Abstract
Objective: To investigate associations between physical activity and risk factors for cardiovascular disease (CVD), subclinical atherosclerosis, and disease activity in patients with early and long-standing rheumatoid arthritis (RA).
Method: This cross-sectional study included 84 patients with early and 37 with long-standing RA (disease duration, mean ± sd: 1.4 ± 0.4 and 16.3 ± 2.3 years, respectively). Physical activity was measured using a combined accelerometer and heart-rate monitor. Further assessments were disease activity (erythrocyte sedimentation rate, Disease Activity Score in 28 joints), functional ability (Health Assessment Questionnaire), risk factors for CVD (blood lipids, i.e. triglycerides, high-density lipoprotein, low-density lipoprotein; blood glucose, blood pressure, sleeping heart rate, waist circumference, body mass index, and body fat), and subclinical atherosclerosis (pulse-wave velocity, augmentation index, and carotid intima–media thickness).
Results: Physical activity variables did not differ between patients with early and long-standing RA. However, 37% of the patients with early and 43% of those with long-standing RA did not reach the World Health Organization’s recommended levels of moderate to vigorous physical activity (MVPA). In a final multiple regression model, adjusted for age, gender, disease duration, and activity monitor wear time, higher total physical activity was associated with lower body fat and higher functional ability. With the same adjustments, more time spent in MVPA was associated with lower high-density lipoprotein and lower sleeping heart rate.
Conclusions: Physical activity was associated with more favourable risk factors for CVD. However, many patients were physically inactive, stressing the importance of promoting physical activity in RA.
Rheumatoid arthritis (RA) is associated with an excess risk for cardiovascular disease (CVD), attributable partly to traditional cardiovascular risk factors (Citation1–Citation3) and partly to the systemic inflammation present in RA (Citation2, Citation4). An insufficient level of physical activity has also been presented as a contributory factor to the CVD morbidity in RA (Citation5–Citation8). Physical activity, defined as ‘any bodily movement produced by skeletal muscles that results in energy expenditure’ (Citation9), is strongly protective against CVD morbidity and mortality in the general population, and has the potential of affecting cardiovascular risk factors (Citation10, Citation11) as well as inflammation (Citation12). The World Health Organization (WHO) public health recommendations for physical activity in adults include 150 min per week of moderate-intensity or 75 min per week of vigorous-intensity aerobic activity, or an equivalent of both (Citation13). Furthermore, there seems to be a dose–response relationship between physical activity and health, with health benefits arising even at lower levels of physical activity compared to being sedentary (Citation14).
Several studies, using objective methods for measuring physical activity, present insufficient levels among RA patients according to public health recommendations (Citation8, Citation15–Citation17). In comparisons with healthy controls, the results have been inconclusive, although some studies report lower levels of physical activity among the patients (Citation16, Citation18–Citation22). Current knowledge suggests that physical activity may have beneficial effects on cardiovascular health in patients with RA (Citation8, Citation15, Citation19, Citation21–Citation23), but since many patients do not reach sufficient physical activity levels (Citation24), it is a major concern in the management of RA patients to measure, encourage, and facilitate physical activity. A premise for adequate, professional advice is knowledge of the frequency, intensity, and duration of physical activity in a broad spectrum of RA patients and knowledge about its relationship to CVD risk factors and cardiovascular health in RA. Studies presenting objectively measured physical activity and the associations with cardiovascular risk factors are few, especially those including patients with early disease. Thus, the aim of this cross-sectional study was to investigate associations between objectively measured physical activity and risk factors for CVD, measures of subclinical atherosclerosis, and disease activity in patients with early and long-standing RA.
Method
Patients
Since 1995, all individuals in the county of Västerbotten, Sweden, with RA (i.e. symptomatic for < 12 months), fulfilling the American College of Rheumatology criteria for RA (Citation25), and being at least 18 years of age, have been included in the early arthritis cohort at the Department of Rheumatology in Umeå, Sweden. They have been followed by a team including medical and health professionals. Medical treatment has followed the standard care, aiming at remission, while the objective of rehabilitation has been to minimize impairment and disability, by providing information about how to manage different consequences of the disease and by encouraging physical activity.
