Abstract
Objective: To analyse healthcare utilization, loss of productivity, and disease activity in relation to presence of anti-citrullinated protein antibodies (ACPAs).
Method: In total, 447 ACPA-positive and 224 ACPA-negative patients from two early rheumatoid arthritis cohorts, recruited 1996–1998 (cohort 1) and 2006–2009 (cohort 2), were followed during 3 years. Data on disease activity were collected, and patients reported healthcare utilization and days lost from work. Disease activity, healthcare costs, and loss of productivity were compared between ACPA groups. Linear regression was performed, controlling for confounders.
Results: Healthcare costs did not differ significantly by ACPA status (EUR 3214 for vs EUR 2174 for ACPA-positive vs ACPA-negative patients in cohort 1, ns; EUR 4150 vs EUR 3820 in cohort 2, ns). Corresponding values for loss of productivity were EUR 9148 vs EUR 7916 (ns) and EUR 5857 vs EUR 5995 (ns). Total prescription of traditional disease-modifying anti-rheumatic drugs was higher in cohort 2 than in cohort 1. Methotrexate prescription was higher in ACPA-positive patients, but biologics did not differ significantly between ACPA groups. Disease activity was significantly more improved in cohort 2, but there was no difference in achieving remission in relation to ACPA status. In cohort 1, 25% of ACPA-positive patients were in remission vs 31% of ACPA-negative (ns) and in cohort 2, 55% vs 60% (ns).
Conclusions: With increasing drug treatment for both ACPA-positive and ACPA-negative patients, outcome in ACPA-positive was no more severe than in ACPA-negative patients. Healthcare costs and loss of productivity were similar in the two groups.
Rheumatoid arthritis (RA) is a progressive inflammatory disease associated with disability and reduced working capacity. The burden of the disease has a great impact on the individual and their families but also on society as a whole (Citation1, Citation2). Treatment strategies have undergone large changes over the past two decades with increasing use of conventional disease-modifying anti-rheumatic drugs (cDMARDs) early in the disease course as well as access to biological drugs (Citation3).
The presence of serological biomarkers, particularly anti-citrullinated protein antibodies (ACPAs), has been shown to be associated with the development of a more severe disease and progressive joint damage (Citation4, Citation5). It has also been suggested that response to DMARD treatment may vary depending on whether patients are ACPA positive or ACPA negative (Citation6). ACPA has a high specificity for RA and is increasingly being used in routine testing of biomarkers at the point of diagnosis (Citation7).
Numerous studies have evaluated healthcare utilization, loss of productivity, and development of disease activity in patients with early RA (Citation8–10). Few, however, have evaluated the economic burden of the disease in relation to ACPA status. In a longitudinal study, Boer et al evaluated outcome in ACPA-positive and ACPA-negative patients, using self-reported impairments, including functional disability and restrictions at work as outcomes, and found no differences in disease burden between ACPA-positive and ACPA-negative patients (Citation11). Mennini et al developed a model estimating annual costs for ACPA-positive patients (Citation12) and Shafrin et al reported that ACPA-positive patients had higher direct costs compared to ACPA-negative patients during the first year after diagnosis (Citation13). The present study was conducted to analyse the development of costs and disease activity during the first 3 years in patients with early RA, in relation to the presence of ACPAs.
Method
Patients
Ten rheumatology units in Sweden, corresponding to a catchment area of approximately 1 million inhabitants, participated in this multicentre study. Between 1996 and 2009, two early (≤ 1 year) RA cohorts were consecutively recruited (Citation2). Cohort 1 was enrolled in 1996–1998 and cohort 2 in 2006–2009. All units had similar inclusion criteria and all patients fulfilled at least four out of seven criteria according to the 1987 revised American College of Rheumatology (ACR) criteria (Citation14) or had morning stiffness ≥ 60 min, symmetrical joint affection, and arthritis in small joints (fingers, hands, wrists, toes). Both cohorts were followed prospectively at regular follow-ups.
