Abstract
Objectives: Pain is the most common and troublesome complaint in rheumatoid arthritis (RA). This study aimed to assess the prevalence and clinical implications of unacceptable pain in an inception cohort of patients with RA.
Method: This study followed 477 patients from the BARFOT (Better Anti-Rheumatic FarmacOTherapy) early RA cohort for 15 years. Unacceptable pain was defined as ≥ 40 mm on a visual analogue scale for pain, while tolerable pain denoted no pain or pain below this cut-off, according to the patient acceptable symptom state concept.
Results: Unacceptable pain was frequent. At the 15 year follow-up visit, 34% had unacceptable pain. Patients with unacceptable pain had, compared with patients with tolerable pain, significantly more disease activity, worse patient global assessment, and worse function on the Health Assessment Questionnaire and Signals of Functional Impairment, but the degree of joint destruction was similar. Disease-modifying anti-rheumatic drug treatment was similar, but patients with unacceptable pain were more often treated with corticosteroids. At 15 years, patients with unacceptable pain who were in remission (33%) had less inflammation and better function than those not in remission, suggesting the presence of non-inflammatory causes of pain.
Conclusions: In this cohort of patients with RA, pain was frequent and severe, with negative effects on experienced health and function. Unacceptable pain was frequent and occurred also in patients in remission, indicating that pain in RA is multifactorial and should always be regarded as an important concern in itself. The cause of pain should be recognized and treated appropriately.
Pain in patients with rheumatoid arthritis (RA) is the most common and troublesome complaint, occurring frequently in many sites in the musculoskeletal system, and causing fatigue, disability, and impaired quality of life (Citation1, Citation2).
Pain may be relieved by effective suppression of the inflammatory process by disease-modifying anti-rheumatic drugs (DMARDs), but frequently significant pain remains, suggesting the presence of causes of pain other than current inflammation (Citation3).
Pain in RA may be nociceptive, elicited by tissue damage, as in joint inflammation. This may result in peripheral sensitization with hyperalgesia. Nociceptive signals are processed in the central nervous system (CNS) and may lead to central sensitization. Pain in RA may also be nociplastic. Nociplastic pain lacks evidence of tissue damage, is processed within the CNS, and tends to be chronic and widespread. Fibromyalgia is a key example of central sensitization due to nociplastic pain. Pain in osteoarthritis and low back pain are considered to depend on both nociceptive and nociplastic mechanisms. Neuropathic pain, a further pain mechanism in RA, is often caused by a nerve lesion, such as that in a carpal tunnel syndrome (Citation2–5).
Pain in patients with RA is measured in various ways, mostly by a validated self-reporting visual analogue scale (VAS) (Citation6). The mean change over time is frequently calculated to assess improvement. However, it may be quite difficult to decide whether a certain change in a patient-reported outcome measure (PROM) is relevant to the patient or not. To avoid this bias, the concept of the patient acceptable symptom state (PASS) was introduced (Citation7). For this, the highest acceptable level of the PROM in question is calculated, based on the answers to the following question to the patient: ‘If you were to remain for the rest of your life as you were during the last 48 hours, would this be acceptable or unacceptable for you?’ (Citation8).
Pain has been assessed by the PASS concept in several studies on musculoskeletal diseases, e.g. osteoarthritis (Citation7, Citation8–10). The cut-offs for PASS pain have been found to be similar over the various diagnoses, about 30–40 mm on a VAS.
The aims of the present study were to assess the frequency and impact of unacceptable pain in an inception cohort of patients with RA followed for 15 years and to study differences in clinical features between patients with and without unacceptable pain after 15 years. Furthermore, the possible relevance of having unacceptable pain and yet being in remission after 15 years was addressed. In addition, possible predictors of unacceptable pain were searched for.
Method
Patients
In total, 839 patients with RA were included in the BARFOT (Better Anti-Rheumatic FarmacOTherapy) early RA cohort between the years 1993 and 1999. All patients fulfilled the American Rheumatism Association 1987 revised criteria for the classification of RA (Citation11) and had, according to the protocol, a disease duration of no more than 12 months. Of these patients, 477 had attended the predetermined follow-up visit after 15 years and form the present study material. The rate of missing values was generally tolerably small. Thus, at baseline and after 15 years, respectively, pain VAS was missing in 11 and 15 cases, 28-joint Disease Activity Score with three variables (DAS28-3) in 84 and 11, patient global assessment (PatGA) in five and 12, number of swollen and tender joints in 82 and six each, erythrocyte sedimentation rate (ESR) in two and five, Health Assessment Questionnaire (HAQ) data in nine and 25 cases, and radiographs in 20 and 76 cases.
