Abstract
Objectives: To study whether female patients with active rheumatoid arthritis (RA) have myocardial abnormalities and whether progression of myocardial involvement can be attenuated by disease-modifying anti-rheumatic drugs (DMARDs).
Method: Cardiac magnetic resonance (cMR; 1.5 or 3.0 T), including late gadolinium enhancement (LGE), T1 relaxation time, and ventricular functions, was performed in 30 patients with untreated active early RA starting first DMARDs, and 28 patients with chronic RA with inadequate response to conventional synthetic DMARDs starting biological DMARDs. cMR was repeated in RA patients 1 year later. cMR was conducted once in 22 fibromyalgia (FM) subjects and in 35 healthy volunteers serving as controls. All subjects were non-smoking females without coronary heart disease, heart failure, or diabetes.
Results: Compared with controls, 58 RA patients had slightly lower ventricular function, although in the normal range, and longer T1 time at baseline. None of the FM subjects had LGE, but it was frequent in RA (67%). During the 1 year DMARD treatment, Disease Activity Score based on 28-joint count–C-reactive protein declined, ventricular functions tended to improve, but the number of patients with LGE remained unchanged. However, the number of LGE-positive heart segments either decreased or stayed the same in 91% of RA patients. In early RA patients, achieving tight remission was associated with LGE stabilization, after adjustment for age, metabolic syndrome, baseline inflammatory activity, and leisure-time physical activity.
Conclusion: Treatment targeted to tight remission in early stages of RA seems to be important to prevent not only joint damage but also myocardial abnormalities.
In patients with rheumatoid arthritis (RA), cardiovascular diseases (CVDs) have a major impact on mortality, with a 40–50% increased risk of CVD death (Citation1, Citation2). CVD remains an important cause of death. In a 2018 paper, as much as 34% of patients with incident RA diagnosed from 1995 to 2002 died up to 31 December 2013 because of CVD (Citation3). RA patients have high rates of heart failure and coronary heart disease (CHD) (Citation4). The risk of myocardial infarction in RA patients is comparable to that of patients with diabetes (Citation5). CVDs in RA patients are frequently subclinical and sudden cardiac death is more common than in healthy people (Citation6). RA patients have twice the risk, compared with those without RA, of developing congestive heart failure (CHF) unexplained by common cardiovascular (CV) risk factors or CHD (Citation7). The development of CVD events has been attributed to classical CV risk factors and RA characteristics such as seropositivity and disease activity (Citation8). Myocardial function has been shown to correlate with RA activity in treatment-naïve RA patients (Citation9). Pro-inflammatory cytokines seem to play an important role in the development of myocardial dysfunction (Citation10).
In studies reporting cardiac magnetic resonance (cMR) findings in RA, active disease has been linked to myocardial abnormalities (Citation11–13). In patients with chronic rheumatoid arthritis (CRA), treatment with biological disease-modifying anti-rheumatic drugs (bDMARDs) has improved myocardial function, as visualized by cMR (Citation14, Citation15) and by echocardiography (Citation16).
Contrast-enhanced cMR using the delayed enhancement technique [late gadolinium enhancement (LGE)] is widely applied in the clinical work-up of myocardial diseases. Ischaemic and non-ischaemic cardiomyopathies display characteristic enhancement patterns (Citation17). In the evaluation of diffuse myocardial inflammation and fibrosis, native T1 mapping has become a promising non-invasive cMR tool, in which diffuse myocardial damage represents as high T1 values (Citation18).
We have previously shown patients with active RA, compared with controls, to have more often prolonged T1 relaxation times, frequent LGE, and lower myocardial function on cMR (Citation19). Here, we report the 1 year re-examination results of these patients, after they have been treated with first DMARDs [patients with early rheumatoid arthritis (ERA)] or with bDMARDs (patients with CRA). We hypothesized that, in parallel with intensive DMARD treatment and reduction of RA activity, the progression of myocardial involvement observed in the patients with active RA (Citation19) can be prevented or, possibly, the changes alleviated.
Method
Patients
Of the original 60 female RA patients (Citation19), re-examination at follow-up was performed on 58 patients, and the results are presented here. The study population comprised two patient groups: (i) 30 patients with untreated active ERA who had started their first DMARDs, either conventional synthetic DMARDs (csDMARDs) or bDMARDs; and (ii) 28 patients with CRA who had had an inadequate response to csDMARDs and were candidates for bDMARDs. As gender-matched controls, there were 22 fibromyalgia (FM) subjects and 35 healthy volunteers. RA patients were recruited prospectively from the Department of Rheumatology, Helsinki University Hospital; FM patients from the Department of Rheumatology, Helsinki University Hospital, as well as through the Finnish Rheumatism Association; and healthy volunteers from the hospital staff.