Clinical assessments
During the first year, clinical and laboratory data were collected at inclusion, after 3 and 6 months, and thereafter every 6 months, using similar instruments and questionnaires in both cohorts, as described elsewhere (Citation2, Citation15). In brief, tender and swollen joint counts were registered, erythrocyte sedimentation rate (ESR) was analysed, patient’s global assessment of disease activity was estimated on a 100 mm visual analogue scale (VAS), and the 28-joint Disease Activity Score (DAS28) was calculated. ACPAs were analysed by enzyme-linked immunosorbent assays and the cut-off limit was set at 25 U/mL. Serum levels of C-reactive protein (CRP) were analysed and patients completed the Health Assessment Questionnaire (HAQ) and the EuroQol 5 Dimensions (EQ-5D) (Citation16, Citation17). In addition, EQ-VAS (0–100) was completed, with 0 corresponding to worst imaginable health and 100 to best imaginable health. Drug treatment decisions were made according to the physician’s preference, and ongoing, instituted, and withdrawn medication was registered at all visits.
Health economic questionnaire
Besides a baseline questionnaire including age, gender, marital status, educational level, and employment status, patients were provided with health economic questionnaires every 6 months. The questionnaires were kept as diaries and patients reported prospectively all outpatient visits, admissions to hospital, surgical procedures, and days lost from work. Dosage and frequency of prescribed drugs were reported, as well as drugs bought over the counter. In case complementary medicine, such as herbal medication and chiropractic therapy, was used, this was also reported.
Costs
All healthcare utilization was RA related; hence, visits to the physician, nurse, and physiotherapist/occupational therapist were RA related, as well as all surgery (total joint replacements, foot-, hand, elbow surgery, etc.). Regarding hospitalizations, only those related to RA were included in RA-related hospitalization. However, since there is an obvious possibility for overlapping between RA-related and non-RA-related hospitalizations, both groups were included in the calculations. Hospitalization costs were also added together and compared between ACPA groups.
Costs were calculated using the official Swedish County Council tariffs for outpatient visits, including administration costs, laboratory analyses, radiographs, and salaries of relevant staff involved in the visit (Citation18). Costs for surgery, including a standardized number of hospital days, were calculated according to the diagnostic-related coding system (Citation19). Additional hospitalization, besides standard care, was calculated as additional days in hospital. Drug costs were calculated using dosage and duration multiplied by unit costs using market wholesale prices (Citation20). Indirect costs were calculated using the human capital approach, estimating the value of lost production during the entire period of absence, similarly for sick leave and for disability pension, using the average labour salary plus taxes of all gainfully employed full-time Swedish workers (Citation21). To describe loss of productivity similarly for the patients, days were recalculated to full-time days, for instance 1 full-time day is equal to 2 days at 50% or 4 days at 25%. Healthcare in Sweden is financed through taxation and, except for a minor annual co-payment of 2338 SEK (EUR 228), all prescription medications, including biological drugs, are free of charge during the rest of the year. In the present study, a societal perspective was applied, including all costs, regardless of payer. Costs in both cohorts were adjusted for inflation to 2018 using the Swedish Consumer Price Index and converted to 2018 euros, using the average exchange rate in 2018, i.e. EUR 1 = 10.2567 SEK (Citation22).
Statistics
Continuous variables are reported as means with standard deviations (sd) and categorical variables as numbers and proportions. Differences were analysed by the Student’s t-test, chi-squared test, or Fisher’s exact test. Mean values were used as an informative measure for resource use data (Citation23). As some cost distributions were skewed, bootstrapping with 2000 replications with 95% confidence interval (CI) from bootstrapped samples was performed. Multivariate linear regression was performed to adjust for covariates. The level of significance was set at p < 0.05. Analyses were performed using IBM SPSS version 25.0.
Ethical considerations
All patients gave written informed consent to participate. The study protocol was approved by the local ethics committees associated with the participating hospitals.