The study patients had similar baseline characteristics to the 362 patients who were lost to follow-up, with the exception that the study patients were younger (mean ± sd age 51 ± 13 vs 65 ± 15 years; p < 0.001), had better function measured by the Swedish version of the Stanford HAQ (Citation12) (mean ± sd 0.95 ± 0.58 vs 1.05 ± 0.69; p < 0.023), and were more often women (67% vs 60%; p < 0.028). The main reason for being lost to follow-up was death (114 patients).
Most patients had not been treated with conventional disease-modifying anti-rheumatic drugs (cDMARDs) or glucocorticoids before inclusion. The patients were treated according to their rheumatologists’ discretion.
Clinical assessments
Pain was measured on a VAS, i.e. a 100 mm continuous horizontal line, with the anchors ‘no pain’ and ‘worst possible pain’. The patient was asked to mark on this line ‘the average amount of pain experienced during the last week’.
Unacceptable pain was defined as ≥ 40 mm on the VAS for pain, while tolerable pain denotes no pain or pain below this cut-off according to the PASS concept (Citation8).
The PatGA was reported on a VAS anchored with ‘very good health’ and ‘very bad health’, answering the question ‘how is your health overall with respect to your arthritis?’
Disease activity was measured by the Disease Activity Score calculated on 28 joints (DAS28) (Citation13). Since pain is known to be highly correlated with PatGA, the three-variables index, DAS28-3, omitting PatGA, was preferred. This composite index includes the number of swollen and tender joints, and the ESR, which was analysed by the Westergren method (Citation14). Remission was defined as DAS28-3 < 2.6 (Citation15), the same level as for DAS28 (Citation15).
Impaired function of daily life activities was measured by the HAQ (range 0–3). A value of ≥ 1.0 was used to indicate significant disability (Citation16).
Impaired physical function was assessed by Signals of Functional Impairment (SOFI), a performance index of physical function (Citation17). SOFI examines four items of hand function, three of upper limb, and four of lower limb function. The score ranges from 0 to 44 (best to worst).
Anti-citrullinated protein antibodies (ACPAs) were detected by the anti-cyclic citrullinated peptide (anti-CCP2) antibody enzyme-linked immunosorbent assay (ELISA) kit from Euro Diagnostica (Malmö, Sweden). A titre > 25 U/mL was regarded as positive.
Radiographic assessment
Posterior–anterior radiographs of the hands and feet were assessed according to the van der Heijde modification of the Sharp score (SvHS) (Citation18), comprising total SvHS (range 0–448), erosion score (range 0–280), and joint space narrowing (JSN) score (range 0–168). Radiographic progression from inclusion to 15 years was defined as a change in SvHS of more than 15 (i.e. more than 1 unit per year), based on the assumption that a change of 1 unit per year is the lowest value of minor radiographic change (Citation19).
Statistical analysis
Statistical analyses were performed using SPSS version 21.0 statistical software (IBM Corp., Armonk, NY, USA). To test the differences between groups for continuous variables, the Student’s t-test or the Mann–Whitney U test was used, while the chi-squared test was used for proportions. Spearman’s rank correlation coefficient was used to assess the relationships between two continuous variables.
A multiple logistic regression analysis of the ability of baseline variables to predict unacceptable pain at 15 years was performed. Age, gender, disease duration, smoking habits, anti-CCP, ESR, pain, number of swollen and tender joints, HAQ, SOFI, erosion score, and JSN score were considered and tested in bivariate analyses. The possibility of collinearity was considered. Two variables, baseline pain and PatGA, had a correlation coefficient of r ≥ 0.6; therefore, PatGA was excluded from the model.
Variables associated with unacceptable pain at 15 years with a significance level of p < 0.10 were introduced in the model. Otherwise, the significance tests were two tailed and conducted at the 0.05 level of significance.