To minimize the possibility of a patient having any other disorder than RA that could have an impact on the myocardium, the study population comprised non-smoking (if ex-smoker, stopped ≥ 10 years ago), non-diabetic females under the age of 70 years, and without a history of CHD, uncontrolled hypertension, episode of hyperthyroidism, severe arrhythmias, chronic atrial fibrillation, severe obesity [body mass index (BMI) > 35 kg/m2], renal failure, cardiac valvular disease, idiopathic cardiomyopathy, or CHF.
Clinical evaluation
At baseline before starting DMARD and at follow-up after 1 year DMARD treatment, RA patients underwent clinical evaluation supplemented with laboratory tests (see below) and 12-lead electrocardiography (ECG), with a review of hospital records as well as contrast-enhanced cMR. In CRA patients, subcutaneous fat aspirate was taken for screening of amyloidosis at baseline. FM subjects were evaluated once, including clinical evaluation, laboratory tests, ECG, and contrast-enhanced cMR. For ethical reasons, healthy controls underwent cMR without any contrast agent. Healthy controls had no clinical evaluation or laboratory tests.
Rheumatoid factor (RF), anti-citrullinated peptide antibody (ACPA), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), haemoglobin A1c (HbA1c), total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides (TGs), plasma urate, plasma troponin T (TnT), and N-terminal pro-B-type natriuretic peptide (proBNP) were measured by the hospital laboratory (HUSLAB clinical laboratory of Helsinki University). We also measured plasma levels of high-sensitivity CRP (hs-CRP), plasma amyloid A (AA), glycoprotein YKL-40, resistin, E-selectin, visfatin, and interleukin-6 (IL-6). Concentrations of YKL-40, resistin, E-selectin, visfatin, and IL-6 were measured by enzyme-linked immunosorbent assay (ELISA). The detection limits and interassay coefficients of variation were, respectively, 7.8 pg/mL and 1.3% for YKL-40, 7.8 pg/mL and 1.7% for resistin, 23.4 pg/mL and 4.3% for E-selectin, 0.1 ng/mL and 4.1% for visfatin, and 0.2 pg/mL and 8.6% for IL-6.
We used modified preliminary American College of Rheumatology (ACR) criteria for remission (Citation20), as used previously (Citation21). According to these criteria, an RA patient was considered to be in tight remission if five out of six variables (morning stiffness < 15 min, no fatigue, joint pain, tender joints, swollen joints or tendons, ESR < 30 mm/h) were present and no swollen (66 joint count) or tender (68 joint count) joints existed. To evaluate remission, we also recorded the Disease Activity Score based on 28-joint count–C-reactive protein (DAS28-CRP), and calculated the treatment response by the European League Against Rheumatism (EULAR) response criteria (Citation22, Citation23).
According to the harmonized criteria (Citation24), subjects with three or more of the following criteria were classified as having metabolic syndrome: (i) increased waist circumference (≥ 88 cm); (ii) elevated fasting total TGs (≥1.7 mmol/L or treatment for dyslipidaemia); (iii) low fasting HDL cholesterol (≤ 1.29 mmol/L or treatment for dyslipidaemia); (iv) systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mmHg or use of antihypertensive medication; and (v) fasting plasma glucose ≥ 5.6 mmol/L or use of medication for hyperglycaemia.
cMR technique and image analysis
All RA patients and FM controls underwent a clinical scan protocol. cMR with contrast agent and motion-corrected native T1 maps was performed on RA patients with either a 1.5 T or 3.0 T magnetic resonance imaging (MRI) scanner (Citation19). Our institution had T1 mapping sequence available for the years 2012–2013 only in the 3.0 T scanner and afterwards only in the 1.5 T scanner. FM subjects were imaged with the 3.0 T and healthy volunteers with the 1.5 T scanner. In each RA and FM patient, the three left ventricle (LV) short axis sections were divided into 17 segments according to American Heart Association (AHA) guidelines (Citation25). The pattern and location of LGE were visually estimated with the AHA model. cMR images were analysed in consensus with two experienced cardiac radiologists (SK and MH) without knowledge of the clinical data of RA and FM patients (Citation19). cMR volumetric measurements were assessed by a cardiac radiologist (SK). To assess interobserver reproducibility, two observers (SK and MH) independently analysed LGE images. Intraclass correlation coefficients (ICCs) using a two-way mixed effects model with absolute agreement were used to calculate interobserver reproducibility. LGE analysis had strong to very strong reproducibility (n = 59), with ICC values varying from 0.77 to 0.92.