Results
In cohort 1, 320 patients were included; and in cohort 2, 463 patients. ACPA tests were available in 241 patients (75%) in cohort 1 and in 430 patients (93%) in cohort 2, in total 671 patients. The proportions of ACPA-positive patients were similar in the cohorts: 65% in cohort 1 and 68% in cohort 2. Baseline clinical and demographic characteristics by ACPA status are presented in .
Table 1. Baseline clinical and demographic characteristics by anti-citrullinated protein antibody (ACPA) status in the two cohorts.
ACPA-positive patients were younger than ACPA-negative patients in both cohorts. There were small differences in disease activity between the groups; these differences were unexpectedly in favour of ACPA-positive patients, who had better EQ-VAS in cohort 1 and lower DAS28 score and lower CRP in cohort 2 compared to ACPA-negative patients.
At 3 year follow-up, complete clinical data and complete health economic questionnaires were available in 151 out of 241 patients (63%) in cohort 1 and 231 out of 430 (54%) in cohort 2. Patients were gradually lost to follow-up for reasons such as having difficulties with transportation or having moved from the area. In addition, a number of patients chose to remain only in the clinical study and declined to participate further in the health economic part, because of ‘too many questionnaires’. In cohort 2, patients remaining in the study were older than patients lost to follow-up (60 vs 56 years); otherwise, there were no differences in educational level, marital status, level of sick leave, or disability pension between patients remaining in the study and patients lost to follow-up. Baseline data for DAS28, HAQ, CRP, EQ-5D, and EQ-VAS were also similar in patients remaining in the study at 3 year follow-up and patients lost to follow-up.
Cohort 1 versus cohort 2
Patients in cohort 2 were treated more aggressively than patients in cohort 1, and improvements in DAS28, HAQ, pain, and EQ-5D were more pronounced in cohort 2. Direct costs were higher in cohort 2, owing to higher drug costs and more frequent outpatient visits. The increase in direct costs was partly offset by decreasing costs for loss of productivity, but total costs remained basically unchanged and did not differ between the cohorts.
Disease activity in ACPA-positive versus ACPA-negative patients
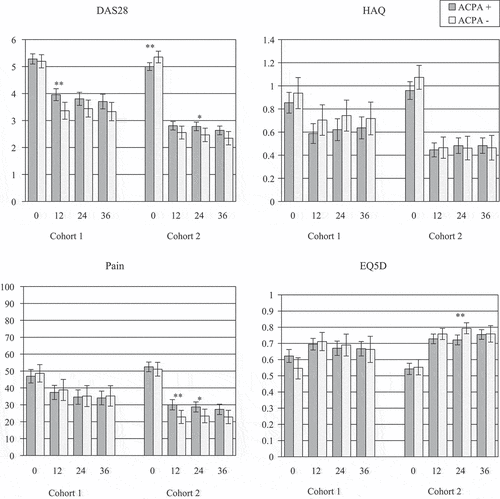
Disease activity was significantly improved over time for all patients. DAS28 was higher in ACPA-positive patients compared to ACPA-negative patients at 1 year follow-up in cohort 1 and at 2 year follow-up in cohort 2, but at 3 year follow-up, there was no difference in DAS28 between groups in either of the cohorts. Disability, as measured by HAQ, did not differ significantly between the groups at any follow-up. In cohort 2, ACPA-positive patients reported more pain at 1 and 2 year follow-up and lower EQ-5D at 2 year follow-up compared to ACPA-negative patients, but otherwise, there were no significant differences between ACPA-positive and ACPA-negative patients ().
Figure 1. 28-Joint Disease Activity Score (DAS28), Health Assessment Questionnaire (HAQ), pain, and EuroQol 5 Dimensions (EQ-5D) in anti-citrullinated protein antibody (ACPA)-positive and ACPA-negative patients over 3 years in cohort 1 and cohort 2. The x-axis represents months. *p < 0.05, **p < 0.01.
