consolidations with peripheral and peribronchial predominance and an air cyst (white arrowhead).
Diagnostic abnormalities
Pulmonary diagnostics had been performed in 225 (86%) patients, of which chest radiography was most frequently performed (). Chest radiography was performed in 24 (96%) and 13 (93%) patients with pulmonary involvement assumed to be or possibly related to pSS, respectively. In 18 (46%) of the patients in both groups, abnormalities were seen on the radiographs, including bronchopathy, atelectasis, reticular opacities, fibrotic residual features, and pleural effusion.
Table 2. Pulmonary diagnostics.
CT or HRCT was performed in 24 (96%) and in 10 (71%) of the patients with pulmonary involvement assumed to be or possibly related to pSS, respectively. The (HR)CT scans of both groups showed the following features: ground-glass opacities (n = 10; 29%), nodular thickening (n = 8; 24%), thin-walled air cysts (n = 6; 18%), atelectasis (n = 5; 15%), reticular interstitial pattern (n = 4; 12%), pleural thickening (n = 4; 12%), pleural effusion (n = 3; 9%), and thickening of interlobular septa (n = 2; 6%). Honeycombing was not seen in any of the patients. Ground-glass opacities were predominantly present in the lower lobes of the lungs. When air cysts were present, there were often 10 cysts or more (n = 4), with a diffuse distribution. Three examples of interstitial pneumonia on HRCT seen in this cohort are shown in .
PFTs were performed in 24 (96%) and in 10 (71%) of the patients with pulmonary involvement assumed to be or possibly related to pSS, respectively. PFTs of patients with possible or assumed pulmonary involvement in pSS showed restriction more often than obstruction: n = 10 (36%) and n = 7 (21%) respectively.
Pulmonary manifestations not related to pSS
Current pulmonary manifestations which were supposed not to be related to pSS were present in 29 patients (11%) (). Most common was asthma (n = 14), followed by smoking-related conditions: COPD (n = 6), emphysema (n = 3), and bronchiectasis and/or bronchopathy (n = 3). One patient, with bronchiectasis and bronchopathy, had residual defects after a lobectomy for lung cancer. Three patients had undefined features: isolated mildly reduced DLCO with a history of smoking, atelectasis with pleural effusion, and one patient with a stable hyperdense structure in the mediastinum.
Pulmonary domain of the ESSDAI
In total, 16 patients (6%) had a positive ESSDAI for the pulmonary domain: four patients with low, 11 patients with moderate, and one patient with high activity. Pulmonary involvement in pSS was not scored in the ESSDAI of patients with long-lasting features (> 1 year) which were assumed to be related to damage (n = 15), MALT lymphoma which was rated in another domain (lymphadenopathy and lymphoma; n = 2), or when (HR)CT and/or PFTs had not been recently performed (n = 6).
Comparison of patients with and without pulmonary involvement
Patients with pulmonary involvement were significantly older, had a longer disease duration and higher ESSDAI score, and more often had a positive salivary gland biopsy (focus score ≥ 1) than patients without pulmonary involvement. Patients with pulmonary involvement less often had positive ANAs. No significant differences in gender, smoking status, or other laboratory parameters were found (). When analysing only patients with activity for the pulmonary domain of the ESSDAI, comparable results were found, except that no significant difference was found in time since diagnosis and a significant difference was found in paraproteinaemia (). Additional patient characteristics of pSS patients with and without ILD are shown in Supplementary table S2.
Table 3. Characteristics of primary Sjögren’s syndrome (pSS) patients with and without pulmonary involvement and with active pulmonary involvement according to the pulmonary domain of the EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI).
Prevalence of COPD
COPD was clinically diagnosed in nine (3.4%) of the entire population, of which six cases (2.3%) were smoking related and three (1.1%) were non-smoking related. In the 147 patients who underwent a PFT, forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) < 0.70 was present in 22 (15%) of patients.
Discussion
In this large cross-sectional retrospective cohort study, we investigated the prevalence of different types of pulmonary involvement in pSS in daily clinical practice and rated them according to the pulmonary domain of the ESSDAI.