Statistical analysis
The characteristics are presented as means with standard deviation (sd) for continuous variables and as frequencies with percentages for categorical variables. Statistical comparisons between the groups were made using chi-squared and Fisher’s exact tests for categorical variables and t-test or permutation-type tests for continuous variables. The changes (repeated measures) within groups were analysed by applying the t-test, permutation test, or Wilcoxon signed-rank test. Factors associated with the evolution of the number of segments affected by LGE were evaluated by ordinal logistic regression. The significance of the changes in myocardial functions was corrected by multiplicity using Hommel’s multiple comparison procedure because functions had noticeable intercorrelation. Hommel’s adjustment was used because it is more powerful than alternative procedures, including the Bonferroni, Holm’s, and Hochberg’s procedures. Statistical analysis was performed with SPSS version 22.0 (SPSS, Chicago, IL, USA) and Stata version 15.0 (Stata Corp, College Station, TX, USA). Statistical analyses concerning the interobserver reproducibility of LGE findings in cMR were carried out with SPSS version 23 (IBM, Armonk, NY, USA).
Results
Patient characteristics
The 58 female RA patients had fewer classical CV risk factors and a lower prevalence of metabolic syndrome than the FM subjects (). The mean ± sd age (48 ± 8 years) and mean ± sd BMI (25 ± 4 kg/m2) of the healthy volunteers were comparable to those of RA patients (p = 0.780 and p = 0.927, respectively). The majority of the RA patients were RF positive (84%) and/or ACPA positive (90%). Of all 58 RA patients, 47% had erosions at baseline, in 17% of ERA patients and in 81% of CRA patients (p < 0.001). Extra-articular manifestations were observed in 33% of the whole RA patient group, but were more frequent in CRA than in ERA patients (50% vs 17%; p = 0.007). Amyloid deposits in subcutaneous fat aspirate were detected in one CRA patient.
Table 1. Characteristics of rheumatoid arthritis (RA) patients and fibromyalgia (FM) subjects at baseline
In line with the Finnish guidelines for the treatment of RA (https://www.kaypahoito.fi/en), 77% of ERA patients started with a csDMARD combination as the first DMARD treatment, most commonly the combination of methotrexate, sulphasalazine, and hydroxychloroquine (in 60%). At 1 year follow-up, treatment with a combination of csDMARDs was slightly more frequent (80%) and 47% of the ERA patients were on the combination of methotrexate, sulphasalazine, and hydroxychloroquine. Two ERA patients started bDMARDs and stayed on the same bDMARD during the follow-up (Supplementary table S1). In the CRA group, 50% used combination csDMARDs to which a bDMARD [tumour necrosis factor (TNF) inhibitor in more than 80% of patients] was added (Supplementary table S2). At baseline, prednisolone was started in 37% of ERA patients and, of the CRA patients, 61% used prednisolone. At follow-up, prednisolone was used by 17% and 43%, respectively.
Over the study period, DAS28-CRP declined significantly in both RA groups; the decrease in various inflammatory markers was more pronounced in ERA than in CRA patients ().
Table 2. Rheumatoid arthritis (RA) disease activity, with levels of cytokines, cardiac markers, and classical cardiovascular risk factors
According to the modified preliminary ACR criteria, 16 (53%) ERA patients and two (7%) CRA patients reached tight remission. Of the ERA patients, 25 (83%) reached DAS28-CRP remission and, of the CRA patients, 15 (54%). Good EULAR response was reached by 67% and 41%, respectively.
cMR findings
LGE
LGE was a frequent finding in RA patients. It was observed in 39 patients (67%) at baseline and in 38 (66%) at follow-up. shows a cMR image of an ERA patient with LGE. None of the FM patients had LGE. In multivariate binary logistic regression analysis, LGE at baseline was associated with DAS28-CRP [odds ratio (OR) 2.01, 95% confidence interval (CI) 1.09 to 3.70; p = 0.025] and age (OR 1.07, 95% CI 1.01 to 1.13; p = 0.012), but not with metabolic syndrome (p = 0.230) and leisure-time physical activity (p = 0.967) (Supplementary table S3). Of the ERA patients, LGE was found in 70% (n = 21) at baseline and in 73% (n = 22) at follow-up. The corresponding figures for CRA patients were 64% (n = 18) and 57% (n = 16).