Costs in ACPA-positive versus ACPA-negative patients
In cohort 1, ACPA-positive patients had higher costs for nurses in all 3 years and for surgery in year 1 compared to ACPA-negative patients. In cohort 2, ACPA-positive patients had higher costs for physician visits than ACPA-negative patients in year 3 (EUR 1050 vs EUR 741, respectively, p = 0.029). Costs for analgesics were higher in all years for ACPA-positive patients and costs for cDMARDs were higher in year 2 (EUR 227 vs EUR 115, p = 0.002) and year 3 (EUR 263 vs EUR 125, p = 0.001). Hospitalizations were calculated as RA related or non-RA related. However, since there is an obvious possibility for overlap between RA-related and non-RA-related hospitalizations, both groups were included in the calculations. Hospitalization costs were also added together and compared between the groups, but did not differ significantly between ACPA-positive and ACPA-negative in either cohort. Total direct costs did not differ significantly between the ACPA groups at any follow-up, in either cohort (). Neither did costs for sick leave and disability pension at any time-point differ significantly between ACPA-positive and ACPA-negative patients. Total costs in cohort 1 were EUR 12 905 for ACPA-positive patients and EUR 10 534 for ACPA-negative patients (ns). Corresponding values in cohort 2 were EUR 10 446 and EUR 10 246, respectively (ns) ().
Table 2. Direct costs (EUR) in anti-citrullinated protein antibody (ACPA)-positive and ACPA-negative patients during 3 years after diagnosis.
Table 3. Indirect costs (EUR) in anti-citrullinated protein antibody (ACPA)-positive and ACPA-negative patients during 3 years after diagnosis, and separately for patients < 65 years of age.
Table 4. Disease-modifying anti-rheumatic drug (DMARD) prescription in anti-citrullinated protein antibody (ACPA)-positive and ACPA-negative patients at 3 year follow-up.
Annual costs were calculated for patients with complete data at each annual follow-up.
Limiting cost calculations to only patients with complete health economic data during all 3 years gave similar results. Indirect costs were also calculated, excluding patients over the age of 65 years, but no significant difference between ACPA-positive and ACPA-negative patients in either cohort was found (). A full table including mean differences with 95% confidence intervals is available in the supplementary material (Supplementary table S1).
Drugs in ACPA-positive versus ACPA-negative patients
The difference in medication between ACPA-positive and ACPA-negative patients was very evident in cohort 1, where almost half of the ACPA-negative patients had no DMARD medication at all during the first 3 years. There was no significant difference in DMARD prescription between the groups at the time-point of inclusion, but after 1, 2, and 3 years, ACPA-positive patients in cohort 1 had significantly higher DMARD prescription compared to ACPA-negative patients (p = 0.001). In contrast, in cohort 2, DMARD prescription was similar in the two ACPA groups. Although slightly decreasing over the years, DMARD prescription was still significantly higher for ACPA-negative patients in cohort 2 compared to cohort 1. Conventional DMARDs in double and triple combinations, most often methotrexate + hydroxychloroquine and methotrexate + hydroxychloroquine + sulfasalazine, respectively, were more common in ACPA-positive than in ACPA-negative patients. Combination treatment increased gradually in ACPA-positive patients while it remained rather stable at a lower level in ACPA-negative patients. Biological drugs were more common in ACPA-positive than in ACPA-negative patients, but the difference was non-significant ().
Figure 2. Disease-modifying anti-rheumatic drug prescription in anti-citrullinated protein antibody (ACPA)-positive and ACPA-negative patients in both cohorts at baseline and after 12, 24, and 36 months. The x-axis represents months.
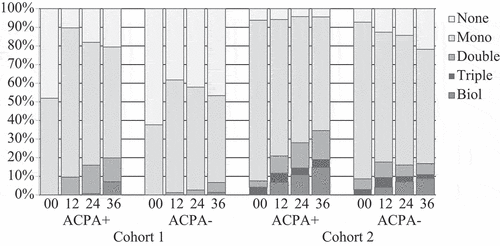
At the 3 year follow-up, ACPA-positive patients had higher prescription of methotrexate in both cohorts and higher prescription of hydroxychloroquine in cohort 2, compared to ACPA-negative patients. In cohort 1, biological drugs were prescribed to 7% of ACPA-positive patients compared to 1% of ACPA-negative patients (ns). Corresponding values for cohort 2 were 15% and 9% (ns). In cohort 1, all patients were prescribed tumour necrosis facto (TNF) biologics, while in cohort 2, 31 patients had TNF biologics and 15 had non-TNF biologics. There was, however, no difference between ACPA-positive and ACPA-negative patients, regardless of treatment with TNF versus non-TNF biologics ().