Ethics approval and consent to participate
All patients gave their informed consent to the study, which was performed in accordance with the Helsinki Declaration. The following ethics committees approved the study: Lund University (LU 154-95), Göteborg University (Gbg M 45-95), Linköping University (Li 123-95), and Karolinska Institutet (KI 153-95).
Results
Baseline demographic and clinical characteristics
shows the baseline demographic and clinical characteristics of all participating patients. The mean ± sd age was 51 ± 13 years and disease duration 7 ± 3 months; 67% were women, 25% were current smokers, and 57% were anti-CCP positive. Disease activity was high and function impaired.
Table 1. Baseline demographic and clinical characteristics for all patients and separately for the patients with and without unacceptable pain at baseline.
Unacceptable pain
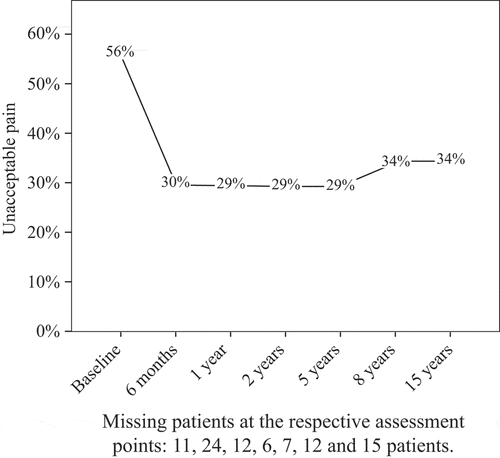
At baseline, the prevalence of unacceptable pain was 56%, decreasing to 30% after 6 months, after which it was fairly stable until 15 years, when 34% of the patients had unacceptable pain ().
Figure 1. Frequencies of unacceptable pain in the participating 477 patients at the predefined follow-up visits from inclusion into the study (baseline) to 15 years.
At baseline, the patients with unacceptable pain had higher disease activity and more impaired function than the patients with tolerable pain. Initial treatment with corticosteroids and DMARDs was more often given to patients with unacceptable pain.
At the five follow-up visits, after 1, 2, 5, 8, and 15 years, 34% never had unacceptable pain, while 38% had unacceptable pain at one or two visits, 23% at three or four visits, and 5% at all five visits.
Unacceptable pain and outcome at the 15 year follow-up visit
After 15 years, 75% of the patients with unacceptable pain were women compared with 63% of those with tolerable pain (p = 0.012). Regarding age, presence of anti-CCP2, disease duration, and current smoking at inclusion, no significant differences were observed. As shown in , after 15 years the disease activity by DAS28-3 was significantly higher in patients with unacceptable pain than in patients with tolerable pain (mean ± sd 3.3 ± 1.4 vs 2.6 ± 1.0; p = 0.001), and this was the case also for the PatGA (58 ± 19 vs 16 ± 15 mm; p = 0.001). In addition, VAS pain correlated strongly with VAS PatGA at all follow-up visits. At 15 years, the correlation coefficient was 0.87 (p = 0.001).
Table 2. Difference in disease characteristics after 15 years between patients with unacceptable pain and patients with tolerable pain.
Sixty-six per cent of the patients with unacceptable pain had significant disability at 15 years compared with 18% of the patients with tolerable pain (p = 0.001). The mean ± sd HAQ and SOFI at 15 years were significantly worse in patients with unacceptable pain than in patients with tolerable pain (1.2 ± 0.6 vs 0.5 ± 0.6 and 10 ± 6 vs 6 ± 6, respectively; p = 0.001 for both comparisons).
The mean SvHS, erosion scores and JSN scores at all follow-up visits were similar in the groups with unacceptable and with tolerable pain. Unacceptable pain at 15 years was similarly frequent in patients with SvHS progression and patients with no progression (51% vs 49%, respectively; p = 0.99).
At the 15 year visit, treatment with conventional and biological disease-modifying anti-rheumatic drugs (cDMARDs and bDMARDs) was similar in patients with and without unacceptable pain, except that sulfasalazine was slightly less frequently used in the patients with tolerable pain (0.9% vs 6.3%; p = 0.048).