We found pulmonary involvement assumed to be related to pSS in 10% of patients and pulmonary manifestations possibly related to pSS in 5% of patients. This prevalence is in line with two reviews reporting pulmonary involvement in approximately 10–20% of the pSS patients (Citation1, Citation23). However, our prevalence of 10–15% is probably an underestimation. Pulmonary tests, other than chest radiography, were usually only performed when pulmonary symptoms were present. Symptoms are not always correlated with abnormalities on HRCT (Citation14). Therefore, pulmonary involvement in asymptomatic patients was not included.
In the present study, ILD was the most prevalent pulmonary manifestation related to pSS, being slightly more common than airway abnormalities. The most common type of ILD was NSIP, in concordance with previous studies in pSS (Citation2, Citation23–Citation27). Fibrotic NSIP was present in four of the seven patients with NSIP, also in concordance with previous studies which showed that fibrotic NSIP was more common than non-fibrotic NSIP (Citation23, Citation26). Some studies suggested that airway abnormalities are more common in pSS but are often subclinical (Citation1, Citation28). When we include traction bronchiectasis secondary to ILD, we also found a higher prevalence of airway abnormalities than ILD. Airway abnormality is a broad concept and it can be difficult to differentiate whether it is related to pSS or other causes of airway abnormality, such as recurrent pneumonia.
In our cohort, we found a COPD prevalence of only 3.4%. However, in many patients no recent PFTs had been performed, and in patients who did undergo a PFT, FEV1/FVC < 0.70 was present in 15% of patients. These data suggest that the prevalence of COPD is probably underestimated, and that even in patients with COPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria, COPD is often not clinically diagnosed. Two studies by one research group, including one longitudinal study in which a PFT was performed in all participants, showed a COPD prevalence of 37–41% in pSS patients, which was much higher compared to the general population (Citation29, Citation30). Even in never-smokers, the prevalence of COPD was 30% (Citation30). This high prevalence could partly be explained by the large number of subclinical COPD patients when performing a PFT in all patients. In addition, a cut-off of FEV1/FVC < 0.70 probably leads to an overestimation of COPD in elderly patients (Citation31). Despite these limitations, it is likely that COPD is common in pSS patients. In line with these findings, a retrospective cohort study showed that the incidence of COPD was 1.4-fold greater in pSS patients than in non-SS patients (Citation32). A 2017 study showed no significant difference in pulmonary symptoms in pSS patients with or without concomitant COPD, suggesting that other factors, such as airway sicca, are more important in eliciting such symptoms in pSS patients (Citation33). Another study showed an increase in specific cytokines in sputum of never-smoking pSS patients, suggesting a specific ongoing inflammatory process in the airways among pSS patients (Citation34).
In previous studies, the prevalence of obstruction and restriction in PFT in pSS patients with pulmonary involvement has varied. Some studies reported that the most common finding is restriction (Citation35), while others suggested that obstruction may be more common (Citation12, Citation28, Citation30). We found restriction more often than obstruction, which can be explained by the high prevalence of ILD in pSS.
The ESSDAI was developed to measure systemic disease activity in pSS patients (Citation18, Citation19). In our cohort, 6% of the 262 pSS patients had a positive ESSDAI on the pulmonary domain: 2% had low, 4% moderate, and 0% (one patient) high activity. In the study by Nilsson et al, including 51 consecutive pSS patients with pulmonary tests, 55% had a positive ESSDAI on the pulmonary domain: 39% had low, 14% moderate, and 2% high activity (Citation30). This large difference in pulmonary activity according to the ESSDAI scores is probably based on similar reasons to the difference in pulmonary involvement in pSS. In their study, all patients were assessed by PFT and (HR)CT, while in the current study additional pulmonary tests were performed only when there was a clinical suspicion of lung involvement. Furthermore, the ESSDAI was calculated retrospectively in almost all patients from our cohort. This may have resulted in an underestimation of the ESSDAI scores.
The ESSDAI is a good instrument for rating active pulmonary involvement, but the ESSDAI score is not a complete reflection of pulmonary complaints caused by pulmonary involvement in pSS. A history of active pulmonary involvement in pSS can also cause stable pulmonary complaints as a result of residual defects such as fibrosis. These defects are not rated in the ESSDAI, because stable, long-lasting features present for > 1 year are assumed to be no longer active. However, it can be difficult to determine whether pulmonary involvement is stable, when no previous tests are available. Longitudinal, prospective studies are needed to accurately determine the percentage of patients with active and progressive pulmonary involvement according to the ESSDAI. MALT lymphomas in the lungs and lymphadenopathy in the mediastinum are not included in the pulmonary domain of the ESSDAI (since they are scored in the lymphadenopathy domain), even though they can cause pulmonary complaints.