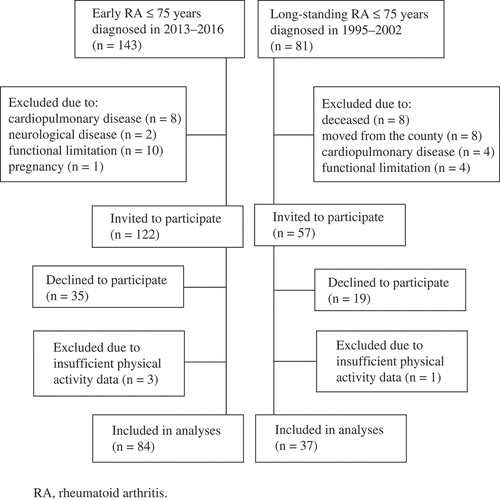
From the early arthritis clinic, all patients with early disease (12–24 months), diagnosed in 2013–2016, and all patients with long-standing disease, diagnosed during 1995–2002, still living in the county of Västerbotten, were consecutively invited to participate in this study (). Exclusion criteria were being > 75 years of age, severe diseases, and functional limitations, hindering physical activity to a higher extent than limitations caused by RA. The inclusion and exclusion process is presented in . Data were collected during 2015–2017. All patients gave their written informed consent to participate in accordance with the Helsinki Declaration. The study was approved by the Ethical Committee of the University in Umeå, Sweden (Dnr 2014/356-31).
Demographic and disease-related data
Disease duration was defined as time since diagnosis. Disease activity was assessed by the 28-joint Disease Activity Score (DAS28) (Citation26). Rheumatoid factor (RF) was analysed using the Waaler Rose test (a titre of 1/80 corresponds to a positive value), and anti-citrullinated protein antibody (ACPA) using enzyme-linked immunosorbent assays for anti-cyclic citrullinated peptide antibodies type 2. Pain during the week preceding the visit was assessed using a visual analogue scale (VAS). Current medication was noted. The Stanford Health Assessment Questionnaire (HAQ) was used to measure functional ability (Citation27). Joint and muscle function was evaluated using Signals of Functional Impairment (SOFI), a performance index comprising 12 items for hand, upper and lower extremity function (Citation28). A score of less than 12 reflects mild impairment (Citation28).
Traditional cardiovascular risk factors and atherosclerosis
Plasma fasting levels of cholesterol (mmol/L), triglycerides (mmol/L), and high-density lipoprotein (HDL) (mmol/L) were analysed in clinical routine in an accredited laboratory by Cobas cholesterol/triglyceride/HDL reagents on Cobas c701 platforms. Blood glucose (mmol/L) was analysed according to routine methods. Any history of CVD, tobacco use, or diabetes mellitus was registered according to the patients’ reports and records. Hypertension was registered when patients were treated with anti-hypertensives.
Dual-energy X-ray absorptiometry (DXA) was used to assess body composition (Lunar Prodigy X-ray Tube Housing Assembly, Brand BX-1L, Model 8743, and Lunar iDXA Forma; GE Medical Systems, Madison, WI, USA) (Citation29). Waist circumference was measured midway between the lower costal margin and the iliac crest, with the patient standing and the arms hanging freely. The measurement was made to the nearest 5 mm and performed at the end of a normal expiration (Citation30).
Early signs of atherosclerosis were measured by pulse-wave analysis (PWA) and carotid ultrasonography. Arteriograph Type TL2 v. 3.0.0.3 (TensioMed, Budapest, Hungary) was used for the PWA, which presents measures of systolic (SBP), diastolic (DBP) and aortic systolic blood pressure (SBPao), aortic pulse pressure (PPao), pulse-wave velocity (PWV), and augmentation index (AIx). The PWV and AIx are measures of arterial stiffness and endothelial function (Citation31). After patients had rested supine in a quiet room for 10 min, three measurements were taken in the right arm, with the average values noted. The carotid ultrasonography measured the carotid intima–media thickness (cIMT), using a Seqoia 512 ultrasound system [Siemens (Acuson) Corp., Upplands-Väsby, Sweden] with a 9L transducer (Citation32). The cIMTs of the right and left common carotid artery (CCA) were measured along 1 cm long longitudinal segments of the CCA just proximal to the carotid bulb on three end-diastolic images [defined by the R wave on an attached three-lead electrocardiogram (ECG)] and mean values were noted.