Figure 1. Cardiac magnetic resonance images of a 57-year-old patient with early RA. (A) Short-axis view of the heart showing linear late gadolinium enhancement (LGE) of the basal interventricular septum, segments 2–3 (arrows), at baseline imaging. (B) At follow-up imaging, LGE had cleared. LV, left ventricle
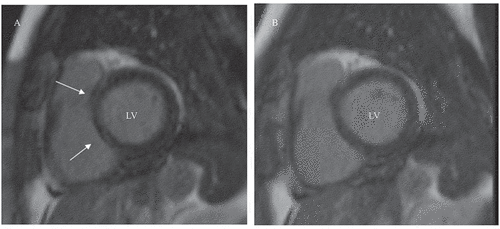
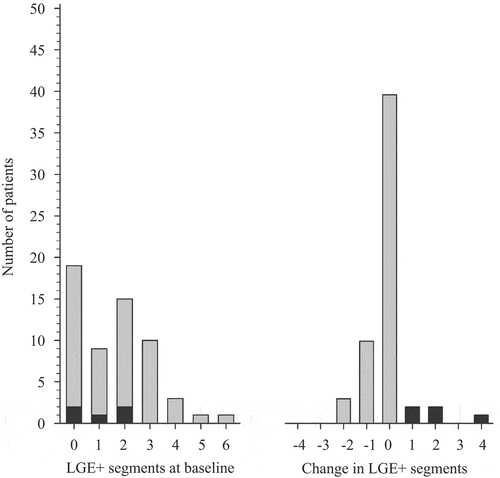
LGE was most frequently observed in basal and mid-inferior segments of the LV at baseline and at follow-up (Supplementary figure S1A–C). The number of LGE-positive segments of the heart, and the change in the number of segments during follow-up, are shown in . In 91.4% of the 39 RA patients with LGE, the number of LGE-positive segments either stayed at the same level or decreased. The number of LGE-positive segments increased in five RA patients (8.6%, 95% CI 2.9 to 19.0). All of these patients were RF- and/or ACPA-positive ERA patients.
Figure 2. Late gadolinium enhancement (LGE) on cardiac magnetic resonance in rheumatoid arthritis (RA) patients. Grey columns represent number of RA patients with LGE-positive segments of the heart according to the American Heart Association (Citation25), in whom LGE-positive segments decreased or stayed at the same level as at baseline at follow-up. Black columns represent RA patients in whom LGE-positive segments increased
The one CRA patient with amyloid deposits in subcutaneous fat aspirate had LGE both at baseline and at follow-up cMR, but the number of LGE-positive segments declined from three to two affected segments.
LGE stabilization and tight remission
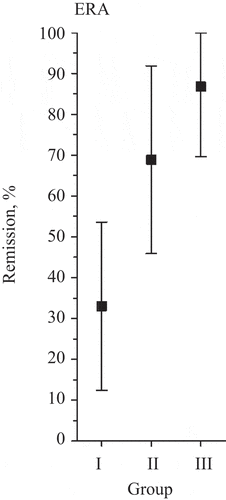
We divided the ERA patients into three groups based on the number of LGE-positive heart segments during the course of the study. Group I comprised patients who had LGE at baseline with the number of LGE-positive segments increasing or remaining the same during follow-up. Group II comprised patients who had LGE neither at baseline nor at follow-up. Group III comprised patients who had LGE at baseline with the number of affected segments decreasing during follow-up. Tight remission was associated with improvement in LGE-positive segments of the heart (Group III) in patients with ERA (OR 21.30, 95% CI 1.76 to > 100; p = 0.016), evaluated by ordinal logistic regression adjusted for age, metabolic syndrome, baseline DAS28-CRP, and leisure-time physical activity ( and Supplementary table S4). We could not conduct such calculations for CRA patients, because only two of them reached tight remission.
