Abstract
Objectives: Low body weight is an easily assessable cause of Raynaud’s phenomenon (RP), and is frequently overlooked by clinicians. We aim to investigate the association of low body weight (body mass index < 18.5 kg/m2), involuntary weight loss, and nutritional restrictions with the presence of RP.
Method: Participants from the Lifelines Cohort completed a validated self-administered connective tissue disease questionnaire. Subjects who reported cold-sensitive fingers and biphasic or triphasic colour changes were considered to suffer from RP. Patient characteristics, anthropometric measurements, and nutritional habits were collected. Statistical analyses was stratified for gender.
Results: Altogether, 93 935 participants completed the questionnaire. The prevalence of RP was 4.2% [95% confidence interval (CI) 4.1–4.4%], and was three-fold higher in women than in men (5.7% vs 2.1%, p < 0.001). Subjects with RP had a significantly lower daily caloric intake than those without RP. Multivariate analysis, correcting for creatinine level, daily caloric intake, and other known aetiological factors associated with RP, revealed that low body weight [men: odds ratio (OR) 5.55 (95% CI 2.82–10.93); women: 3.14 (2.40–4.10)] and involuntary weight loss [men: OR 1.32 (1.17–1.48); women: 1.31 (1.20–1.44)] were significantly associated with the presence of RP. Low-fat diet was also associated with RP in women [OR 1.27 (1.15–1.44)].
Conclusion: Low body weight and prior involuntary weight loss are associated with an increased risk of RP in both men and women. This study emphasizes that low body weight and weight loss are easily overlooked risk factors for RP, and should be assessed and monitored in subjects with RP.
Raynaud’s phenomenon (RP), which is a common disorder, is characterized by an episodic discoloration of the extremities in response to cold exposure or emotional stress (Citation1, Citation2). Raynaud’s phenomenon is common in young females and may be associated with several underlying connective tissue diseases (CTDs), including systemic sclerosis. Although patients presenting with RP are usually screened for features of CTDs, easily measurable causes of RP, such as low body weight or recent weight loss, are often overlooked.
In clinical practice, many patients with RP are underweight, have recently lost weight, or have dietary restrictions, potentially leading to malnutrition. Several case reports have demonstrated that RP can concomitantly occur in certain eating disorders (Citation3, Citation4). In addition, it was reported that patients with anorexia nervosa suffering from RP exhibit nailfold capillaroscopic findings typical of a CTD (Citation5). Collectively, these findings suggest that low body weight increases a patient’s susceptibility to RP. However, studies investigating the relationship between low body weight, weight loss, and RP are lacking.
The objective of the current study is to investigate the association of low body weight, involuntary weight loss, and dietary restrictions with the presence of RP in the general population. In addition, we will investigate other factors that are associated with low body weight, including diets based on low-fat and low-carbohydrate intake. To gain a clear understanding on the relationship between low body weight and RP, we will adjust for factors associated with low body weight [i.e. creatinine level (as a surrogate of muscle mass) and daily caloric intake] and other known aetiological factors associated with RP [i.e. age, hormonal status (in women), known CTD, smoking, and use of medication].
Method
Participants
In this cross-sectional study, we analysed data from the Lifelines Cohort Study. Lifelines is a prospective, multidisciplinary population-based cohort study, examining the health and health-related behaviours of 167 729 people from three generations, living in the north of the Netherlands (Citation6). Inclusion of the participants started in 2013, and all participants will be followed for 30 years. Prior to the first visit at the Lifelines outpatient clinic, all participants were asked to complete a self-administered questionnaire, and to provide (self-reported) information about their medical history, current diseases, use of medication, and health behaviour. During the first visit all participants underwent a general clinical examination, followed by blood and urine collection. All participants were aged ≥ 18 years, and subjects were only excluded from the current study if the CTD screening questionnaire (for details, see below) was missing. The Lifelines Cohort Study was conducted according to the principles of the Declaration of Helsinki and approved by the local medical ethics review committee of the University Medical Centre Groningen (UMCG), and all participants provided written informed consent to participate in this study.
Data collection
The Lifelines Cohort is a comprehensive source of demographic, physical, biomedical, behavioural, and psychological data. Information on the data access procedure can be found at http://www.Lifelines.nl. For the current study, several baseline patient characteristics were obtained, e.g. gender, age, smoking behaviour (current smoking was defined as smoking at the time of study entry or in the past month), hormonal status (i.e. pre vs post-menopausal, receiving hormonal contraceptives, receiving hormonal treatment other than contraceptives), and medication use (beta-blocking agents, immunosuppressive drugs, and central-acting sympathomimetics).
