Abstract
Objective: This paper describes the baseline demographics, clinical characteristics, and patient-reported outcomes (PROs) according to clinical phenotype of patients with early psoriatic arthritis (PsA) for the purpose of creating a decision support system for daily clinical practice.
Method: Patients with newly diagnosed PsA were included in the Dutch south west Early Psoriatic ARthritis (DEPAR) study. No classification criteria were applied, to ensure collection of real-world data on demographics, medication, clinical characteristics, and PROs. An IT infrastructure facilitated data collection.
Results: We described 527 patients, categorized according to the clinical phenotype stated by the rheumatologist at the time of diagnosis, namely monoarthritis (15%), oligoarthritis (40%), polyarthritis (23%), enthesitis (10%), axial disease (2%), and dactylitis (10%). Overall psoriasis severity was mild and 83 patients (16%) had no psoriasis. Short-term sick leave (> 1 day per 4 weeks) was 17% and long-term sick leave (> 4 weeks) was 4%. The group with phenotype enthesitis reported the longest duration of complaints, had the highest fatigue scores, and contained the highest percentage of patients with a Hospital Anxiety and Depression Scale (HADS) anxiety score ≥ 8 and depression score ≥ 8.
Conclusion: PsA patients presenting at outpatient clinics in the Netherlands had a mild degree of psoriasis, with impairment of quality of life and work productivity. Most patients presented with phenotype oligoarthritis. Those presenting with phenotype enthesitis more often reported scores suggestive of an anxiety or depression disorder and fatigue. It is important for attending rheumatologists to be aware of these differences when assessing patients with PsA.
Psoriatic arthritis (PsA) is a chronic heterogeneous disease, characterized by inflammation of the skin and musculoskeletal system, resulting in psoriasis, arthritis, enthesitis, and dactylitis (Citation1–3). In recent years, researchers have tried to gain more insight into PsA by conducting cohort studies and randomized controlled trials (RCTs) (Citation4–7). These RCTs used specific inclusion, exclusion, and classification criteria for the recruitment of patients, resulting in baseline data not necessarily applicable to the PsA population seen in daily clinical practice (Citation8, Citation9). To date, not many real-world cohorts with early PsA patients exist (Citation4–6). However, to develop support algorithms for treatment, which can also aid in the process of shared decision making, real-world data on the clinical presentation of PsA patients are needed.
To fulfil this unmet need, the Dutch south west Early Psoriatic ARthritis (DEPAR) study was set up. The DEPAR study collects real-world data on disease activity and long-term outcomes of early PsA patients to develop a decision support system for shared decision making and treatment in daily clinical practice.
In this paper, we describe the baseline demographics, clinical characteristics, and patient-reported outcomes (PROs) according to the clinical phenotype of patients with early PsA in the DEPAR study.
Method
Patients and setting
Newly diagnosed patients with PsA [aged ≥ 18 years, with no current treatment with disease-modifying anti-rheumatic drugs (DMARDs) for joint complaints, and sufficient knowledge of the Dutch language] were invited to participate in the DEPAR study. The diagnosis was made by rheumatologists and based on expert opinion; no classification criteria were applied, to ensure enrolment of a patient sample representative of daily clinical practice. Patients were excluded from the study if arthritis, enthesitis, and/or dactylitis was treated with DMARDs or corticosteroids prior to the first study visit. Patients were recruited in centres in the south-west of the Netherlands (one academic hospital, 10 general hospitals, and one treatment centre specializing in rheumatic care).
For this analysis, baseline data were used from patients included between July 2013 and April 2018. Written informed consent was obtained from all participants according to the Declaration of Helsinki. The study was approved by the local medical research ethics committee of Erasmus MC, University Medical Centre Rotterdam, the Netherlands (MEC-2012-549).
Clinical phenotypes
Upon enrolment, treating rheumatologists categorized each patient according to the predominant clinical features. These categories were monoarthritis, oligoarthritis, polyarthritis, enthesitis, axial, and dactylitis. This manner of categorization was preferred over classification criteria, since the latter are primarily intended for research and not daily clinical practice. Also, it was expected that applied treatment would differ between phenotypes, making the documentation of the clinical phenotypes by the rheumatologist essential for the decision support system.
Data collection
Data were collected by research nurses at baseline and every 3 months during the first year, every 6 months in the second year, and once a year thereafter. All research nurses involved in data collection were trained by the study team, with refreshment courses being offered annually. During the study visits, data were collected on demographics (age, gender, work status, education, smoking, alcohol status), medical history, comorbidities and family history, rheumatic medication history and concomitant medication (e.g. for psoriasis).