Figure 3. Tight remission is associated with stabilization in late gadolinium enhancement (LGE)-positive segments of the heart in early rheumatoid arthritis (ERA). The graph shows the proportion of patients with ERA reaching tight remission according to preliminary American College of Rheumatology criteria in relation to the number of heart segments affected by LGE during the study. Group I comprised 16 patients who had LGE at baseline with the number of LGE-positive segments increasing or remaining the same during follow-up. Group II comprised seven patients who had LGE neither at baseline nor at follow-up. Group III comprised seven patients who had LGE at baseline with the number of LGE-positive segments decreasing during follow-up. The odds ratio was calculated by ordinal logistic regression. LGE stabilization was adjusted for age, metabolic syndrome, baseline Disease Activity Score based on 28-joint count–C-reactive protein, and leisure-time physical activity
At baseline, RA patients with LGE, compared with those without, had a higher mean level of plasma AA (mean ± sd 258.8 ± 328.1 mg/mL vs 84.6 ± 142.6 mg/mL; p = 0.007), YKL-40 (86.0 ± 50.2 ng/mL vs 57.2 ± 24.6 ng/mL; p = 0.013), and IL-6 (11.7 ±12.2 pg/mL vs 4.6 ± 5.9 pg/mL; p = 0.012). No significant differences were observed in hs-CRP, resistin, E-selectin, or visfatin levels (data not shown). Changes in inflammatory markers over the study period showed no significant association with changes in LGE (data not shown).
Ventricular functions
Compared with FM subjects or healthy controls, RA patients had, at baseline, lower ventricular functions, although they were in the normal range (). Over the study period, there was a trend towards improved ventricular functions (), which was, however, not statistically significant following correction for multiple testing. No statistically significant differences were observed in LV mass (mean ± sd) between RA patients (52 ± 11 mg/m2) and FM patients (49 ± 6.8 mg/m2; p = 0.166) or between RA patients and healthy volunteers (52 ± 7 mg/m2; p = 0.958). No significant change in LV mass from baseline to follow-up occurred in RA patients (data not shown). No signs of cardiac amyloidosis were observed in one CRA patient with amyloid deposits in subcutaneous fat aspirate.
Table 3. Findings on cardiac magnetic resonance in rheumatoid arthritis (RA) patients at baseline, in fibromyalgia (FM) subjects, and in healthy volunteers
T1 relaxation time
At baseline, T1 relaxation time (mean ± sd), studied with 1.5 T MRI, was longer in 22 RA patients compared with 35 healthy volunteers (1038 ± 90 ms vs 978 ± 32 ms; p = 0.006), but no significant difference was observed compared with 12 FM subjects (1010 ± 37 ms; p = 0.222). At baseline, T1 relaxation time studied with 3.0 T MRI showed no significant difference in 29 RA patients compared with 10 FM subjects (1125 ± 80 ms vs 1068 ± 123 ms; p = 0.201).
Discussion
In 58 female patients with active RA, focal myocardial abnormalities, as visualized by LGE, were frequent. In FM controls, no such finding was observed. Compared with FM controls and healthy volunteers, lower ventricular functions, although in the normal range, were observed in patients with active RA before the start of new DMARDs. The 1 year remission-targeted DMARD treatment, which in the majority of patients was a combination DMARD therapy, resulted in decreased RA activity, and no progression of focal myocardial abnormalities was observed in the majority of patients. In 30 ERA patients, tight remission was associated with stabilization in focal myocardial abnormalities after adjustment for age, metabolic syndrome, baseline DAS28, and leisure-time physical activity.
Our RA patients were treated frequently with combination DMARDs targeting tight remission, and the target of treatment was a joint decision made by the patient and the physician, in line with EULAR 2019 recommendations (Citation26). Of the ERA patients, 60% started a combination of three csDMARDs (methotrexate, sulphasalazine, and hydroxychloroquine). In combination therapy or monotherapy, methotrexate was started by 97% of ERA patients, in parallel with EULAR 2019 recommendations, which state that methotrexate should be part of the first treatment strategy (Citation26).
In the literature, few cMR studies have reported the effect of bDMARD treatment on the myocardium in patients with CRA (Citation14, Citation15). To the best of our knowledge, no prospective cMR studies on early RA exist. The present study reports myocardial findings not only in CRA, but also in early active untreated RA, before starting the first DMARD and after 1 year of intensive DMARD treatment.