Physical activity
Physical activity was measured using the Actiheart monitor (CamNtech, Cambridge, UK), a waterproof combined heart rate and movement sensor, applied to the left side of the chest by two standard ECG electrodes (Red Dot 2560; 3M, St Paul, MN, USA) (Citation33). The monitor sampled ECG signals and uniaxial accelerations, which were stored in 30 s intervals during the recordings. Before performing long-term recordings, a signal test was performed in accordance with the instructions of the manufacturer (Citation34). The patients were instructed to wear the monitor day and night for seven consecutive days and to change the ECG electrodes at least once during the week. They were encouraged to continue with their habitual activities as usual.
The data recorded by the monitor were cleaned of potential measurement noise and analysed regarding:
total volume of physical activity, presented as mean accelerometer counts per minute
time spent in moderate to vigorous physical activity (MVPA), as a percentage of wear time and as minutes per day
the proportion meeting the WHO recommendations for physical activity, as daily minutes of MVPA ≥ 21.4 min (150 min/week) (Citation13)
sedentary time, as a percentage of wear time.
Heart rate ≥ 1.75 × resting heart rate was determined as a cut-off for moderate to vigorous intensity, representing the energy expenditure of ≥ 3 metabolic equivalents (METs) (Citation35). Resting heart rate was calculated as the median value of the 30 lowest heart rates registered for each day with valid measurements. Participants not reaching the recommended levels of MVPA ≥ 21.4 min/day were considered physically inactive. Sedentary time was calculated using intervals of valid heart rate data and zero accelerometer counts per minute. A day with at least 600 min of valid heart rate measurements was considered a valid day. Sleeping heart rate was defined as the highest heart rate registered during the 30 min with the lowest heart rate during every 24 h wearing the monitor. The mean values of the sleeping heart rates during the valid days were noted.
Statistics
Descriptive statistics are presented as median with interquartile range (Q1–Q3), mean with standard deviation (sd), or number with percentage, as appropriate. Patients were dichotomized into groups with early (< 2 years) and long-standing (diagnosed during 1995–2002) RA. Differences between groups were analysed using the Mann–Whitney U-test, unpaired t-test, or chi-squared test based on the distribution and type of data. Associations between the two dependent variables, i.e. total volume of physical activity (mean accelerometer counts per minute) and time spent in MVPA (percentage of wear time), and the independent variables, i.e. variables reflecting disease activity, CVD risk factors, and atherosclerosis, were analysed using univariate linear regression analysis. In the next step, every independent variable was analysed in a separate model, adjusted for age, gender, disease duration, and Actiheart wear time. In the third step, multiple linear regression modelling was performed to determine the presence of significant and independent factors associated with the total volume of physical activity and time spent in MVPA, using variables with a p-value ≤ 0.2 in the univariate regression analysis and adjusting for age, gender, disease duration, and Actiheart wear time. Because of the skewed distribution, the dependent variables were log-transformed (log) in the regression analyses. Since there was no difference between patients with early and long-standing RA in the different variables of physical activity, as well as age, gender, and DAS28, the two groups were combined in the analyses of associations. Missing data were considered to be random. The level of significance was set at a p-value of < 0.05. Statistical calculations were performed using SPSS version 24 (IBM Corp., Armonk, NY, USA).
Results
Study population
The study included 121 patients: 84 with early and 37 with long-standing RA. Descriptive, disease, and CVD-related data are presented in . The mean (range) age was 56.1 (21.0–75.0) years in the group with early RA and 58.4 (38.0–73.0) years in the group with long-standing RA. Patients invited, but not participating in the study did not differ from those who participated in terms of gender, age, disease activity, or functional ability (data not shown).
Table 1. Descriptive data and measures reflecting disease activity, traditional cardiovascular disease (CVD) risk factors, subclinical atherosclerosis, and physical activity in 121 patients with early or long-standing rheumatoid arthritis (RA).