There was no difference in achieving remission (DAS28 < 2.6) in relation to ACPA status. At 3 year follow-up, 25% of ACPA-positive patients in cohort 1 were in remission compared to 31% of ACPA-negative patients (ns). In cohort 2, results were substantially improved, but the similarities between ACPA-positive and ACPA-negative patients were maintained, with 55% of ACPA-positive patients and 60% of ACPA-negative patients being in remission at 3 year follow-up (ns). Multivariate linear regression analyses were performed, adjusting for covariates to determine whether ACPA-positive and ACPA-negative patients differ in terms of direct and indirect costs. ACPA status did not, however, turn out to be a significant explanatory variable in the regression model (data not shown).
Discussion
There has been a general tendency towards paying increased attention to ACPA-positive patients, and the presence of a positive ACPA test has been associated with the development of more severe disease, increasing disability, and joint damage (Citation4, Citation5). In the present study, however, outcome over 3 years turned out to be similar in ACPA-positive and ACPA-negative patients. At the time-point of diagnosis, disease activity was even slightly worse in ACPA-negative patients, with worse DAS28 and worse CRP levels compared to ACPA-positive patients. This discrepancy has, however, also been reported by others and is likely to depend on differences in diagnostic criteria according to inclusion by 1987 or 2010 criteria (Citation11, Citation24). In the present study, patients were included according to the 1987 revised ACR criteria (Citation14).
The two cohorts were recruited 10 years apart and accordingly demonstrate the consequences of changing treatment strategies with early interventions and more aggressive drug treatment during the past few decades. Hence, disease activity was overall more improved in cohort 2, but despite higher levels of remission in cohort 2 compared to cohort 1, similarities in remission were maintained. In cohort 1, 25% of ACPA-positive patients were in remission compared to 31% of ACPA-negative patients (ns), and in cohort 2, corresponding levels were 55% versus 60% (ns). Disease activity at 3 year follow-up was similar and there were no differences in costs by ACPA status. Costs for healthcare and costs for loss of productivity were similar in the ACPA groups at 3 year follow-up. These findings are in line with a study from the Netherlands, where Boer et al analysed self-reported impairments, including functional disability and restrictions at work, over 4 years in ACPA-positive and ACPA-negative patients, and showed that outcome in ACPA-positive RA was not more severe than in ACPA-negative RA (Citation11). By contrast, Shafrin et al found, in a 1 year follow-up, that ACPA-positive patients had significantly higher costs and the difference by ACPA status was mostly driven by the costs of higher use of cDMARDs and biologics in ACPA-positive patients (Citation13).
In the present study, ACPA-positive patients in cohort 1 had significantly higher prescription of cDMARDs, compared to ACPA-negative patients, while in cohort 2, DMARD prescription was basically similar in the two groups. It is possible that ACPA-positive patients needed more aggressive treatment owing to more severe disease. It could, however, also be possible that the ACPA-positive RA diagnosis per se became an incentive to the physician to prescribe more aggressive drugs. In neither group, however, did prescription of biologics differ by ACPA status. At 3 year follow-up in cohort 2, 15% of ACPA-positive patients were prescribed biologics compared to 9% of ACPA-negative patients (ns). The early prescription of DMARDs in mono, double, and triple combinations was high in cohort 2, approximately 95%, and this may explain the relatively low prescription of biologics (Citation25).