Clinical data were collected on swollen and tender joint counts (SJC 66 and TJC 68 joints, respectively), enthesitis at clinical examination [Leeds Enthesitis Index (LEI) (Citation10) and Maastricht Ankylosing Spondylitis Enthesitis Score (MASES) (Citation10)], and psoriasis [Psoriasis Area and Severity Index (PASI)] (Citation11). A visual analogue scale (VAS) score was reported by the research nurse, which assessed the severity of the patient’s arthritis, psoriasis, and arthritis–psoriasis combined (Citation12). C-reactive protein (CRP) levels were analysed by the laboratory of the respective inclusion site.
Patients completed multiple questionnaires within 1 week before or after their visits. These PROs assess a wide variety of domains. Symptoms were assessed through the Visual Analogue Scale (VAS) and Bristol Rheumatoid Arthritis Fatigue (BRAF) questionnaires (Citation12, Citation13). Skin was assessed with the VAS questionnaire and Skindex-17 (Citation12, Citation14). Disease impact and functioning were assessed with the VAS questionnaire (Citation12), Health Assessment Questionnaire (HAQ) (Citation15), and Hospital Anxiety and Depression Scale (HADS) (Citation16, Citation17). General quality of life was assessed with the 36-item Short Form Health Survey (SF-36) (Citation18) and EuroQol 5 Dimensions (EQ-5D) (Citation19). PsA-specific quality of life was assessed with the Psoriatic Arthritis Quality of Life (PsAQoL) questionnaire (Citation20, Citation21). Lastly, work performance was evaluated using the iMTA Productivity Cost Questionnaire (iPCQ) (Citation22). Detailed information on each instrument can be found in Supplementary file S1. All data presented in the tables were collected by research nurses at baseline. The differentiation into clinical PsA phenotypes was made by the rheumatologist at first clinical presentation.
IT infrastructure
To reduce the additional burden on medical staff, an information technology (IT) infrastructure for data collection was put in place in 2013 (). One central server for data collection was approved by all participating hospitals. All the certificates (ISO 27001, ISO 27799, and NEN 7510) required for data communication in healthcare organizations were present (https://www.enovationgroup.com).
Figure 1. Data flow of the Dutch south west Early Psoriatic ARthritis (DEPAR) study: patient-reported outcomes (PROs), clinical data, laboratory data, and medication from the hospital’s electronic patient records were collected and integrated
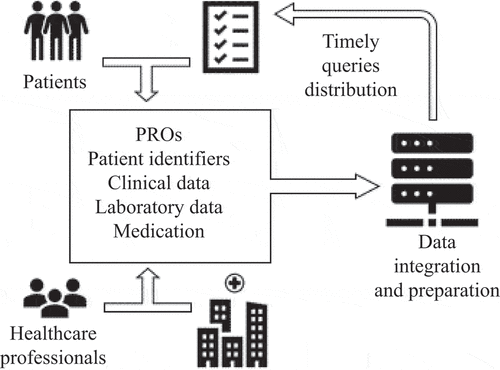
Questionnaires were developed using an opensource Limesurvey software (https://www.limesurvey.org). The logistic support for the distribution of the questionnaires was organized using opensource GemsTracker (GT) software (GEneric Medical Survey Tracker, https://gemstracker.org).
Statistical methods
Patient characteristics, clinical characteristics, and PROs were described using simple descriptive techniques fitted to the distribution of each variable. Analyses were performed in Stata 15.1 (StataCorp, College Station, TX, USA).
Results
Total cohort
In April 2018, 527 patients with newly diagnosed PsA were included. Demographic and clinical characteristics at baseline are shown in . Mean age was 50.2 ± 13.5 years (mean ± sd), 52% were female, and mean body mass index (BMI) was 28.3 ± 5.2 kg/m2. The median self-reported duration of complaints was 11 months [interquartile range (IQR) 4–33]. Approximately 50% (n = 261) of all patients had a positive family history, meaning that at least one first-degree family member was diagnosed with PsA. Ninety-eight (22%) of 449 patients were active smokers at the time of diagnosis. Follow-up of 1 year was available for 355 (78%) of 456 patients who could have had a follow-up of 1 year.