CTD screening questionnaire
Lifelines participants completed a number of questionnaires, including a CTD screening questionnaire, during the follow-up assessment, which took place within 3 years of the baseline assessment [median 25 months, interquartile range (IQR) 23–31 months]. This validated questionnaire comprised 30 questions designed to identify potential CTDs, including the presence of RP (Citation7, Citation8). The two-panel methodology was applied to translate the English CTD questionnaire. A bilingual panel agreed on the best Dutch translations for the items. Subsequently, the questionnaire was presented to a lay panel for consideration. This panel consisted of both men and women of different ages and average level of education (non-medical) to ensure that the language being used in the final version would be understood by potential respondents, and that it was in everyday Dutch. The presence of RP was assessed using five specific questions of the CTD questionnaire, namely: ‘Are your fingers unusually sensitive to the cold?’(question 1), ‘Have your fingers ever shown any unusual colour changes in the cold?’ (question 2), ‘If your fingers have ever shown any unusual colour changes in the cold, was the colour white?’ (question 3), ‘If your fingers have ever shown any unusual colour changes in the cold, was the colour blue/purple?’ (question 4), and ‘If your fingers have ever shown any unusual colour changes in the cold, was the colour red?’ (question 5). Subjects who answered the first two questions (questions 1 and 2) with a ‘yes’ were considered to possibly be suffering from RP, and participants who answered both questions with a ‘yes’ and reported biphasic or triphasic discoloration of the fingers were classified as definite RP (Citation9). In addition, subjects who answered the first two questions with a ‘no’ were considered as non-RP participants.
Dietary assessment
To assess factors associated with low body weight, a 110-item food frequency questionnaire was used. This questionnaire was designed to assess food intake over the previous month (Citation10). In addition, daily energy and macronutrient intake [daily caloric intake (kcal), daily intake of carbohydrates (g/day), and daily intake of fat (g/day)] was calculated based on the answers provided on the food frequency questionnaire at baseline. A low-fat diet was defined as a diet in which < 30% of the total calories were derived from fat (Citation11, Citation12), a low-carbohydrate diet was defined as a diet in which < 26% of the total calories were derived from carbohydrates (Citation13), and low-calorie intake was defined as < 1000 kcal/day. Serum creatinine was measured on a Roche Modular P chemistry analyser (Roche, Basel, Switzerland) and was used as a surrogate measure of muscle mass, as previously demonstrated (Citation14, Citation15). Other weight-loss specific questions included are: ‘What was your lowest weight in the last 5 years?’, ‘Are you currently on a diet to lose weight?’, and ‘Have you ever experienced involuntary weight loss (6 kg in 6 months, or 3 kg in 1 month)?’
Physical assessments
At baseline, participants were invited to visit one of 12 Lifelines research sites to undergo a physical examination and a series of tests. Anthropometric measurements including height (cm), weight (kg), and body mass index (BMI) were conducted by nurses during the study visit. BMI was categorized into two groups (BMI < 18.5 kg/m2 and BMI ≥ 18.5 kg/m2).
Statistical analysis
Statistical analysis was performed with SPSS version 22 (released 2013; IBM Corp., Armonk, NY, USA). Results were expressed as numbers of subjects (percentages) mean ± sd, or median [interquartile range (IQR)] for categorical, normally, and non-normally distributed data, respectively. The chi-squared test, independent samples t-test, and Mann-Whitney U-test were used as appropriate to compare characteristics between participants with and those without RP. Binary logistic regression with the presence of definite RP (no/yes) as the dependent variable was performed [presented as odds ratio (OR) and 95% confidence interval (CI)] to assess the association between BMI, weight loss, and the presence of RP. Since BMI and weight loss can cause problems of collinearity if included together, these variables were included separately in the model. Variables that were statistically significant in the univariate analyses were further analysed in multivariate logistic regression (enter method) to correct for potential confounders [creatinine level (as a surrogate measure of muscle mass), daily caloric intake, age, known CTD, smoking, and use of medications (beta-blocking agents, immunosuppressive drugs, and central-acting sympathomimetics)]. In women, we additionally corrected for hormonal status (pre- vs post-menopausal, receiving hormonal contraception, receiving hormonal treatment other than contraception). Current literature has shown that BMI and weight loss may be influenced by daily caloric intake and muscle mass (creatinine was used as a surrogate of muscle mass) and therefore we decided to correct for these parameters (Citation16, Citation17, Citation18, Citation19). We also corrected for other known aetiological factors associated with RP, such as age, known CTD, hormonal status, smoking, and use of medication (i.e. beta-blocking agents, immunosuppressive drugs, and central-acting sympathomimetics). All analyses were stratified for gender (effect modifier). A p-value below 0.05 was considered as statistically significant.