Physical activity in early and long-standing RA
The registrations of physical activity were made during all seasons of the year, except for the month of July. Actiheart wear time was median (Q1–Q3) 1054 (848–1130) min/day in the group with early, and 1034 (914–1223) min/day in the group with long-standing disease. The number of valid days in the groups with early and long-standing disease was median (Q1–Q3) 6.0 (5.0–7.0) days and 7.0 (5.5–7.5) days, respectively. presents variables of physical activity, as measured by Actiheart, in patients with early and long-standing RA. No differences were found between the groups in any of the variables. According to the current recommendation of 21.4 min/day of MVPA (150 min/week), 31 (37%) of the patients with early and 16 (43%) of the patients with long-standing RA did not reach the recommended levels of physical activity. The chi-squared test revealed no statistical difference between the groups (p = 0.55).
Variables associated with total volume of physical activity
Linear regression analysis was performed to explore the associations between independent variables reflecting disease activity, traditional risk factors for CVD, and subclinical atherosclerosis, and the dependent variable (log) total volume of physical activity (). Older age, higher erythrocyte sedimentation rate (ESR), and higher HAQ scores were associated with lower total physical activity. Furthermore, a higher total volume of physical activity was inversely associated with a number of CVD risk factors as well as subclinical atherosclerosis (). Subsequently, when the separate variables were adjusted for age, gender, disease duration, and Actiheart wear time, significant associations remained for all variables, except for triglycerides, aortic PP, PWV, AIx, and cIMT ().
Table 2. Linear regression models among 121 patients with early or long-standing rheumatoid arthritis, with (log) total physical activity (mean accelerometer counts per minute) as the dependent variable.
In the next step, multiple linear regression modelling was performed with the same adjustments (). The final model explained 39% of the variation in total physical activity (). Lower body fat was still associated with higher total physical activity. The association with ESR was no longer evident, but the patients with higher age, female gender, and lower functional ability were less physically active.
Table 3. Multiple linear regression model explaining 39% of the variation in (log) total physical activity (mean accelerometer counts per minute) in 118 patients with early or long-standing rheumatoid arthritis.
Variables associated with MVPA
Linear regression analysis was performed with (log) MVPA as the dependent variable. More time spent in MVPA was associated with younger age, and further with higher HDL, and lower low-density lipoprotein (LDL), blood glucose, waist circumference, sleeping heart rate, and PWV (). After the same adjustments, i.e. age, gender, disease duration, and Actiheart wear time, for each variable, more time spent in MVPA was associated with lower HAQ score, and significant associations remained for HDL, blood glucose, and sleeping heart rate ().
Table 4. Linear regression coefficients for variables in relation to (log) moderate to vigorous physical activity (MVPA) (% of wear time) as the dependent variable in 121 patients with early or long-standing rheumatoid arthritis.
In a multiple linear regression model, adjusted for the same variables (age, gender, disease duration, and Actiheart wear time), higher HDL level and lower sleeping heart rate were associated with more time spent in MVPA (). Patients with lower functional ability spent less time in MVPA. The model explained 24% of the variation in MVPA.
Table 5. Multiple linear regression model explaining 24% of the variation in (log) moderate to vigorous physical activity (MVPA) (% of wear time) in 119 patients with early or long-standing rheumatoid arthritis.
Discussion
In this study, total physical activity as well as more time spent in MVPA were associated with more favourable measures of several risk factors for CVD and measures of subclinical atherosclerosis. A few reports have examined the associations between objectively measured physical activity and cardiovascular risk profile in patients with RA, presenting associations between lower levels of physical activity and increased risk of cardiovascular events (Citation7, Citation21), metabolic syndrome (Citation21), higher blood pressure, lower insulin sensitivity (Citation8), dyslipidaemia (Citation22), increased body mass index (BMI) (Citation8, Citation19), and obesity (Citation15, Citation23). Our results showed that the total amount of physical activity, i.e. regardless of intensity, was inversely associated with more CVD risk factors than time spent in higher intensities. Total physical activity was associated with lower values for triglycerides, blood glucose, BMI, body fat, waist circumference, sleeping heart rate, peripheral SBP and DBP, and aortic SBP, while time spent in MVPA was associated with more beneficial measures of HDL, LDL, blood glucose, waist circumference, and sleeping heart rate. Thus, it seems as though physical activity at lower intensities than the recommended moderate level is also associated with better cardiovascular health in RA patients.