In the current study, older age and longer disease duration were found to be associated with pulmonary involvement in pSS. Three previous studies also reported that older age at disease onset and/or longer disease duration were associated with a higher risk of pulmonary involvement in pSS (Citation13, Citation36, Citation37). A prospective study published in 2017 found that age of non-sicca onset was higher in pSS patients with pulmonary involvement (Citation38). No clear explanation can be given. However, in the general population the prevalence of bronchiectasis and interstitial lung abnormalities seems to increase with age (Citation39). Another prospective study showed a male predominance in pSS patients with ILD (Citation40). We could not confirm this finding.
Unexpectedly, we found a significantly lower prevalence of ANA positivity and lower RF levels in patients with active pulmonary involvement, while paraproteinaemia was more common in this group. There were no significant differences in other laboratory parameters between patients with and without pulmonary involvement. In contrast, Palm et al found that anti-La/SSB positivity, hypergammaglobulinaemia, RF positivity, and lymphopenia were more prevalent in patients with pulmonary involvement (Citation20). We cannot explain these different results. In the current study, the IgG titre was lower in patients with pulmonary involvement than in patients with no pulmonary involvement, and this difference almost reached statistical significance. A possible explanation may be that the higher use of immunosuppressive medication in patients with pulmonary involvement influenced the IgG titre in patients with pulmonary involvement.
In this study, clinical data from visits in 2015 were used. However, we also included the results of the most recently performed diagnostic tests. Patients who were asymptomatic in 2015 therefore may have had symptoms in the past, when the radiographies or PFTs were performed. In addition, all chest radiographs were included, regardless of the indications. For example, some patients underwent X-rays to screen for infectious diseases prior to immunosuppressive treatment, or as part of preoperative screening.
The strength of this study is the inclusion of a large number of consecutive pSS patients from daily clinical practice, resulting in a cohort that is representative of the general pSS population. Our hospital has a multidisciplinary SS expertise centre. The main limitation of the present study is the retrospective design, which resulted in some missing data. Not all radiographs and (HR)CT were uniformly reported by different radiologists and various indications. Sometimes, there was no report at all, in the case of imported external diagnostics from other hospitals. However, an experienced pulmonologist (GDN) specializing in ILD reviewed the diagnostics of those cases, to limit the influence of interobserver variability.
Based on our results, we would like to underline the clinical relevance of early recognition for treatment of pulmonary involvement in pSS. Based on the high prevalence reported in our study and the possible consequences for treatment of pulmonary involvement in pSS, we strongly recommend screening for pulmonary involvement in pSS patients in daily clinical practice. Our advice for screening is to perform a pulmonary anamnesis, PFT, and chest radiograph in every pSS patient, and HRCT if indicated. More prospective studies are needed to accurately determine the proportion of patients with active and progressive pulmonary involvement in pSS and the cost- effectiveness of screening for pulmonary involvement.
Conclusion
In this large cross-sectional study, pulmonary involvement was found in 10–15% of the patients, comprising 10% assumed to be related to pSS and 5% possibly related to pSS. Most common was ILD, especially NSIP, followed by lymphocytic interstitial pneumonia and organizing pneumonia. UIP was not seen in any of the patients. Of all pSS patients, 6% were scored as active on the pulmonary domain of the ESSDAI, most with moderate activity. These findings underline the need for awareness of pulmonary involvement in patients with pSS in daily clinical practice.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Supplementary table S1. Characteristics of the pSS study population.
Supplementary table S2. Characteristics of pSS patients with and without ILD.
Please note that the editors are not responsible for the content or functionality of any supplementary material supplied by the authors. Any queries should be directed to the corresponding author.
Supplemental Material
Download PDF (171.7 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Kreider M, Highland K. Pulmonary involvement in Sjögren syndrome. Semin Respir Crit Care Med 2014;35:255–64.
- Egashira R, Kondo T, Hirai T, Kamochi N-Y, Yakushiji M, Yamasaki F, et al. CT findings of thoracic manifestations of primary Sjögren syndrome: radiologic-pathologic correlation. Radiographics 2013;33:1933–49.