Corticosteroid use at 15 years was significantly more frequent in patients with unacceptable pain than in patients with tolerable pain (30% vs 15%, respectively; p = 0.001). This difference in corticosteroid use applied only to patients in remission, among whom 24% of those with unacceptable pain used corticosteroids compared with 9% of those with tolerable pain (p = 0.011).
Unacceptable pain in patients in remission versus not in remission
After 15 years of follow-up, 49% of the patients were in remission and 51% of the patients were not.
Among the patients with unacceptable pain at 15 years, 33% were in remission and 67% were not. The patients who were not in remission had, compared with those in remission, longer disease duration before inclusion into the study (mean ± sd 7.3 ± 4.0 vs 6.1 ± 2.7 months; p = 0.025) and were more often smokers (30% vs 14%; p = 0.03). shows that the patients not in remission had more disease activity (mean ± sd DAS28-3 4.0 ± 1.1 vs 1.9 ± 0.4; p = 0.001), worse PatGA (mean ± sd 61 ± 18 vs 50 ± 19 mm; p = 0.001), and worse function by HAQ (1.3 ± 0.6 vs 1.0 ± 0.6; p = 0.07) and by SOFI (10 ± 7 vs 8 ± 5; p = 0.049), while mean SvHS was not significantly different between groups.
Table 3. Patients with unacceptable pain after 15 years: clinical and demographic differences at 15 years between patients in remission and patients not in remission.
Unacceptable pain versus tolerable pain in patients in remission at 15 years
When examining the patients in remission after 15 years, 51 (23%) of these patients had unacceptable and 170 (77%) had tolerable pain. Unacceptable pain was associated with higher age (mean ± sd 54 ± 13 vs 47 ± 13 years; p = 0.002), worse function by HAQ (1.0 ± 0.6 vs 0.4 ± 0.5; p = 0.001) and SOFI (8 ± 5 vs 5 ± 4; p = 0.001), and impaired well-being, PatGA (50 ± 19 vs 14 ± 13 mm; p = 0.001), but not with disease activity or radiographic changes ().
Table 4. Patients in remission after 15 years: clinical and demographic differences at 15 years between patients with unacceptable pain and patients with tolerable pain.
Predictors of unacceptable pain
A multiple logistic regression analysis of the ability of baseline variables to predict unacceptable pain at 15 years was performed.
The analysis showed that female gender and baseline pain VAS independently predicted unacceptable pain at 15 years ().
Table 5. Prediction of unacceptable pain at 15 years: bivariate and multivariate analyses.
Discussion
The aim of this study was to assess the prevalence and clinical implications of unacceptable pain in patients after 15 years with RA. Pain is generally regarded as a major risk factor for poor outcome of RA (Citation2). The present study lends support to this opinion in showing that unacceptable pain was frequent throughout the disease course and present also in patients in clinical remission. Unacceptable pain was found to be closely associated with impaired well-being as well as function.
Pain is commonly measured by a VAS. However, an improvement in a PROM, such as pain, may be less important for the patient if the change does not mean that an acceptable state has been achieved (Citation7). Therefore, we used the PASS concept, by which unacceptable pain was defined as ≥ 40 mm on the pain VAS, while pain below this cut-off was experienced as tolerable (Citation8). Other cut-offs defining an acceptable state have been proposed. Thus, Wolfe and Michaud (Citation20) studied pain in RA using a national data bank comprising 12 090 patients. They found a pain VAS of 20 mm or less as acceptable pain, i.e. the cut-off occurring in patients who considered themselves very satisfied with their health. Furthermore, Moore et al (Citation21) reason that ‘only no worse than mild pain is acceptable in clinical practice’ and found evidence for a cut-off of 30 mm.
The prevalence of unacceptable pain was fairly stable over time and was 34% at the 15 year follow-up. We found some observations in the literature concerning the frequency of unacceptable pain in RA similar to that in the present study. In a cross-sectional study from 2017, unacceptable pain was described in 39% of 531 patients with RA having a mean disease duration of 2.6 years (Citation10). In another study, 31% of 2808 patients had not achieved PASS 1 year after disease onset (Citation9). In agreement with our findings, Ödegård et al (Citation22) report from an inception cohort study over 10 years that more than 30% of the patients had a pain VAS ≥ 40 mm at all points in time. Thus, unacceptable pain appears to be frequent during the course of the disease.