Results
Patient characteristics
In total, 152 020 participants were included in the Lifelines Cohort Study. The CTD questionnaire was completed by 93 935 (61.8% of total) (), with a mean age of 45.6 ± 12.9 years, of whom 56 122 (59.7%) were women and 37 813 (40.3%) men. Further characteristics of the responders can be found in . Of the non-responders (participants who were sent the questionnaire but declined to complete, total n = 58 085), 32 901 (56.5%) were women and the mean age was 43.1 ± 13.4 years.
Figure 1. Overview of the reported discoloration of the hands. A total of 93 935 subjects completed the questionnaire, of whom 10.6% reported monophasic discoloration. Of the subjects who reported monophasic discoloration, 4.2% also reported biphasic or triphasic discoloration. CTD, connective tissue disease
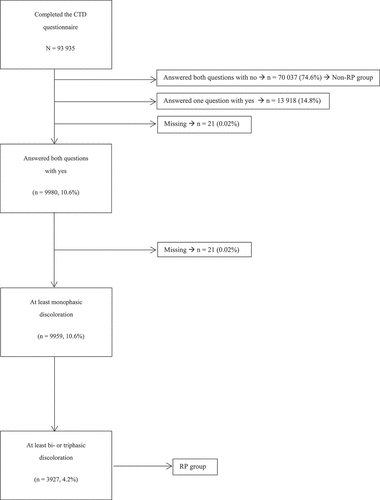
Table 1. Patient characteristics of the total group, and participants with and without Raynaud’s phenomenon (RP)
Prevalence of RP
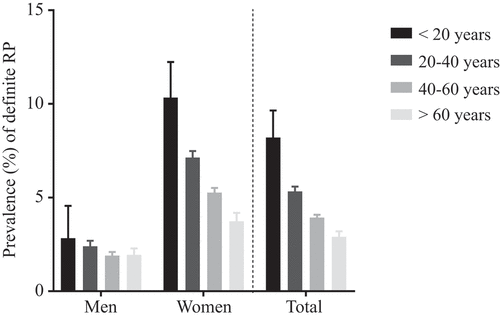
shows the prevalence of RP in our cohort. In total, 3927 participants reported at least biphasic discoloration, and thus were classified as definite RP. The overall prevalence rate of definite RP was found to be 4.2% (95% CI 4.1–4.4). Regarding the age-specific prevalence rates, we observed that the prevalence of definite RP decreases with increasing age. Participants aged 18–20 years showed the highest prevalence rate [men 2.5% (95% CI 1.3–4.7%), women 10.2% (8.5–12.3%), p < 0.001], and prevalence was found to decline with age.
Differences in anthropometric measurements and dietary assessment in participants with and without RP
Subjects with RP had a lower current weight than subjects without RP (p < 0.001). In addition, low body weight (BMI < 18.5 kg/m2) was more frequently found in subjects with RP than in those without RP (RP 2.3%, non-RP 0.5%, p < 0.001). The lowest (self-measured) weight reported in the last 5 years was 8 kg lower in subjects with RP than in subjects without RP [RP median 65 kg (IQR 58–73 kg), non-RP 73 kg (64–83 kg), p < 0.001). In addition, subjects with RP more frequently reported having suffered from involuntary weight loss (RP 5.3%, non-RP 2.6%, p < 0.001). With regard to nutritional information, subjects with RP had a significantly lower daily caloric intake than subjects without RP (RP 1798 kcal/day, non-RP 1911 kcal/day, p < 0.001). However, the number of subjects who were currently on a diet to lose weight did not differ between groups (p = 0.645). Creatinine levels, as a surrogate of muscle mass, were also found to be significantly lower in subjects with RP [RP median 70 µmol/L (IQR 63–77 µmol/L), non-RP 73 µmol/L (65–82 µmol/L), p < 0.001).