In univariate regression, a higher total amount of physical activity was associated with more beneficial values for AIx, PWV, and cIMT, reflecting a lower burden of subclinical atherosclerosis in more physically active patients. The association with lower AIx is in concordance with results presented by Crilly and Wallace (Citation36). It seems as though the beneficial effects of physical activity on vascular health seen in the general population (Citation37) are also evident in patients with RA, which is further verified by improved endothelial function after a 6 month exercise period (Citation38).
In the present study, older patients, women, and those more functionally disabled spent less time in physical activity, in line with previous research on associations with age (Citation39), gender (Citation39), and functional disability (Citation8, Citation21, Citation39, Citation40). Higher disease activity, measured by ESR, was associated with less time spent being physically active. However, the association was weak and no longer evident in the final model. Research regarding the relationship between disease activity and physical activity shows inconclusive results (Citation41, Citation42). Khoja et al found that higher disease activity, measured by DAS28, was weakly or moderately associated with more time spent sedentary and in lower intensity activities but, in line with our results, no association was found for time spent in MVPA (Citation8). Hernández-Hernández et al found that change in disease activity, measured by DAS28, over 6 months was inversely associated with change in objectively assessed physical activity (Citation21). Physical activity has been described as having the potential for decreasing inflammation (Citation24, Citation43), shown by decreased disease activity after exercise interventions (Citation44, Citation45). Inflammation, with its accompanying symptoms, may be a barrier to physical activity, but if the patient can overcome this barrier and be physically active, they may experience an anti-inflammatory effect. Further research is required to better understand the interaction between disease activity and physical activity in patients with RA.
The median duration of MVPA was 34 min/day in patients with early RA and 26 min/day in long-standing RA, which is above the recommended 21.4 min/day (150 min/week). However, the variation in time spent in MVPA was considerable, and 37% of patients with early and 43% with long-standing RA did not reach the recommended weekly 150 min of MVPA and were thus considered physically inactive. As far as we know, this is the first study of RA patients using the Actiheart monitor to register physical activity. Studies of RA patients using other objective methods show various results. A study from the USA found that patients with mean disease duration of 14 years, aged 55 years, spent a median of 14 min/day in MVPA, which was considerably lower than our results (Citation46). In England, Fenton et al found a mean of 18 min/day of MVPA in patients aged 55 years with disease duration of 7 years (Citation7). By contrast, in a study by Khoja et al, patients from the USA, with a median age of 58 years and median disease duration of 14 years, spent a median of 36 min/day in MVPA (Citation8). The variation between studies may partly be explained by differences in disease severity. Furthermore, comparisons are hampered by the use of different activity monitors, with differences in device sensitivity, sampling, and data filtering, as well as the use of proprietary algorithms for handling data. In addition, comparing heart rate monitors to accelerometers is difficult since each method measures different parameters of physical activity behaviour and the relationship between the two aspects can vary between and within people.
In 10 European countries, the level of physical activity was estimated in healthy men and women using the Actiheart monitor (Citation47). Of the Swedish participants (96 women and 98 men, mean age for both genders 52 years), the women spent a median of 86 min/day and the men 112 min/day in MVPA. The level of MVPA was considerably higher in these healthy men and women compared with the patients included in our study, despite the small differences in age.
Studies presenting objectively measured physical activity in patients with early disease are sparse, but in patients with a symptom duration of 43 months at the time of diagnosis and treatment initiation, no difference in physical activity was found compared with controls after 3 months of disease-modifying anti-rheumatic drug (DMARD) therapy (Citation40). In a Swedish randomized controlled study on self-assessed physical activity, including 228 patients with mean disease duration of 21 months, close to 50% reached the WHO recommendations for MVPA at baseline (Citation48). The higher proportion of patients meeting the recommendations in our study may be due to differences in methodology (Citation49), but also to the information on and support for physical activity offered by the team in the early arthritis clinic.