Disease activity assessed by DAS28 was higher in the group of patients with unacceptable pain than in those with tolerable pain, both at inclusion and after 15 years. This has also been observed in previous studies and the pain may conceivably be interpreted as caused by inflammation. However, in one study on chronic widespread pain (ChWP) in RA (Citation23), ChWP was more associated with DAS28 and HAQ than with objective evidence of inflammation, such as swollen joint count and elevated acute-phase reactants. Similarly, in a report by Bilberg et al (Citation24), a high proportion of women with early RA had significant pain and high DAS28 in spite of otherwise only minor objective signs of inflammation. These observations suggest that the pain in these patients may also have non-inflammatory components. An explanation for this may be that DAS28 could be inflated by non-inflammatory pain via increased PatGA levels (Citation24, Citation25). Therefore, we have used DAS28-3 to reduce this impact of PatGA. However, tender joints are weighted higher than swollen joints in the calculation of DAS28-3; that is: DAS28-3 = [0.56 * sqrt(TJC28) + 0.28 * sqrt(SJC28) + 0.70*ln(ESR)] * 1.08 + 0.16. Therefore, even by DAS28-3 there could still be some influence of non-inflammatory pain.
Of the patients with unacceptable pain, about two-thirds of such pain occurred in patients not in remission at 15 years. These patients differed from those in remission in having more disease activity as well as worse experienced health and worse function. Most of the pain in these patients was apparently related to persistent inflammation. However, as many as one-third were in remission at the 15 year visit, indicating that not all pain in RA is related to ongoing inflammatory activity. Furthermore, the patients with unacceptable pain, who were in remission, had worse well-being (worse PatGA) and had more impaired function compared with the group with tolerable pain. These results suggest that pain in patients with RA has different backgrounds. In addition to nociceptive pain from joint inflammation and osteoarthritis, pain may be nociplastic inducing central sensitization, as in fibromyalgia, a prevalent condition in RA (Citation3, Citation5, Citation26). It should be noted that the present study was not designed to account for all possible causes of non-inflammatory pain but emphasizes the necessity to identify such causes in patients with RA in order to provide optimal management.
At 15 years, the group of patients with unacceptable pain had significantly worse PatGA than patients with an acceptable pain state. The link between pain and PatGA has previously been noticed (Citation23) and pain has been described as the single most important determinant of patient-experienced well-being (Citation25). This opinion is also supported by the fact that pain in the present study strongly correlated with PatGA at 15 years (r = 0.87). Pain has been reported to predict functional capacity (Citation27). Indeed, pain in RA has been said to be ‘a more important cause of disability than structural joint damage’ (Citation26). This statement is supported by the present findings that both HAQ and SOFI were significantly associated with unacceptable pain, and is further illustrated by the fact that significant disability was closely related to unacceptable pain. Thus, the data presented here have revealed a considerable impact of unacceptable pain on patient-experienced health and function.
Although synovitis causes pain and joint damage, radiological progression after 15 years was similar in the two pain groups. This is in agreement with the data of others (Citation3). This may be explained by the presence of multiple causes of pain in RA. Thus, the inflammatory process causing nociceptive pain, associated with joint damage, may be balanced by the nociplastic pain caused by non-inflammatory processes unrelated to joint damage.
Pain in RA has been reported to be aggravated by joint damage (Citation2). However, this may not be of major importance. Thus, erosions due to inflammation and JSN caused by coexisting osteoarthritis have been found to be associated, but only to a minor degree, with pain (Citation26).
bDMARDs as well as cDMARDs have been found to decrease pain in RA (Citation5). However, in this study after 15 years, unacceptable pain was similarly frequent among patients with or without DMARDs. In contrast, corticosteroid use was more common in patients with unacceptable pain (30%) than in patients with tolerable pain (15%) (p = 0.001). When split by remission at 15 years, the difference applied only for patients in remission. This may be explained by attempts to treat pain not related to inflammation.