Association between BMI and RP
The interaction term between BMI and gender was found to be statistically significant (p = 0.01), which indicates that gender is an effect modifier. Therefore, the univariate and multivariate analyses were stratified for gender and are presented in and B, respectively.
Figure 3. (A) Univariate and (B) multivariate analysis of the relationship between the presence of Raynaud’s phenomenon and low body weight, involuntary weight loss, body weight (kg), low-carbohydrate diet, low-fat diet, and low-calorie intake, shown for men and women. In the multivariate analysis we corrected for creatinine level (as a surrogate measure of muscle mass), daily caloric intake, age, known connective tissue disease., smoking, and use of medication (i.e. beta-blocking agents, immunosuppressive drugs, and central-acting sympathomimetics). In women, we additionally corrected for hormonal status (pre- vs post-menopausal, receiving hormonal contraception, receiving hormonal treatment other than contraception)
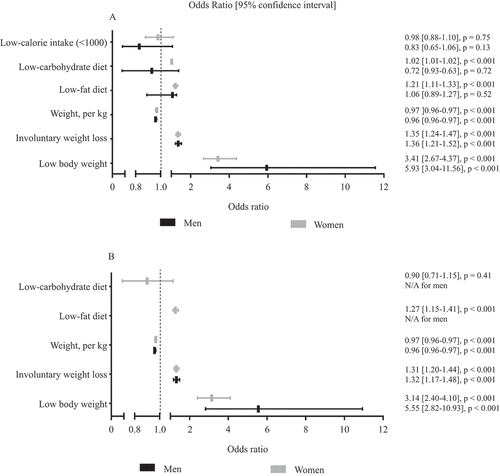
In men, the univariate analysis showed that weight (kg), low body weight (BMI < 18.5 kg/m2), and involuntary weight loss were significantly associated with the presence of RP. The multivariate analyses revealed that, after correction for potential confounders, these variables remained significantly associated with the presence of RP () [weight OR 0.96 (95% CI 0.96–0.97), low body weight OR 5.55 (2.82–10.93), involuntary weight loss OR 1.32 (1.17–1.48)].
In women, the univariate analyses showed that weight (kg), low body weight, involuntary weight loss, low-carbohydrate diet, and low-fat diet were significantly associated with the presence of RP. After adjustment for potential confounders, these variables, with the exception of low-carbohydrate diet, remained significantly associated with the presence of RP [weight OR 0.97 (95% CI 0.96–0.97), low body weight OR 3.14 (2.40–4.10), involuntary weight loss OR 1.31 (1.20–1.44), and low-fat diet OR 1.27 (1.15–1.41)].
Discussion
To the best of our knowledge, this is largest study to investigate the association between low body weight, involuntary weight loss, dietary restriction, and RP in the general population. We clearly demonstrated that low body weight and prior involuntary weight loss are significantly associated with an increased risk of RP in both men and women. In addition, low-fat diet was found to be associated with RP in women, but not in men. These associations remained present after correction for known aetiological factors associated with RP and other factors that might influence body weight. Conversely, low-calorie intake (< 1000 kcal/day) was not found to be associated with RP. Collectively, our results underline the importance of body weight and nutritional issues as easily overlooked associates of RP.
Subjects with RP had a lower daily caloric intake (approximately 100 kcal lower) than those without RP. The number of subjects who were currently on a diet to lose weight did not differ between groups. In addition, we demonstrated that underweight subjects and subjects with involuntary weight loss had an increased odds of RP, independently of age, current smoking, creatinine level (as a surrogate measure of muscle mass), daily caloric intake, and hormonal status (in women). However, studies focusing on the relationship between BMI and RP are very limited, and, therefore, the exact causal relationship remains poorly understood. For instance, it could hypothesized that substantial weight loss (or a BMI < 18.5 kg/m2) increases a person’s susceptibility to RP. A possible explanation is that weight loss may lead to a loss of subcutaneous and perivascular adipose tissue, and subsequently increases the susceptibility to vasospastic events. In support of this, it has been shown that perivascular adipose tissue alters the balance between endothelium-dependent vasodilator and vasoconstrictor substances (e.g. nitric oxide and endothelin-1) (Citation20), which further underlines the importance of BMI (and more importantly recent weight loss) in the occurrence of RP. In addition, a previous study showed that low body weight was also associated with an increased risk of cardiovascular disease (CVD) (Citation21). The authors stated that the increased risk of CVD could potentially be explained by the poor nutritional status and the decline in muscle mass in underweight subjects (Citation21). Furthermore, it was previously reported that subjects with low muscle mass were at increased mortality risk (Citation22). Although only a surrogate marker of muscle mass, the current study found that subjects with RP had lower levels of creatinine. Therefore, it could be hypothesized that subjects with low muscle mass may also have a higher susceptibility to developing RP. Collectively, these results underline the importance of investigating body composition to differentiate the separate contributions of muscle and fat mass as possible contributory factors for RP. Given that an increase in body weight was found to be associated with a slightly decreased odds of RP, future studies should also focus on the potentially protective effects of fat.