We detected LGE, representing focal myocardial abnormalities, in 67% of patients with active RA at baseline, but in none of the FM controls, in accordance with a previous cross-sectional study on CRA patients (Citation11). Here, we observed that over the 1 year study period, no statistically significant changes appeared in the number of RA patients with LGE. However, the number of LGE-positive segments of the heart decreased or stabilized at follow-up compared to baseline in the majority of RA patients. It has been reported that LGE represents scarring, fibrosis, and inflammation (Citation27). One explanation for the persistence of LGE may be that LGE was caused by myocardial fibrosis and, therefore, LGE was not cleared in spite of the decreased inflammatory activity. Increasing age seems to have an impact on LGE, as also shown here. Another explanation may be that, although RA activity decreased significantly, there still may have been residual inflammation causing myocardial abnormality. The latter explanation is supported by our observation that tight remission in ERA patients was associated with stabilization of focal myocardial involvement. If we had followed our RA population, especially ERA patients, for a longer duration, we might have seen an improvement in LGE.
Myocardial abnormalities have been a common finding in autopsied RA patients (Citation28). Protein citrullination, induced by smoking and periodontitis, is an important triggering event in patients with a genetic background favouring the development of RA. In one autopsy-based study, the authors stated that staining for citrullination was higher in the myocardial interstitium of RA patients than in patients with other diseases, a finding that could link autoimmunity to the known increased incidence of myocardial dysfunction and heart failure in RA (Citation29). In a study reporting LV function by conventional echocardiography and speckle-tracking echocardiography in methotrexate-treated early RA, persistently elevated ACPA was associated with worsening in global longitudinal strain over 2 years (Citation30). This is probably also reflected here, as all our RA patients in whom the number of LGE-affected segments increased over time were RF and/or ACPA positive. Thus, RF and/or ACPA positivity may be an indicator for a high risk of myocardial involvement.
Compared with healthy volunteers, the patients with active RA had longer T1 relaxation time, suggesting diffuse myocardial abnormality, in accordance with findings in a previous cross-sectional study of 39 RA patients (Citation11). No statistically significant trend in T1 relaxation time over the study period was detected.
Before starting DMARDs, the 58 female patients with active RA had normal ventricular functions. Compared with healthy volunteers and FM controls, ventricular functions were slightly lower. This was true despite the FM controls having more classical CV risk factors than the RA patients. Even mild impairment in myocardial function, such as heart failure with preserved LV ejection fraction, has been linked, in non-RA populations, to increased mortality (Citation31). Over the study period, no worsening of ventricular functions was observed in our RA patients, but there was a slight, although non-significant, tendency towards improvement. This was true especially for our ERA patients, who more frequently reached remission than the CRA patients and of whom a majority were treated with combination csDMARDs. If the follow-up time had been longer, there might have been more pronounced changes in myocardial functions.
In ERA patients, proBNP improved over time, maybe reflecting an improvement of myocardial function. Biological therapy targeted towards inhibition of TNF has been associated with worsening of CHF and is contraindicated in patients with New York Heart Association (NYHA) III–IV CHF. None of our CRA patients, the majority of whom were treated with TNF inhibitors, developed CHF. This is in line with a study in which patients with active RA showed increased LV function on echocardiography along with a decrease in endothelin-1, IL-6, and amino-terminal fragment of proBNP levels after treatment with infliximab (Citation16). Bradham et al (Citation32) also showed that CRA patients with low disease activity, 49% of whom were on TNF inhibitor treatment, had LV functions, LGE, and T1 mapping results similar to controls. Contrary to prospective cMR studies in which tocilizumab improved myocardial function in patients with a 30 month duration of RA (Citation14, Citation15), we were unable to find any statistically significant improvement in ventricular functions in our CRA patients, although there was a positive trend over time. In the studies referred to above (Citation14, Citation15), the reduction in DAS28 score was more significant and reached a lower level after tocilizumab treatment than was observed in our CRA patients. One explanation for this discrepancy may be that our CRA patients had much longer RA duration and persistent disease activity despite the use of bDMARDs, and this may have allowed the development of more fibrotic changes of the myocardium, compared with the cases described previously (Citation14, Citation15).
One limitation of our study is that we started the cMR studies with a 3.0 T scanner, but then had to switch to a 1.5 T scanner in the later part of the study. Another limitation is the small number of RA patients divided into two groups. Furthermore, we studied only female patients in order to minimize the risk of CHD. The mean age of RA patients differed from that of FM controls, but not significantly. However, the mean age of RA patients was very close to that of healthy controls. The follow-up time was also rather short.