In this study, female gender and baseline pain predicted unacceptable pain after 15 years of follow-up. Altawil et al, using a modified Poisson regression model, found HAQ to be a risk factor for unacceptable pain 1 year after inclusion into the study (Citation9). Otherwise, we have not been able to find any studies on RA addressing factors predictive of unacceptable pain. In the study by Ödegård et al (Citation22), the course of pain over 10 years was independently predicted by female gender, anxiety, disease activity, and physical function. However, grip strength, not HAQ, was used as a measure of physical function in the prediction model. Even though, in the present study, the variables reflecting function did not appear as independent predictors of unacceptable pain, they were bivariately strongly associated with unacceptable pain after 15 years ().
The fact that active inflammation does not seem to be a major predictor of future pain is compatible with several studies demonstrating a discrepancy between pain and disease activity (Citation2, Citation3, Citation27). The present data agree with these findings and suggest that this may be explained by a multifactorial origin of pain in RA. Bearing this in mind, it becomes more and more evident that focus should be placed on all unacceptable pain that does not respond to treatment with cDMARDs or bDMARDs. The cause of pain should be identified and appropriately treated. In addition to optimal drug treatment, psychotherapy may be appropriate for patients with anxiety and depression, as it is known to decrease pain (Citation28–30). Moreover, reports supporting the pain-relieving effects of re-establishing or increasing physical activity levels and aerobic fitness should be considered (Citation26).
A limitation of the study is that it is a post-hoc analysis and not designed for evaluating pain. However, all data used were available in the original database. A further limitation is that the database does not contain information on pain-associated comorbidities such as fibromyalgia and osteoarthritis. A strength is that this study was based on a well-defined cohort of patients followed over the long term at predefined intervals, using a structured protocol.
Conclusion
Pain is a frequent and severe cause of distress during the long-term course of RA, having considerable negative effects on experienced health, function, and quality of life. Unacceptable pain also occurs in patients in remission, indicating that pain in RA is multifactorial. Therefore, pain in RA should always be regarded as an important concern per se, which means that the cause of pain should be recognized and proper treatment initiated accordingly.
Acknowledgements
The BARFOT study group is greatly acknowledged for valuable contributions to this article.
We express our thanks to Dr Kristina Albertsson for skilful reading of the X-ray films.
The work was supported by grants from the Swedish Rheumatism Association and the Foundation for Assistance to Disabled People in Skåne (Stiftelsen för Bistånd åt Rörelsehindrade i Skåne).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Salaffi F, Carotti M, Grassi W. Health-related quality of life in patients with hip or knee osteoarthritis: comparison of generic and disease-specific instruments. Clin Rheumatol 2005;24:29–37.
- Sarzi-Puttini P, Salaffi F, Di Franco M, Bazzichi L, Cassisi G, Casale R, et al. Pain in rheumatoid arthritis: a critical review. Reumatismo 2014;66:18–27.
- McWilliams DF, Walsh DA. Pain mechanisms in rheumatoid arthritis. Clin Exp Rheumatol 2017;35:94–101.
- Salaffi F, Di Carlo M, Carotti M, Sarzi-Puttini P. The effect of neuropathic pain symptoms on remission in patients with early rheumatoid arthritis. Curr Rheumatol Rev 2019;15:154–61.
- Salaffi F, Giacobazzi G, Di Carlo M. Chronic pain in inflammatory arthritis: mechanisms, metrology, and emerging targets-A focus on the JAK-STAT pathway. Pain Res Manag 2018;2018:8564215.
- Lati C, Guthrie LC, Ward MM. Comparison of the construct validity and sensitivity to change of the visual analog scale and a modified rating scale as measures of patient global assessment in rheumatoid arthritis. J Rheumatol 2010;37:717–22.
- Tubach F, Ravaud P, Baron G, Falissard B, Logeart I, Bellamy N, et al. Evaluation of clinically relevant states in patient reported outcomes in knee and hip osteoarthritis: the patient acceptable symptom state. Ann Rheum Dis 2005;64:34–7.
- Tubach F, Ravaud P, Martin-Mola E, Awada H, Bellamy N, Bombardier C, et al. Minimum clinically important improvement and patient acceptable symptom state in pain and function in rheumatoid arthritis, ankylosing spondylitis, chronic back pain, hand osteoarthritis, and hip and knee osteoarthritis: results from a prospective multinational study. Arthritis Care Res (Hoboken) 2012;64:1699–707.