Patients with long-standing disease could be expected to be less physically active and to spend more time sedentary compared with patients with early disease. Somewhat surprisingly, we report no difference between the groups, and disease duration was not associated with the physical activity parameters analysed. In contrast, Prioreschi et al found disease duration to be negatively correlated with physical activity (Citation19). In our study, mean age was almost the same in the two groups, and measures of DAS28, HAQ, and SOFI indicated low disease activity and severity in both groups, pointing towards good preconditions for physical activity despite several years of disease. Since the mean age was similar in both groups, the patients with long-standing RA represent a group that was diagnosed when they were younger compared with the patients with early RA. Higher age at disease onset has been associated with a more rapid decline in functional ability as well as higher age-adjusted mortality (Citation50). This may have caused a selection bias due to exclusion of older patients. The improvements in medical treatment over the years imply a more efficient reduction of disease activity for those patients diagnosed in recent years. However, except for the number of swollen joints, no measure reflecting disease activity differed significantly between the groups, and no significant associations were found between treatment with biological agents and the physical activity variables in the groups with early or long-standing disease (data not shown). Apart from the medical treatment, the two groups were all treated in a similar way, with regular visits to the early arthritis clinic during the first years of disease and with information on the importance and promotion of physical activity.
The participants in the present study were recruited from the early arthritis clinic, which is the only reference centre for patients with RA in the Västerbotten county. In addition, there were no differences in age, gender, disease activity, or functional ability between participants and those denying participation. Consequently, this cohort is representative of the prevalent seropositive and adult population with RA in the county without severe comorbidity. The major limitation of this study is the cross-sectional design, which hinders analyses of causal relationships.
Conclusion
In this study, physical activity behaviour was similar in patients with early and long-standing RA; however, insufficient levels of physical activity were found in a substantial proportion of patients. Total physical activity as well as more time spent in MVPA were associated with more favourable measures of risk factors for CVD and subclinical atherosclerosis, and thus physical activity at any level seems to be beneficial. Patients with functional disability were less physically active. These results stress the importance of promoting physical activity in patients with RA and paying extra attention to patients with lower functional ability.
Acknowledgements
The authors thank Professor Tommy Olsson for the loans of the Actiheart monitors, Associate Professor Bo Carlberg for lending us the Arteriograph, and Professor Solbritt Rantapää Dahlqvist for valuable contribution to the data. Furthermore, we thank biomedical scientists Emma Nyman and Elisabeth Lundström, and nurses Camilla Ring and Lena Uddståhl for skilled measurements of cIMT and DXA.
Grants were provided from The King Gustav V’s 80-Year Fund, the County Council of Västerbotten, the Medical Faculty of Umeå University, and the Swedish Rheumatism Association. In particular, we want to thank the local district of the Swedish Rheumatism Association for generous support.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Boyer JF, Gourraud PA, Cantagrel A, Davignon JL, Constantin A. Traditional cardiovascular risk factors in rheumatoid arthritis: a meta-analysis. Joint Bone Spine 2011;78:179–83.
- Innala L, Moller B, Ljung L, Magnusson S, Smedby T, Sodergren A, et al. Cardiovascular events in early RA are a result of inflammatory burden and traditional risk factors: a five year prospective study. Arthritis Res Ther 2011;13:R131.
- Crowson CS, Rollefstad S, Ikdahl E, Kitas GD, van Riel P, Gabriel SE, et al. Impact of risk factors associated with cardiovascular outcomes in patients with rheumatoid arthritis. Ann Rheum Dis 2018;77:48–54.
- Wallberg-Jonsson S, Johansson H, Ohman ML, Rantapaa-Dahlqvist S. Extent of inflammation predicts cardiovascular disease and overall mortality in seropositive rheumatoid arthritis. A retrospective cohort study from disease onset. J Rheumatol 1999;26:2562–71.
- Elkan AC, Hakansson N, Frostegard J, Hafstrom I. Low level of physical activity in women with rheumatoid arthritis is associated with cardiovascular risk factors but not with body fat mass-a cross sectional study. BMC Musculoskelet Disord 2011;12:13.