- Sarkar PK, Patel N, Furie RA, Talwar A. Pulmonary manifestations of primary Sjögren’s syndrome. Indian J Chest Dis Allied Sci 2009;51:93–101.
- Mathai SC, Danoff SK. Management of interstitial lung disease associated with connective tissue disease. BMJ 2016;352:h6819.
- Ramos-Casals M, Brito-Zerón P, Seror R, Bootsma H, Bowman SJ, Dörner T, et al. Characterization of systemic disease in primary Sjögren’s syndrome: EULAR-SS Task Force recommendations for articular, cutaneous, pulmonary and renal involvements. Rheumatology (Oxford) 2015;54:2230–8.
- Stojan G, Baer AN, Danoff SK. Pulmonary manifestations of Sjögren’s syndrome. Curr Allergy Asthma Rep 2013;13:354–60.
- Ma D, Lu H, Qu Y, Wang S, Ying Y, Xiao W. Primary Sjögren’ s syndrome accompanied by pleural effusion: a case report and literature review. Int J Clin Exp Pathol 2015;8:15322–7.
- Ienopoli S, Carsons SE. Extraglandular manifestations of primary Sjögren’s syndrome. Oral Maxillofac Surg Clin North Am 2014;26:91–9.
- Shahane A. Pulmonary hypertension in rheumatic diseases: epidemiology and pathogenesis. Rheumatol Int 2013;33:1655–67.
- Travis WD, Costabel U, Hansell DM, King TE, Lynch DA, Nicholson AG, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733–48.
- Gruden JF, Panse PM, Leslie KO, Tazelaar HD, Colby TV. UIP diagnosed at surgical lung biopsy, 2000-2009: HRCT patterns and proposed classification system. Am J Roentgenol 2013;200:W458–67.
- Uffmann M, Kiener HP, Bankier AA, Baldt MM, Zontsich T, Herold CJ. Lung manifestation in asymptomatic patients with primary Sjögren syndrome: assessment with high resolution CT and pulmonary function tests. J Thorac Imaging 2001;16:282–9.
- Yazisiz V, Arslan G, Ozbudak IH, Turker S, Erbasan F, Avci AB, et al. Lung involvement in patients with primary Sjögren’s syndrome: what are the predictors? Rheumatol Int 2010;30:1317–24.
- Franquet T, Giménez A, Monill JM, Díaz C, Geli C. Primary Sjögren’s syndrome and associated lung disease: CT findings in 50 patients. AJR 1997;169:655–8.
- Ohno Y, Koyama H, Yoshikawa T, Seki S. State-of-the-art imaging of the lung for connective tissue disease (CTD). Curr Rheumatol Rep 2015;17:69.
- Koyama M, Johkoh T, Honda O, Mihara N, Kozuka T, Tomiyama N, et al. pulmonary involvement in primary Sjögren’ s syndrome. Spectrum of pulmonary abnormalities and computed tomography findings in 60 patients. J Thorac Imaging 2001;16:290–6.
- Chen M-H, Chou H-P, Lai C-C, Chen Y-D, Chen M-H, Lin H-Y, et al. Lung involvement in primary Sjögren’s syndrome: correlation between high-resolution computed tomography score and mortality. J Chinese Med Assoc 2014;77:75–82.
- Seror R, Ravaud P, Bowman SJ, Baron G, Tzioufas A, Theander E, et al. EULAR Sjogren’s syndrome disease activity index: development of a consensus systemic disease activity index for primary Sjogren’s syndrome. Ann Rheum Dis 2010;69:1103–9.
- Seror R, Bowman SJ, Brito-Zeron P, Theander E, Bootsma H, Tzioufas A, et al. EULAR Sjögren’s syndrome disease activity index (ESSDAI): a user guide. RMD Open 2015;1:e000022.
- Palm O, Garen T, Berge Enger T, Jensen JL, Lund M-B, Aalokken TM, et al. Clinical pulmonary involvement in primary Sjogren’s syndrome: prevalence, quality of life and mortality-a retrospective study based on registry data. Rheumatology (Oxford) 2013;52:173–9.
- Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European consensus group. Ann Rheum Dis 2002;61:554–8.
- Shiboski SC, Shiboski CH, Criswell LA, Baer AN, Challacombe S, Lanfranchi H, et al. American College of rheumatology classification criteria for Sjögren’s syndrome: A data-driven, expert consensus approach in the Sjögren’s international collaborative clinical alliance cohort. Arthritis Care Res 2012;64:475–87.