In our study, the association of low body weight and involuntary weight loss with the presence of RP remained present after correction for hormonal status (including hormonal treatment other than contraceptives) in women. This indicates that the causal pathway in which BMI affects RP is not solely mediated by hormonal factors in women, and that other unknown factors may have a greater role in this pathway. We found that a low-fat diet was associated with an increased risk of RP in women. This could indicate a poor nutritional status (Citation23). However, studies investigating dietary restrictions in RP do not exist and, therefore, the clinical relevance of this association is merely speculative.
This is the largest epidemiological study to assess the prevalence of RP in the general population. Garner et al conducted a meta-analysis and reported an overall prevalence of definite RP ranging between 1.6% and 7.2% (Citation24). This is in accordance with our findings; however, other studies have reported much lower and also higher prevalence rates (Citation25, Citation26). The large variation in prevalence rates could be explained by regional differences, as previous studies have clearly demonstrated that the prevalence of RP is increased in colder regions of the world (Citation27). In support of this, Fraenkel et al and Brand et al reported prevalences of 7.8% and 7.2%, respectively, in Boston (USA) (Citation1, Citation28). In countries with a warmer climate, the prevalence of RP was estimated to range between 2% and 6% (Citation29, Citation30, Citation31). The variation in reported prevalence rates can also be explained by differences in the definition of RP. For instance, only 39% of the previously conducted studies investigating the prevalence of RP used a precise definition of RP (Citation24). Without a universally agreed upon definition, studies may overestimate or underestimate the true prevalence of RP (Citation32, Citation33). The definition used in the current study was comparable to several previously conducted studies (Citation26, Citation33, Citation34), and is in accordance with the definition used by the UK Scleroderma Study Group (Citation9).
The limitations of this study include the cross-sectional design, which does not allow the investigation of causality. In addition, the primary outcome parameters were solely based on questionnaires, which could have introduced bias into our paper. However, this is a population-based study, which was designed to answer multiple research questions, and was not set up only for the purpose of the current paper. Since a large sample of the general population of the northern parts of the Netherlands was included, the clinical information that could feasibly be assessed in such a large sample was limited. In line with this limitation, more specific assessment of CTD-specific data (including nailfold videocapillaroscopy) was not feasible at that point. Therefore, other approaches for the diagnosis of RP, as previously suggested by others, were not deemed possible (Citation33, Citation35, Citation36, Citation37) Furthermore, the self-reported nature of the CTD questionnaire might lead to an overestimation of the prevalence of RP. However, the CTD questionnaire used in the current study had been previously validated, and was found to have a high sensitivity and specificity for screening large populations (Citation7). Furthermore, the CTD questionnaire was completed within 3 years following the baseline assessment. Although variables assessed at baseline (e.g. body weight) could have changed over time, we believe that given the large sample size of this study this would only minimally influence our results. In addition, occupational exposure was not included in the current study. Therefore, the role of environmental factors (e.g. use of vibrating tools) remains unknown. Nevertheless, our study provides crucial information on the true burden of RP in the general population and its relationship with low body weight. Our study should raise awareness among physicians to pay more attention to body weight and nutritional issues as possible treatable causes of RP.
Conclusion
This large population-based cohort study clearly demonstrated that low body weight and prior involuntary weight loss are significantly associated with an increased risk of RP in both men and women. The current study underlines the importance of these possibly treatable associates of RP, and further emphasizes that BMI and weight changes should be carefully assessed and monitored in subjects presenting with RP.
Data availability
The dataset used during the current study is available at http://www.Lifelines.nl. Information on the data access procedure can be found at http://www.Lifelines.nl.