Table 1. Demographic characteristics of the Dutch south west Early Psoriatic ARthritis (DEPAR) study cohort (n = 527)
Table 2. Clinical characteristics of the Dutch south west Early Psoriatic ARthritis (DEPAR) study cohort (n = 527)
Patients had a median (IQR) of 2 (0–4) swollen and 3 (1–7) tender joints. Enthesitis according to the LEI was present in 217 patients (41%) and according to the MASES in 188 patients (36%). Dactylitis of ≥ 1 digits was present in 113 patients (21.4%). Eighty-three patients (16%) had no psoriasis. In cases where psoriasis was present, the median (IQR) PASI score was 2.6 (1–4.7). Twenty-seven patients (5%) had a PASI score above 10 ().
The majority of questionnaires were filled in by > 80% of patients. The mean ± sd VAS on pain was 47.1 ± 26.1. The mean total BRAF score was 21.5 ± 14.4, of which physical fatigue was the highest scoring dimension. The impact of psoriasis and joints on daily life was measured with the VAS, and had a median of 22 (IQR 5–46) and mean ± sd of 46.9 ± 26.9, respectively. Eighty-one patients (19%) had a HADS anxiety score ≥ 8 and 73 patients (17%) a HADS depression score ≥ 8. A score ≥ 8 indicates patients who, given their current state, are suggestive of the presence of an anxiety disorder or depression (Citation17). Patients had a mean score of 39.1 ± 8.6 on the physical component scale of the SF-36 and 47.8 ± 10.4 on the mental component scale. PsA-specific quality of life, measured with the PsAQoL, had a median score of 4 (IQR 1–10) (). Patients reported a median of 5 days (IQR 0–10) productivity loss per 4 weeks. Short-term sick leave (> 1 day per 4 weeks) occurred in 17% of patients. Long-term sick leave (> 4 weeks) occurred in 4% of patients ().
Table 3. Patient-reported outcomes according to clinical phenotype
Table 4. Work productivity according to phenotype among patients of working age (< 66 years; n = 453)
Clinical phenotypes
The PsA phenotype distribution was monoarthritis in 80 patients (15%), while 210 patients (40%) had oligoarthritis, 119 patients (23%) polyarthritis, 54 patients (10%) enthesitis, 13 patients (2%) axial disease, and 51 patients (10%) dactylitis (). Patients with primarily monoarthritis or dactylitis reported total mean ± sd BRAF scores of 19.4 ± 14.6 and 15.2 ± 13.3, respectively, which were the lowest compared to the other phenotypes. The group of patients with dactylitis also reported the highest median EQ-5D Index Score of 0.82 (IQR 0.78–0.89) (). The group of patients with oligoarthritis consisted of slightly more men (n = 117, 56%). Patients with polyarthritis were more often female (n = 69, 58%) and older (mean age 56.1 ± 13.5 years), and more patients had elevated CRP levels (n = 39, 43%). The group of patients with primarily enthesitis (n = 54, 10%) consisted mostly of women (n = 39, 72%) and had the longest duration of complaints, with a median of 28.1 months (IQR 15.1–97.7). This group reported the highest fatigue scores (mean ± sd BRAF score 27.5 ± 11.7) and contained the most patients with a HADS anxiety score ≥ 8 (n = 15, 33%) and HADS depression score ≥ 8 (n = 10, 22%). Patients with primarily axial disease comprised the smallest group (n = 13, 2%) and reported a median duration of complaints of 21.8 months (IQR 9.3–25.0). This group reported a mean ± sd global VAS score and a VAS score for joints of 56.6 ± 26.2 and 58.9 ± 27.8, respectively, which were the highest of all clinical phenotypes.
Both productivity loss per 4 weeks and short-term sick leave (> 1 day per 4 weeks) were highest in the group of patients with axial complaints. Long-term sick leave (> 4 weeks) occurred in 7% of patients with monoarthritis and was highest in this group of patients ().
Arthritis, enthesitis, and dactylitis scores
In the group of monoarthritis (as categorized according to the attending rheumatologists), patients had a median SJC of 1 (IQR 0–1). Median SJC in the dactylitis group was 1 (IQR 0–2). Out of 54 patients (10%) patients who were grouped as having primarily enthesitis by the rheumatologist, only 35 (65%) had an LEI > 1 and also 35 patients had an MASES > 1 as recorded by the research nurse. Of the 51 patients (10%) characterized as ‘predominantly dactylitis’ by the rheumatologist, 30 (58.8%) had dactylitis according to the research nurse.