- Altawil R, Saevarsdottir S, Wedren S, Alfredsson L, Klareskog L, Lampa J. Remaining pain in early rheumatoid arthritis patients treated with methotrexate. Arthritis Care Res (Hoboken) 2016;68:1061–8.
- Puyraimond-Zemmour D, Etcheto A, Fautrel B, Balanescu A, de Wit M, Heiberg T, et al. Associations between five important domains of health and the patient acceptable symptom state in rheumatoid arthritis and psoriatic arthritis: a cross-sectional study of 977 patients. Arthritis Care Res (Hoboken) 2017;69:1504–9.
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24.
- Ekdahl C, Eberhardt K, Andersson SI, Svensson B. Assessing disability in patients with rheumatoid arthritis. Use of a Swedish version of the Stanford Health Assessment Questionnaire. Scand J Rheumatol 1988;17:263–71.
- Prevoo ML, van ‘t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8.
- Westergren A. Studies of the suspension stability of the blood in pulmonary tuberculosis. Acta Med Scand. 1921;54:247–82.
- Fransen J, Creemers MC, Van Riel PL. Remission in rheumatoid arthritis: agreement of the disease activity score (DAS28) with the ARA preliminary remission criteria. Rheumatology (Oxford) 2004;43:1252–5.
- Wolfe F, Cathey MA. The assessment and prediction of functional disability in rheumatoid arthritis. J Rheumatol 1991;18:1298–306.
- Eberhardt KB, Svensson B, Mortiz U. Functional assessment of early rheumatoid arthritis. Br J Rheumatol 1988;27:364–71.
- van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol 2000;27:261–3.
- van der Heijde D, Landewe R, van Vollenhoven R, Fatenejad S, Klareskog L. Level of radiographic damage and radiographic progression are determinants of physical function: a longitudinal analysis of the TEMPO trial. Ann Rheum Dis 2008;67:1267–70.
- Wolfe F, Michaud K. Assessment of pain in rheumatoid arthritis: minimal clinically significant difference, predictors, and the effect of anti-tumor necrosis factor therapy. J Rheumatol 2007;34:1674–83.
- Moore RA, Straube S, Aldington D. Pain measures and cut-offs - ‘no worse than mild pain’ as a simple, universal outcome. Anaesthesia 2013;68:400–12.
- Odegard S, Finset A, Mowinckel P, Kvien TK, Uhlig T. Pain and psychological health status over a 10-year period in patients with recent onset rheumatoid arthritis. Ann Rheum Dis 2007;66:1195–201.
- Andersson ML, Svensson B, Bergman S. Chronic widespread pain in patients with rheumatoid arthritis and the relation between pain and disease activity measures over the first 5 years. J Rheumatol 2013;40:1977–85.
- Bilberg A, Bremell T, Bjersing J, Mannerkorpi K. High prevalence of widespread pain in women with early rheumatoid arthritis. Scand J Rheumatol 2018;47:447–54.
- Sokka T. How should rheumatoid arthritis disease activity be measured today and in the future in clinical care? Rheum Dis Clin North Am 2010;36:243–57.
- Sarzi-Puttini P, Atzeni F, Salaffi F. Pain in rheumatic diseases: how relevant is it? Reumatismo 2014;66:1–3.
- Gwinnutt JM, Sharp CA, Symmons DPM, Lunt M, Verstappen SMM. Baseline patient reported outcomes are more consistent predictors of long-term functional disability than laboratory, imaging or joint count data in patients with early inflammatory arthritis: a systematic review. Semin Arthritis Rheum 2018;48:384–98.
- Carek PJ, Laibstain SE, Carek SM. Exercise for the treatment of depression and anxiety. Int J Psychiatry Med 2011;41:15–28.
- Gujral S, Aizenstein H, Reynolds CF 3rd, Butters MA, Erickson KI. Exercise effects on depression: possible neural mechanisms. Gen Hosp Psychiatry 2017;49:2–10.
- Schuch FB, Vancampfort D, Rosenbaum S, Richards J, Ward PB, Veronese N, et al. Exercise for depression in older adults: a meta-analysis of randomized controlled trials adjusting for publication bias. Braz J Psychiatry 2016;38:247–54.