One of the strengths of our study is the tight inclusion criteria used. Furthermore, our patients were very compliant. Over the 1 year study period, the treatment responses of RA were excellent and enabled us to analyse factors related to myocardial changes with respect to disease activity.
Conclusion
The goal of RA treatment is to prevent joint damage and comorbidities. Our findings suggest that focal myocardial involvement in RA patients is related to systemic inflammation and can be attenuated or stabilized by intensive treatment of RA. Thus, treating RA patients actively and targeting to remission from the early stages of RA is important to prevent not only joint damage, but also the development of myocardial involvement and possibly premature death.
Supporting information
Additional supporting information may be found in the online version of this article.
Supplementary figure S1. (S1A) LV segments AHA; (S1B) myocardial LGE distribution according to AHA segments, RA patients, baseline; (S1C) myocardial LGE distribution according to AHA segments, RA patients, follow-up.
Supplementary table S1. Medication of early RA patients.
Supplementary table S2. Medication of chronic RA patients.
Supplementary table S3. Association of late gadolinium enhancement at baseline with DAS28-CRP, age, metabolic syndrome, and leisure-time physical activity in patients with rheumatoid arthritis.
Supplementary table S4. Relationship of tight remission at 1 year with changes in late gadolinium enhancement from baseline to 1 year follow-up in patients with early rheumatoid arthritis.
Please note that the editors are not responsible for the content or functionality of any supplementary material supplied by the authors. Any queries should be directed to the corresponding author.
Supplemental Material
Download PDF (864.8 KB)Acknowledgements
We thank all the participating subjects for their willingness to contribute to the study and their cooperation. Ms Terhi Salonen and Mr Touko Kaasalainen are acknowledged for their excellent technical assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Sokka T, Abelson B, Pincus T. Mortality in rheumatoid arthritis: 2008 update. Clin Exp Rheumatol 2008;5:35–61.
- Avina-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum 2008;59:1690–7.
- Pedersen JK, Holst R, Primdahl J, Svendsen AJ, Hørslev-Petersen K. Mortality and its predictors in patients with rheumatoid arthritis: a Danish population-based inception cohort study. Scand J Rheumatol 2018;47:371–7.
- Logstrup BB, Ellingsen T, Pedersen AB, Kjaersgaard A, Botker HE, Maeng M. Development of heart failure in patients with rheumatoid arthritis: a Danish population-based study. Eur J Clin Invest 2018;48:e12915.
- Lindhardsen J, Ahlehoff O, Gislason GH, Madsen OR, Olesen JB, Torp-Pedersen C, et al. The risk of myocardial infarction in rheumatoid arthritis and diabetes mellitus: a Danish nationwide cohort study. Ann Rheum Dis 2011;70:929–34.
- Maradit-Kremers H, Crowson CS, Nicola PJ, Ballman KV, Roger VL, Jacobsen SJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum 2005;52:402–11.
- Nicola PJ, Maradit-Kremers H, Roger VL, Jacobsen SJ, Crowson CS, Ballman KV, et al. The risk of congestive heart failure in rheumatoid arthritis: a population-based study over 46 years. Arthritis Rheum 2005;52:412–20.
- Crowson CS, Rollefstad S, Ikdahl E, Kitas GD, PLCM VR, Gabriel SE, et al. Impact of risk factors associated with cardiovascular outcomes in patients with rheumatoid arthritis. Ann Rheum Dis 2018;77:48–54.
- Logstrup BB, Deibjerg LK, Hedemann-Andersen A, Ellingsen T. Left ventricular function in treatment-naive early rheumatoid arthritis. Am J Cardiovasc Dis 2014;4:79–86.
- Giles JT, Fernandes V, Lima JA, Bathon JM. Myocardial dysfunction in rheumatoid arthritis: epidemiology and pathogenesis. Arthritis Res Ther 2005;7:195–207.
- Ntusi NA, Piechnik SK, Francis JM, Ferreira VM, Matthews PM, Robson MD, et al. Diffuse myocardial fibrosis and inflammation in rheumatoid arthritis: insights from CMR T1 mapping. JACC Cardiovasc Imaging 2015;8:526–36.
- Kobayashi H, Kobayashi Y, Yokoe I, Akashi Y, Takei M, Giles JT. Magnetic resonance imaging-detected myocardial inflammation and fibrosis in rheumatoid arthritis: associations with disease characteristics and N-terminal pro-brain natriuretic peptide levels. Arthritis Care Res (Hoboken) 2017;69:1304–11.