Discussion
In this paper, we described the baseline characteristics according to clinical phenotype of patients with early PsA in the real-world DEPAR study. The 527 patients included so far showed most patients having predominantly arthritis (monoarthritis, oligoarthritis, or polyarthritis). Quality of life was impaired in the entire group of patients. Skin involvement was relatively mild, as was the impact of psoriasis on daily life. The impact of joint inflammation and joint inflammation combined with psoriasis on daily life was comparable. This finding suggests that the degree of joint involvement is more burdensome than psoriasis in early PsA patients presenting at outpatient rheumatological clinics in The Netherlands.
Within the different clinical phenotypes, certain distinctions were observed. Patients with primarily enthesitis carried a higher emotional burden than those with other phenotypes, which may be partly due to them having the longest delay in complaints prior to diagnosis. Most patients in this group had a HADS score suggestive of the presence of an anxiety or depression disorder. Worth mentioning is that this category contained a higher proportion of women, for whom it has been reported that anxiety and depression rates are higher (Citation23, Citation24). In addition, it is known that chronic widespread pain at baseline increases the risk of becoming depressed and decreases the possibility of recovering from anxiety (Citation25).
Patients with primarily monoarthritis or dactylitis experienced less pain and less fatigue, and enjoyed better general health compared to patients with polyarthritis, even though all patients reported a fair amount of pain. The group of patients with an axial clinical phenotype was too small to draw any conclusions from.
Clinical characteristics of our patients were comparable to existing ‘early PsA’ cohorts in terms of age, gender distribution, and number of swollen joints (Citation4–6). Several other PsA cohorts are available, but either have established PsA as possible entry criterion (Citation26, Citation27) or are more stringent in the use of DMARD prescription for PsA (Citation28–31). This makes these samples less comparable to ours. Data on axial disease are scattered. A previous study reported an axial phenotype to occur in a similar range of 3–5% (Citation32). Furthermore, it is known that employment in patients with rheumatic diseases is lower than in the general population (Citation33). It is therefore interesting to note that work productivity in the DEPAR study was already impaired at baseline.
Our observations have shown a discrepancy in the reported presentation of arthritis, dactylitis, and enthesitis as reported by the attending rheumatologist at the time of initial presentation compared to the clinical record of the research nurse at a later point (the inclusion of the patient into the study). The differences can be partly explained by the temporal relationship of the initial presentation and the moment of inclusion into the study. The exact length of time between the initial presentation and baseline visit was not recorded, but in daily practice most patients were seen by the research nurse 0–14 days after diagnosis. From the literature, is known that observer variation is present between different care provider groups (Citation34). It is advisable to be aware of these discrepancies in a real-world cohort.
The limitations of our study include the small number of patients included with primarily axial complaints (n = 13, 2%). This could be ascribed to the method of categorization or could be caused by inflammatory symptoms and signs of the spine being overlooked, resulting in an underestimation of the true proportion. Another reason may be that axial disease in PsA is associated with longer disease duration (Citation32), while in the DEPAR cohort disease duration was short.
Another limitation may be the apparent discrepancy in the reported presentation of arthritis, dactylitis, and enthesitis, as described earlier in the Discussion. From the literature, it is known that PsA symptoms fluctuate throughout time (Citation35). Owing to the logistics of the study, the assessments by the rheumatologist and research nurse were almost never conducted on the same date. This raises the question of which moment provides a more accurate representation of the severity of the symptoms, even though both measurements are considered to be taken at ‘baseline’. In addition, we instructed research nurses to use the validated Leeds Dactylometer on swollen digits (Citation36), which is a tool not used by rheumatologists.
The strengths of our study include the unique combination of clinical data from all participating hospitals, with a wide variety of PROs filled in by patients. The absence of stringent inclusion and exclusion criteria allowed for the enrolment of a representative sample of the PsA population as seen in daily clinical practice. In addition, a relatively high proportion of patients had a 1 year follow-up (n = 355, 78%), and the majority of questionnaires had a response rate of > 80%. We attribute these high rates to the online data collection system, which allows patients to easily access and fill in the questionnaires, and to be sent a reminder if they do not.
Conclusion
The DEPAR study shows that it is feasible to collect real-world clinical data from over 500 patients with early PsA and to enrich it with extensive PROs. The intended use of the data is to develop a support system for treatment and shared decision making in daily clinical practice. To complete this system, we estimate that approximately 1500 patients need to be included.
The baseline real-world data have shown that most PsA patients presented with oligoarthritis, had a mild degree of psoriasis, and quality of life and work productivity were already impaired at baseline. Clinically, a high proportion of patients presenting with the clinical phenotype enthesitis showed scores suggestive of the presence of an anxiety or depression disorder and fatigue. It is important for attending rheumatologists to be aware of these differences when assessing patients with PsA.