Acknowledgements
The authors thank the Lifelines Cohort Study for its services. This research was supported by the NIHR Manchester Biomedical Research Centre.
This study was supported by the Dutch Arthritis Association, by an in-kind grant from ThermoFisher Scientific as part of the IMI JU funded project BeTheCure (contract no. 115142-2), and by a grant from Biobanking and Biomolecular Research Infrastructure (BBMRI)-NL complementation projects.
The Lifelines Cohort Study, and generation and management of GWAS genotype data for the Lifelines Cohort Study, is supported by the Netherlands Organization of Scientific Research NWO (grant 175.010.2007.006), the Ministry of Economic Affairs, the Ministry of Education, Culture and Science, the Ministry for Health, Welfare and Sports, the Northern Netherlands Collaboration of Provinces (SNN), the Province of Groningen, University Medical Center Groningen, the University of Groningen, Dutch Kidney Foundation, and Dutch Diabetes Research Foundation.
Disclosure statement
EB, as an employee of the UMCG, received speaker fees and consulting fees from Roche which were paid to the UMCG. DJM has received financial support for investigator-initiated studies from Actelion, Boehringer Inhelheim, and Sanofi, which was paid to UMCG. The other authors declare no conflicts of interest.
References
- Fraenkel L, Zhang Y, Chaisson CE, Maricq HR, Evans SR, Brand F, et al. Different factors influencing the expression of Raynaud’s phenomenon in men and women. Arthritis Rheum 1999;42:306–10.
- Herrick AL. Pathogenesis of Raynaud’s phenomenon. Rheumatology (Oxford) 2005;44:587–96.
- Yucel B, Kubat Uzum A, Ozbey N, Kamali S, Yager J. Anorexia nervosa and Raynaud’s phenomenon: A case report. Int J Eat Disord 2007;40:762–5.
- Sachs KV, Harnke B, Mehler PS, Krantz MJ. Cardiovascular complications of anorexia nervosa: A systematic review. Int J Eat Disord 2016;49:238–48.
- De Martinis M, Sirufo MM, Ginaldi L. Raynaud’s phenomenon and nailfold capillaroscopic findings in anorexia nervosa. Curr Med Res Opin 2018;34:547–50.
- Stolk RP, Rosmalen JGM, Postma DS, De Boer RA, Navis G, Slaets JPJ, et al. Universal risk factors for multifactorial diseases: LifeLines: a three-generation population-based study. Eur J Epidemiol 2008;23:67–74.
- Karlson EW, Sanchez-Guerrero J, Wright EA, Lew RA, Daltroy LH, Katz JN, et al. A connective tissue disease screening questionnaire for population studies. Ann Epidemiol 1995;5:297–302.
- Karlson EW, Costenbader KH, McAlindon TE, Massarotti EM, Fitzgerald LM, Jajoo R, et al. High sensitivity, specificity and predictive value of the connective tissue disease screening questionnaire among urban African-American women. Lupus 2005;14:832–6.
- Brennan P, Silman A, Black C, Bernstein R, Coppock J, Maddison P, et al. Validity and reliability of three methods used in the diagnosis of Raynaud’s phenomenon. Rheumatology (Oxford) 1993;32:357–61.
- Vinke PC, Corpeleijn E, Dekker LH, Jacobs DR, Navis G, Kromhout D. Development of the food-based Lifelines Diet Score (LLDS) and its application in 129,369 Lifelines participants. Eur J Clin Nutr 2018;72:1111–19.
- Brinkworth GD, Noakes M, Buckley JD, Keogh JB, Clifton PM. Long-term effects of a very-low-carbohydrate weight loss diet compared with an isocaloric low-fat diet after 12 mo. Am J Clin Nutr 2009;90:23–32.
- Tobias DK, Chen M, Manson JAE, Ludwig DS, Willett W, Hu FB. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: A systematic review and meta-analysis. Lancet Diabetes Endocrinol 2015;3:968–79.
- Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med 2003;348:2074–81.
- Schutte JE, Longhurst JC, Gaffney FA, Bastian BC, Blomqvist CG. Total plasma creatinine: an accurate measure of total striated muscle mass. J Appl Physiol Respir Environ Exerc Physiol 1981;51:762–6.