- Flament T, Bigot A, Chaigne B, Henique H, Diot E, Marchand-Adam S. Pulmonary manifestations of Sjögren’s syndrome. Eur Respir Rev 2016;25:110–23.
- Ito I, Nagai S, Kitaichi M, Nicholson AG, Johkoh T, Noma S, et al. Pulmonary manifestations of primary Sjögren’s syndrome. Am J Respir Crit Care Med 2005;171:632–8.
- Reina D, Roig Vilaseca D, Torrente-Segarra V, Cerdà D, Castellví I, Díaz Torné C, et al. Sjögren’s syndrome-associated interstitial lung disease: A multicenter study. Reumatol Clin 2016;12:201–5.
- Luckhardt TR, Fessler BJ. Pulmonary manifestations of primary Sjögren’s syndrome. Indian J Chest Dis Allied Sci 2009;51:93–101.
- Lin DF, Yan S, Zhao Y, Zhang W, Li MT, Zeng XF, et al. Clinical and prognostic characteristics of 573 cases of primary Sjögren’s syndrome. Chin Med J 2010;123:3252–7.
- Papiris SA, Maniati M, Constantopoulos SH, Roussos C, Moutsopoulos HM, Skopouli FN. Lung involvement in primary Sjögren’s syndrome is mainly related to the small airway disease. Ann Rheum Dis 1999;58:61–4.
- Mandl T, Diaz S, Ekberg O, Hesselstrand R, Piitulainen E, Wollmer P, et al. Frequent development of chronic obstructive pulmonary disease in primary SS–results of a longitudinal follow-up. Rheumatology (Oxford) 2012;51:941–6.
- Nilsson AM, Diaz S, Theander E, Hesselstrand R, Piitulainen E, Ekberg O, et al. Chronic obstructive pulmonary disease is common in never-smoking patients with primary Sjogren syndrome. J Rheumatol 2015;42:464–71.
- Mannino DM, Sonia Buist A, Vollmer WM. Chronic obstructive pulmonary disease in the older adult: what defines abnormal lung function? Thorax 2007;62:237–41.
- Shen T-C, Wu B-R, Chen H-J, Lin C-L, Wei C-C, Chen C-H, et al. Risk of chronic obstructive pulmonary disease in female adults with primary Sjögren syndrome. Medicine (Baltimore) 2016;95:e3066.
- Strevens Bolmgren V, Olsson P, Wollmer P, Hesselstrand R, Mandl T. Respiratory symptoms are poor predictors of concomitant chronic obstructive pulmonary disease in patients with primary Sjögren’s syndrome. Rheumatol Int 2017;37:813–18.
- Nilsson AM, Tufvesson E, Hesselstrand R, Olsson P, Wollmer P, Mandl T, et al. Increased B-cell activating factor, interleukin-6, and interleukin-8 in induced sputum from primary Sjögren’s syndrome patients. Scand J Rheumatol 2019;48:149–56.
- Papathanasiou MP, Constantopoulos SH, Tsampoulas C, Drosos AA, Moutsopoulos HM. Reappraisal of respiratory abnormalities in primary and secondary Sjogren’s syndrome. A controlled study. Chest 1986;90:370–4.
- Ramos-Casals M, Solans R, Rosas J, Camps MT, Gil A, Del Pino-Montes J, et al. Primary Sjögren syndrome in Spain. Medicine (Baltimore) 2008;87:210–19.
- Soto-Cardenas M-J, Perez-De-Lis M, Bove A, Navarro C, Brito-Zerón P, Diaz-Lagares C, et al. Bronchiectasis in primary Sjogren’s syndrome: prevalence and clinical significance. Clin Exp Rheumatol 2010;28:647–53.
- Manfredi A, Sebastiani M, Cerri S, Cassone G, Bellini P, Della Casa G, et al. Prevalence and characterization of non-sicca onset primary Sjögren syndrome with interstitial lung involvement. Clin Rheumatol 2017;36:1261–8.
- Lazarus A, Myers J, Fuhrer G. Bronchiectasis in adults : a review. Postgr Med 2008;120:113–21.
- Wang Y, Hou Z, Qiu M, Ye Q. Risk factors for primary Sjögren syndrome-associated interstitial lung disease. J Thorac Dis 2018;10:2108–17.