Supplemental Material
Download PDF (116.1 KB)Acknowledgements
We thank all participating patients, research nurses, and rheumatologists. We also thank Dr Esther Röder for her contribution to the study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supplementary file S1. Detailed information on each instrument.
Please note that the editors are not responsible for the content or functionality of any supplementary material supplied by the authors. Any queries should be directed to the corresponding author.
Additional information
Funding
References
- Scher JU, Ogdie A, Merola JF, Ritchlin C. Preventing psoriatic arthritis: focusing on patients with psoriasis at increased risk of transition. Nat Rev Rheumatol 2019;15:153–66.
- Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med 2017;376:957–70.
- Moll JM, Wright V. Psoriatic arthritis. Semin Arthritis Rheum 1973;3:55–78.
- Niccoli L, Nannini C, Cassara E, Kaloudi O, Susini M, Lenzetti I, et al. Frequency of iridocyclitis in patients with early psoriatic arthritis: a prospective, follow up study. Int J Rheum Dis 2012;15:414–8.
- Lindqvist UR, Alenius GM, Husmark T, Theander E, Holmstrom G, Larsson PT, et al. The Swedish early psoriatic arthritis register – 2-year followup: a comparison with early rheumatoid arthritis. J Rheumatol 2008;35:668–73.
- Theander E, Husmark T, Alenius GM, Larsson PT, Teleman A, Geijer M, et al. Early psoriatic arthritis: short symptom duration, male gender and preserved physical functioning at presentation predict favourable outcome at 5-year follow-up. Results from the Swedish early Psoriatic Arthritis register (SwePsA). Ann Rheum Dis 2014;73:407–13.
- Coates LC, Moverley AR, McParland L, Brown S, Navarro-Coy N, O’Dwyer JL, et al. Effect of tight control of inflammation in early psoriatic arthritis (TICOPA): a UK multicentre, open-label, randomised controlled trial. Lancet 2015;386:2489–98.
- Blonde L, Khunti K, Harris SB, Meizinger C, Skolnik NS. Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther 2018;35:1763–74.
- Kennedy-Martin T, Curtis S, Faries D, Robinson S, Johnston J. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials 2015;16:495.
- Mease PJ. Measures of psoriatic arthritis: tender and Swollen Joint Assessment, Psoriasis Area and Severity Index (PASI), Nail Psoriasis Severity Index (NAPSI), Modified Nail Psoriasis Severity Index (mNAPSI), Mander/Newcastle Enthesitis Index (MEI), Leeds Enthesitis Index (LEI), Spondyloarthritis Research Consortium of Canada (SPARCC), Maastricht Ankylosing Spondylitis Enthesis Score (MASES), Leeds Dactylitis Index (LDI), Patient Global for Psoriatic Arthritis, Dermatology Life Quality Index (DLQI), Psoriatic Arthritis Quality of Life (PsAQOL), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), Psoriatic Arthritis Response Criteria (PsARC), Psoriatic Arthritis Joint Activity Index (PsAJAI), Disease Activity in Psoriatic Arthritis (DAPSA), and Composite Psoriatic Disease Activity Index (CPDAI). Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S64–85.
- Fredriksson T, Pettersson U. Severe psoriasis–oral therapy with a new retinoid. Dermatologica 1978;157:238–44.
- Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S240–52.
- Hewlett S, Dures E, Almeida C. Measures of fatigue: Bristol Rheumatoid Arthritis Fatigue Multi-Dimensional Questionnaire (BRAF MDQ), Bristol Rheumatoid Arthritis Fatigue Numerical Rating Scales (BRAF NRS) for severity, effect, and coping, Chalder Fatigue Questionnaire (CFQ), Checklist Individual Strength (CIS20R and CIS8R), Fatigue Severity Scale (FSS), Functional Assessment Chronic Illness Therapy (Fatigue) (FACIT-F), Multi-Dimensional Assessment of Fatigue (MAF), Multi-Dimensional Fatigue Inventory (MFI), Pediatric Quality Of Life (PedsQL) Multi-Dimensional Fatigue Scale, Profile of Fatigue (ProF), Short Form 36 Vitality Subscale (SF-36 VT), and Visual Analog Scales (VAS). Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S263–86.
- Nijsten TE, Sampogna F, Chren MM, Abeni DD. Testing and reducing Skindex-29 using Rasch analysis: Skindex-17. J Invest Dermatol 2006;126:1244–50.