The present study has several limitations. First, previous studies demonstrate that ACPA positivity is associated with joint damage and radiological progression (Citation4, Citation5, Citation26). This could, however, not be evaluated, since radiological data were not available. Secondly, costs were based upon self-reported data and recall bias cannot be ruled out (Citation27). The questionnaires were, however, distributed at the preceding visit and kept as diaries during the whole period, and the patients provided very detailed information on outpatient visits, medication, hospitalization, and surgical interventions. Part of our collected data could also have been acquired from national register-based data. This was, however, not planned for or done at the time-point of inclusion of cohort 1 (1996). When establishing the second cohort 10 years later, this possibility was discussed but since the intention was to compare the two cohorts, based on the same conditions, costs and disease activity have been evaluated similarly in the cohorts, i.e. by data from self-reported questionnaires and regular clinical follow-ups. Thirdly, all surgery included in the calculations was RA related. It could, however, not be ruled out that some total joint replacements could be due to coexisting osteoarthrosis. Fourthly, since reliable data on intra-articular corticosteroids were not available, only oral steroids were included in cost calculations. Fifthly, the follow-up period is only 3 years and further development in relation to ACPA status may occur over the following years (Citation28).
Despite these limitations, our longitudinal study has several strengths. We followed a relatively large number of patients, evaluating the development of disease activity and costs in relation to the presence of ACPA, during a period of changing treatment strategies, before and after the introduction of biological drugs.
Conclusion
With increasing drug treatment for both ACPA-positive and ACPA-negative patients, outcome in ACPA-positive patients was not more severe than in ACPA-negative patients, and the costs for healthcare and loss of productivity were also similar in the two groups, suggesting that RA patients require attention regardless of ACPA status.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Supplementary table S1. Direct, indirect and total costs (EUR) in ACPA-positive and ACPA-negative patients during 3 years after diagnosis, mean (sd) and p-value for differences between bootstrapped 95% confidence intervals (95% CI).
Please note that the editors are not responsible for the content or functionality of any supplementary material supplied by the authors. Any queries should be directed to the corresponding author.
Supplemental Material
Download PDF (243.8 KB)Acknowledgements
This work was supported by Linköping University and the FRF Foundation, Stockholm, Sweden.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Furneri G, Mantovani LG, Belisari A, Mosca M, Cristiani M, Bellelli S, et al. Systematic literature review on economic implications and pharmacoeconomic issues of rheumatoid arthritis. Clin Exp Rheumatol 2012;30(Suppl 73):S72–84.
- Hallert E, Husberg M, Kalkan A, Bernfort L. Rheumatoid arthritis is still expensive in the new decade: a comparison between two early RA cohorts, diagnosed 1996-98 and 2006-09. Scand J Rheumatol 2016;45:371–8.
- Bansback N, Fu E, Sun H, Guh D, Zhang W, Lacaille D, et al. Do biologic therapies for rheumatoid arthritis offset treatment-related resource utilization and cost? A review of the literature and an instrumental variable analysis. Current Rheumatol Rep 2017;19:54.
- Vencovský J, Machácek S, Sedová L, Kafková J, Gatterová J, Pesáková V, et al. Autoantibodies can be prognostic markers of an erosive disease in early rheumatoid arthritis. Ann Rheum Dis 2003;62:427–30.
- Kroot EJ, de Jong BA, van Leeuwen MA, Swinkels H, van den Hoogen FH, Van’t Hof M, et al. The prognostic value of anti-cyclic citrullinated peptide antibody in patients with recent-onset rheumatoid arthritis. Arthritis Rheum 2000;43:1831–5.
- Seegobin SD, Ma MH, Dahanayake C, Cope AP, Scott DL, Lewis CM, et al. ACPA-positive and ACPA-negative rheumatoid arthritis differ in their requirements for combination DMARDs and corticosteroids: secondary analysis of a randomized controlled trial. Arthritis Res Ther 2014;16:R13.
- Schellekens GA, Visser H, de Jong BA, van den Hoogen FH, Hazes JM, Breedveld FC, et al. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum 2000;43:155–63.
- Birnbaum HG, Pike C, Banerjee R, Waldman T, Cifaldi M. Changes in utilization and costs for patients with rheumatoid arthritis, 1997 to 2006. Pharmacoeconomics 2012;30:323–36.