- Thongprayoon C, Cheungpasitporn W, Kashani K. Serum creatinine level, a surrogate of muscle mass, predicts mortality in critically ill patients. J Thorac Dis 2016;8:E305–11.
- Soini S, Mustajoki P, Eriksson JG. Lifestyle-related factors associated with successful weight loss. Ann Med 2015;47:88–93.
- Pansini F, Cervellati C, Guariento A, Stacchini MA, Castaldini C, Bernardi A, et al. Oxidative stress, body fat composition, and endocrine status in pre- and postmenopausal women. Menopause 2008;15:112–18.
- Jakubowicz D, Barnea M, Wainstein J, Froy O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity 2013;21:2504–12.
- Wysokiński A, Sobów T, Kłoszewska I, Kostka T. Mechanisms of the anorexia of aging—a review. Age (Omaha) 2015;37:9821.
- Houben AJ, Eringa EC, Jonk AM, Serne EH, Smulders YM, Stehouwer CD. Perivascular fat and the microcirculation: relevance to insulin resistance, diabetes, and cardiovascular disease. Curr Cardiovasc Risk Rep 2012;6:80–90.
- Park D, Lee JH, Han S. Underweight: another risk factor for cardiovascular disease? Med (United States) 2017;96:e8769.
- Srikanthan P, Horwich TB, Tseng CH. Relation of muscle mass and fat mass to cardiovascular disease mortality. Am J Cardiol 2016;117:1355–60.
- Siguel EN, Lerman RH. Role of essential fatty acids: dangers in the US department of agriculture dietary recommendations (“pyramid”) and in low-fat diets. Am J Clin Nutr 1994;60:973–4.
- Garner R, Kumari R, Lanyon P, Doherty M, Zhang W. Prevalence, risk factors and associations of primary Raynaud’s phenomenon: systematic review and meta-analysis of observational studies. BMJ Open 2015;5:e006389.
- Bartelink ML, Wollersheim H, van de Lisdonk E, Spruijt R, van Weel C. Prevalence of Raynaud’s phenomenon. Neth J Med 1992;41:149–52.
- Purdie G, Harrison A, Purdie D. Prevalence of Raynaud’s phenomenon in the adult New Zealand population. NZ Med J 2009;122:55–62.
- Maricq HR, Carpentier PH, Weinrich MC, Keil JE, Palesch Y, Biro C, et al. Geographic variation in the prevalence of Raynaud’s phenomenon: a 5 region comparison. J Rheumatol 1997;24:879–89.
- Brand FN, Larson MG, Kannel WB, McGuirk JM. The occurrence of Raynaud’s phenomenon in a general population: the Framingham study. Vasc Med 1997;2:296–301.
- Román Ivorra JA, Gonzálvez Perales JL, Fernández Carballido C, Graña J, Torres MJ. Prevalence of Raynaud’s phenomenon in general practice in the East of Spain. Clin Rheumatol 2001;20:88–90.
- De Angelis R, Salaffi F, Grassi W. Raynaud’s phenomenon: prevalence in an Italian population sample. Clin Rheumatol 2006;25:506–10.
- Onbaşi K, Şahin Í, Onbaşi O, Üstün Y, Koca D. Raynaud’s phenomenon in a healthy Turkish population. Clin Rheumatol 2005;24:365–9.
- Herrick AL. The pathogenesis, diagnosis and treatment of Raynaud phenomenon. Nat Rev Rheumatol 2012;8:469–79.
- Maverakis E, Patel F, Kronenberg DG, Chung L, Fiorentino D, Allanore Y, et al. International consensus criteria for the diagnosis of Raynaud’s phenomenon. J Autoimmun 1999;42:60–5.
- Wigley FM. Raynaud’s phenomenon. N Engl J Med 2002;347:1001–8.
- Smith V, Vanhaecke A, Herrick AL, Distler O, Guerra MG, Denton CP, et al. Fast track algorithm: how to differentiate a “scleroderma pattern” from a “non-scleroderma pattern”. Autoimmun Rev 2019;18:102394.
- Smith V, Herrick AL, Ingegnoli F, Damjanov N, De Angelis R, Denton CP, et al. Standardisation of nailfold capillaroscopy for the assessment of patients with Raynaud’s phenomenon and systemic sclerosis. Autoimmun Rev 2020;19:102458.
- Lis-Świȩty A. Recent advances in the workup and management of Raynaud phenomenon. Polish Arch Intern Med 2019;129:798–808.