- Bruce B, Fries JF. The Health Assessment Questionnaire (HAQ). Clin Exp Rheumatol 2005;23(Suppl 39):S14–18.
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983;67:361–70.
- Snaith RP. The Hospital Anxiety and Depression Scale. Health Qual Life Outcomes 2003;1:29.
- Leung YY, Ho KW, Zhu TY, Tam LS, Kun EW, Li EK. Testing scaling assumptions, reliability and validity of Medical Outcomes Study Short-Form 36 Health Survey in psoriatic arthritis. Rheumatology (Oxford) 2010;49:1495–501.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:727–36.
- McKenna SP, Doward LC, Whalley D, Tennant A, Emery P, Veale DJ. Development of the PsAQoL: a quality of life instrument specific to psoriatic arthritis. Ann Rheum Dis 2004;63:162–9.
- Wink F, Arends S, McKenna SP, Houtman PM, Brouwer E, Spoorenberg A. Validity and reliability of the Dutch adaptation of the Psoriatic Arthritis Quality of Life (PsAQoL) questionnaire. PLoS One 2013;8:e55912.
- Bouwmans C, Krol M, Severens H, Koopmanschap M, Brouwer W, Hakkaart-van Roijen L. The iMTA productivity cost questionnaire: a standardized instrument for measuring and valuing health-related productivity losses. Value Health 2015;18:753–8.
- McLean CP, Asnaani A, Litz BT, Hofmann SG. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res 2011;45:1027–35.
- Albert PR. Why is depression more prevalent in women? J Psychiatry Neurosci 2015;40:219–21.
- Meesters J, Bergman S, Haglund E, Jacobsson L, Petersson IF, Bremander A. Prognostic factors for change in self-reported anxiety and depression in spondyloarthritis patients: data from the population-based SpAScania cohort from southern Sweden. Scand J Rheumatol 2018;47:185–93.
- Gladman DD, Chandran V. Observational cohort studies: lessons learnt from the University of Toronto psoriatic arthritis program. Rheumatology (Oxford) 2011;50:25–31.
- Kalyoncu U, Bayindir O, Ferhat Oksuz M, Dogru A, Kimyon G, Tarhan EF, et al. The psoriatic arthritis registry of Turkey: results of a multicentre registry on 1081 patients. Rheumatology (Oxford) 2017;56:279–86.
- Stekhoven D, Scherer A, Nissen MJ, Grobety V, Yawalkar N, Villiger PM, et al. Hypothesis-free analyses from a large psoriatic arthritis cohort support merger to consolidated peripheral arthritis definition without subtyping. Clin Rheumatol 2017;36:2035–43.
- Mease PJ, Karki C, Palmer JB, Etzel CJ, Kavanaugh A, Ritchlin CT, et al. Clinical characteristics, disease activity, and patient-reported outcomes in psoriatic arthritis patients with dactylitis or enthesitis: results from the Corrona Psoriatic Arthritis/Spondyloarthritis Registry. Arthritis Care Res (Hoboken) 2017;69:1692–9.
- Hetland ML. DANBIO: a nationwide registry of biological therapies in Denmark. Clin Exp Rheumatol 2005;23:S205–7.
- Kvien TK, Heiberg LE, Kaufmann C, Mikkelsen K, Nordvag BY, E Rødevand. A Norwegian DMARD register: prescriptions of DMARDs and biological agents to patients with inflammatory rheumatic diseases. Clin Exp Rheumatol 2005;23(Suppl 39):S188–94.
- Feld J, Chandran V, Gladman DD. What is axial psoriatic arthritis? J Rheumatol 2018;45:1611–13.
- Mau W, Listing J, Huscher D, Zeidler H, Zink A. Employment across chronic inflammatory rheumatic diseases and comparison with the general population. J Rheumatol 2005;32:721–8.
- Gajdos V, Beydon N, Bommenel L, Pellegrino B, de Pontual L, Bailleux S, et al. Inter-observer agreement between physicians, nurses, and respiratory therapists for respiratory clinical evaluation in bronchiolitis. Pediatr Pulmonol 2009;44:754–62.
- Nossent JC, Gran JT. Epidemiological and clinical characteristics of psoriatic arthritis in northern Norway. Scand J Rheumatol 2009;38:251–5.
- Helliwell PS, Firth J, Ibrahim GH, Melsom RD, Shah I, Turner DE. Development of an assessment tool for dactylitis in patients with psoriatic arthritis. J Rheumatol 2005;32:1745–50.