- Uhlig T, Moe RH, Kvien TK. The burden of disease in rheumatoid arthritis. Pharmacoeconomics 2014;32:841–51.
- Husberg M, Bernfort L, Hallert E. Costs and disease activity in early rheumatoid arthritis in 1996-2000 and 2006-2011, improved outcome and shift in distribution of costs: a two-year follow-up. Scand J Rheumatol 2018;47:378–83.
- Boer AC, Boonen A, van der Helm van Mil AHM. Is anti-citrullinated protein antibody-positive rheumatoid arthritis still a more severe disease than anti-citrullinated protein antibody-negative rheumatoid arthritis? A longitudinal cohort study in rheumatoid arthritis patients diagnosed from 2000 onward. Arthritis Care Res 2018;70:987–96.
- Mennini FS, Marcellusi A, Gitto L, Iannone F. Economic burden of rheumatoid arthritis in Italy: possible consequences on anti-citrullinated protein antibody-positive patients. Clin Drug Investig 2017;37:375–86.
- Shafrin J, Tebeka MG, Price K, Patel C, Michaud K. The economic burden of ACPA-positive status among patients with rheumatoid arthritis. J Manag Care Spec Pharm 2018;24:4–11.
- Arnett FC, Edworthy SM, Blich DA, McShane D, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24.
- Hallert E, Husberg M, Jonsson D, Skogh T. Rheumatoid arthritis is already expensive during the first year of the disease. Rheumatology (Oxford) 2004;43:1374–82.
- Ekdahl C, Eberhardt KB, Andersson SI, Svensson B. Assessing disability in patients with rheumatoid arthritis. Scand J Rheumatol 1988;17:263–71.
- EuroQol Group. EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208.
- The County Councils of Sweden. (https://skr.se/), in Swedish. Accessed 15 April 2020.
- Swedish National Board of Health and Welfare. (www.socialstyrelsen.se), in Swedish. Accessed 15 April 2020.
- Swedish Environmental Classification of Pharmaceuticals. (www.fass.se), in Swedish. Accessed 15 April 2020.
- Statistics Sweden. (www.scb.se), in Swedish. Accessed 15 April 2020.
- The Swedish Riksbank. (https://www.riksbank.se), in Swedish. Accessed 15 April 2020.
- Thompson SG, Barber JA. How should cost data in pragmatic randomised trials be analysed? BMJ 2000;320:1197–2000.
- Nordberg LB, Lillegraven S, Lie E, Aga AB, Olsen IC, Hammer HB, et al. Patients with seronegative RA have more inflammatory activity compared with patients with seropositive RA in an inception cohort of DMARD-naïve patients classified according to the 2010 ACR/EULAR criteria. Ann Rheum Dis 2017;76:341–5.
- Ter Wee MM, den Uyl D, Boers M, Kerstens P, Nurmohamed M, van Schaardenburg D, et al. Intensive combination treatment regimens, including prednisolone, are effective in treating patients with early rheumatoid arthritis regardless of additional etanercept: 1-year results of the COBRA-light open-label, randomised, non-inferiority trial. Ann Rheum Dis 2015;74:1233–40.
- Rönnelid J, Wick MC, Lampa J, Lindblad S, Nordmark B, Klareskog L, et al. Longitudinal analysis of citrullinated protein/peptide antibodies (anti-CP) during 5 year follow up in early rheumatoid arthritis: anti-CP status predicts worse disease activity and greater radiological progression. Ann Rheum Dis 2005;64:1744–9.
- Rouf J, Huelsemann JL, Mittendorf T, Handelmann S, von Der Schulenburg JM, Zeidler H, et al. Patient-reported health care utilization in rheumatoid arthritis: what level of detail is required? Arthritis Care Res 2004;51:774–81.
- Paalanen K, Rannio K, Rannio T, Asikainen J, Hannonen P, Sokka T. Does early seronegative arthritis develop into rheumatoid arthritis? A 10-year observational study. Clin Exp Rheumatol 2019;37:37–43.