- Metsios GS, Stavropoulos-Kalinoglou A, Panoulas VF, Wilson M, Nevill AM, Koutedakis Y, et al. Association of physical inactivity with increased cardiovascular risk in patients with rheumatoid arthritis. Eur J Cardiovasc Prev Rehabil 2009;16:188–94.
- Fenton SA, Veldhuijzen van Zanten JJ, Duda JL, Metsios GS, Kitas GD. Sedentary behaviour in rheumatoid arthritis: definition, measurement and implications for health. Rheumatology (Oxford) 2018;57:213–26.
- Khoja SS, Almeida GJ, Wasko MC, Terhorst L, Piva SR. Light intensity physical activity is associated with lower cardiovascular risk factor burden in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2016;68:424–31.
- Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep 1985;100:126–31.
- Arem H, Moore SC, Patel A, Hartge P, Berrington de Gonzalez A, Visvanathan K, et al. Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA Intern Med 2015;175:959–67.
- Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT, et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 2012;380:219–29.
- Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol 2005;45:1563–9.
- WHO. Global recommendations on physical activity for health. (https://www.who.int/dietphysicalactivity/factsheet_adults/en/). Accessed 20 August 2019
- Sattelmair J, Pertman J, Ding EL, Kohl HW 3rd, Haskell W, Lee IM. Dose response between physical activity and risk of coronary heart disease: a meta-analysis. Circulation 2011;124:789–95.
- Lee J, Dunlop D, Ehrlich-Jones L, Semanik P, Song J, Manheim L, et al. Public health impact of risk factors for physical inactivity in adults with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:488–93.
- Huffman K, Pieper C, Hall K, St Clair E, Kraus W. Self-efficacy for exercise, more than disease-related factors, is associated with objectively assessed exercise time and sedentary behaviour in rheumatoid arthritis. Scand J Rheumatol 2015;44:106–10.
- Yu CA, Rouse PC, Veldhuijzen Van Zanten JJ, Ntoumanis N, Kitas GD, Duda JL, et al. Subjective and objective levels of physical activity and their association with cardiorespiratory fitness in rheumatoid arthritis patients. Arthritis Res Ther 2015;17:59.
- Roubenoff R, Walsmith J, Lundgren N, Snydman L, Dolnikowski GJ, Roberts S. Low physical activity reduces total energy expenditure in women with rheumatoid arthritis: implications for dietary intake recommendations. Am J Clin Nutr 2002;76:774–9.
- Prioreschi A, Hodkinson B, Avidon I, Tikly M, McVeigh J. The clinical utility of accelerometry in patients with rheumatoid arthritis. Rheumatology (Oxford) 2013;52:1721–7.
- Hashimoto T, Yoshiuchi K, Inada S, Shirakura K, Wada N, Takeuchi K, et al. Physical activity of elderly patients with rheumatoid arthritis and healthy individuals: an actigraphy study. Biopsychosoc Med 2015;9:19.
- Hernández-Hernández V, Ferraz-Amaro I, Díaz-González F. Influence of disease activity on the physical activity of rheumatoid arthritis patients. Rheumatology (Oxford) 2014;53:722–31.
- AbouAssi H, Connelly MA, Bateman LA, Tune KN, Huebner JL, Kraus VB, et al. Does a lack of physical activity explain the rheumatoid arthritis lipid profile? Lipids Health Dis 2017;16:39.
- Feinglass J, Lee J, Semanik P, Song J, Dunlop D, Chang R. The effects of daily weather on accelerometer-measured physical activity. J Phys Act Health 2011;8:934–43.
- Verhoeven F, Tordi N, Prati C, Demougeot C, Mougin F, Wendling D. Physical activity in patients with rheumatoid arthritis. Joint Bone Spine 2016;83:265–70.
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24.
- Prevoo ML, van ‘t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8.
- Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum 1980;23:137–45.
- Eberhardt KB, Svensson B, Mortiz U. Functional assessment of early rheumatoid arthritis. Br J Rheumatol 1988;27:364–71.
- Pietrobelli A, Formica C, Wang Z, Heymsfield SB. Dual-energy X-ray absorptiometry body composition model: review of physical concepts. Am J Physiol 1996;271:E941–E951.