- Kobayashi Y, Kobayashi H, Giles J, Yokoe I, Nishiwaki A, Takei M. Impact of biological treatment on left ventricular function and morphology in rheumatoid arthritis patients without cardiac symptoms, assessed by cardiac magnetic resonance imaging. Scand J Rheumatol 2017;46:328–9.
- Kobayashi H, Kobayashi Y, Giles JT, Yoneyama K, Nakajima Y, Takei M. Tocilizumab treatment increases left ventricular ejection fraction and decreases left ventricular mass index in patients with rheumatoid arthritis without cardiac symptoms: assessed using 3.0 tesla cardiac magnetic resonance imaging. J Rheumatol 2014;41:1916–21.
- Kobayashi Y, Kobayashi H, Giles JT, Hirano M, Nakajima Y, Takei M. Association of tocilizumab treatment with changes in measures of regional left ventricular function in rheumatoid arthritis, as assessed by cardiac magnetic resonance imaging. Int J Rheum Dis 2016;19:1169–74.
- Kotyla PJ, Owczarek A, Rakoczy J, Lewicki M, Kucharz EJ, Emery P. Infliximab treatment increases left ventricular ejection fraction in patients with rheumatoid arthritis: assessment of heart function by echocardiography, endothelin 1, interleukin 6, and NT-pro brain natriuretic peptide. J Rheumatol 2012;39:701–6.
- Mahrholdt H, Klem I, Sechtem U. Cardiovascular MRI for detection of myocardial viability and ischaemia. Heart 2007;93:122–9.
- Schelbert EB, Messroghli DR. State of the art: clinical applications of cardiac T1 mapping. Radiology 2016;278:658–76.
- Holmstrom M, Koivuniemi R, Korpi K, Kaasalainen T, Laine M, Kuuliala A, et al. Cardiac magnetic resonance imaging reveals frequent myocardial involvement and dysfunction in active rheumatoid arthritis. Clin Exp Rheumatol 2016;34:416–23.
- Pinals RS, Masi AT, Larsen RA. Preliminary criteria for clinical remission in rheumatoid arthritis. Arthritis Rheum 1981;24:1308–15.
- Leirisalo-Repo M, Kautiainen H, Laasonen L, Korpela M, Kauppi MJ, Kaipiainen-Seppanen O, et al. Infliximab for 6 months added on combination therapy in early rheumatoid arthritis: 2-year results from an investigator-initiated, randomised, double-blind, placebo-controlled study (the NEO-RACo study). Ann Rheum Dis 2013;72:851–7.
- van Gestel AM, Prevoo ML, van ‘T Hof MA, van Rijswijk MH. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum 1996;39:34–40.
- Fransen J, van Riel PL. The disease activity score and the EULAR response criteria. Rheum Dis Clin North Am 2009;35:745–57.
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–5.
- Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002;105:539–42.
- Smolen J, Landewé RB, Burmester J, Dougados M, Kerschbaumer A, McInnes I, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:778–86.
- Mavrogeni S, Karabela G, Stavropoulos E, Gialafos E, Sfendouraki E, Kyrou L, et al. Imaging patterns of heart failure in rheumatoid arthritis evaluated by cardiovascular magnetic resonance. Int J Cardiol 2013;168:4333–5.
- Koivuniemi R, Paimela L, Suomalainen R, Leirisalo-Repo M. Cardiovascular diseases in patients with rheumatoid arthritis. Scand J Rheumatol 2013;42:131–5.
- Giles JT, Fert-Bober J, Park JK, Bingham CO, Andrade F, Fox-Talbot K, et al. Myocardial citrullination in rheumatoid arthritis: a correlative histopathologic study. Arthritis Res Ther 2012;14:R39.
- Logstrup BB, Masic D, Laurbjerg TB, Blegvad J, Herly M, Kristensen LD, et al. Left ventricular function at two-year follow-up in treatment-naive rheumatoid arthritis patients is associated with anti-cyclic citrullinated peptide antibody status: a cohort study. Scand J Rheumatol 2017;46:432–40.
- Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol 1999;33:1948–55.
- Bradham W, Ormseth MJ, Elumogo C, Palanisamy S, Liu CY, Lawson MA, et al. Absence of fibrosis and inflammation by cardiac magnetic resonance imaging in rheumatoid arthritis patients with low to moderate disease activity. J Rheumatol 2018;45:1078–84.