- Douketis JD, Paradis G, Keller H, Martineau C. Canadian guidelines for body weight classification in adults: application in clinical practice to screen for overweight and obesity and to assess disease risk. CMAJ 2005;172:995–8.
- Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006;27:2588–605.
- Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr 2008;21:93–111.
- Brage S, Brage N, Franks PW, Ekelund U, Wareham NJ. Reliability and validity of the combined heart rate and movement sensor Actiheart. Eur J Clin Nutr 2005;59:561–70.
- Camntech. The Actiheart user manual (https://www.salusa.se/Filer/Produktinfo/Aktivitet/TheActiheartUserManual.pdf). Accessed 20 August 2019.
- Assah FK, Brage S, Ekelund U, Wareham NJ. The association of intensity and overall level of physical activity energy expenditure with a marker of insulin resistance. Diabetologia 2008;51:1399–407.
- Crilly MA, Wallace A. Physical inactivity and arterial dysfunction in patients with rheumatoid arthritis. Scand J Rheumatol 2013;42:27–33.
- Tanaka H, DeSouza CA, Seals DR. Absence of age-related increase in central arterial stiffness in physically active women. Arterioscler Thromb Vasc Biol 1998;18:127–32.
- Metsios GS, Stavropoulos-Kalinoglou A, Veldhuijzen van Zanten JJ, Nightingale P, Sandoo A, Dimitroulas T, et al. Individualised exercise improves endothelial function in patients with rheumatoid arthritis. Ann Rheum Dis 2014;73:748–51.
- Demmelmaier I, Dufour AB, Nordgren B, Opava CH. Trajectories of physical activity over two years in persons with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2016;68:1069–77.
- Prioreschi A, Hodkinson B, Tikly M, McVeigh JA. Changes in physical activity measured by accelerometry following initiation of DMARD therapy in rheumatoid arthritis. Rheumatology (Oxford) 2014;53:923–6.
- Iversen MD, Frits M, von Heideken J, Cui J, Weinblatt M, Shadick NA. Physical activity and correlates of physical activity participation over three years in adults with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2017;69:1535–45.
- Eurenius E, Brodin N, Lindblad S, Opava CH; PARA Study Group. Predicting physical activity and general health perception among patients with rheumatoid arthritis. J Rheumatol 2007;34:10–15.
- Sveaas SH, Smedslund G, Hagen KB, Dagfinrud H. Effect of cardiorespiratory and strength exercises on disease activity in patients with inflammatory rheumatic diseases: a systematic review and meta-analysis. Br J Sports Med 2017;51:1065–72.
- Hakkinen A, Sokka T, Kotaniemi A, Hannonen P. A randomized two-year study of the effects of dynamic strength training on muscle strength, disease activity, functional capacity, and bone mineral density in early rheumatoid arthritis. Arthritis Rheum 2001;44:515–22.
- Stavropoulos-Kalinoglou A, Metsios GS, Veldhuijzen van Zanten JJ, Nightingale P, Kitas GD, Koutedakis Y. Individualised aerobic and resistance exercise training improves cardiorespiratory fitness and reduces cardiovascular risk in patients with rheumatoid arthritis. Ann Rheum Dis 2013;72:1819–25.
- Semanik P, Lee J, Manheim L, Dipietro L, Dunlop D, Chang RW. Relationship between accelerometer-based measures of physical activity and the Yale physical activity survey in adults with arthritis. Arthritis Care Res (Hoboken) 2011;63:1766–72.
- InterAct Consortium, Peters T, Brage S, Westgate K, Franks PW, Gradmark A. Validity of a short questionnaire to assess physical activity in 10 European countries. Eur J Epidemiol 2012;27:15–25.
- Brodin N, Eurenius E, Jensen I, Nisell R, Opava CH, PARA Study Group. Coaching patients with early rheumatoid arthritis to healthy physical activity: a multicenter, randomized, controlled study. Arthritis Rheum 2008;59:325–31.
- Tierney M, Fraser A, Kennedy N. Physical activity in rheumatoid arthritis: a systematic review. J Phys Act Health 2012;9:1036–48.
- Symmons DP. Epidemiology of rheumatoid arthritis: determinants of onset, persistence and outcome. Best Pract Res Clin Rheumatol 2002;16:707–22.