Abstract
Objectives: To examine whether signs of an active human cytomegalovirus (HCMV) infection are present in affected joints of patients with rheumatoid arthritis (RA).
Method: Polymorphonuclear leucocytes (PMNLs) were obtained from synovial fluid (SF) of 17 RA patients and were analysed for HCMV-pp65 and HCMV-immediate early (IE) proteins using the antigenemia assay. Peripheral blood (PB) and SF obtained from these 17 patients and from 17 additional RA patients (n = 34) were tested for HCMV-IE and pp150 DNA with Taqman polymerase chain reaction. Plasma samples from the patients were analysed for HCMV-immunoglobulin M (IgM) and immunoglobulin G (IgG) by enzyme-linked immunosorbent assay and compared to 71 healthy gender-matched blood donors.
Results: HCMV-pp65 protein was detected in 65% of synovial PMNL samples, but in only 18% of PMNLs from PB. In contrast, HCMV IE protein was not found in any of the analysed PMNL samples. On the DNA level, HCMV-IE and pp150 DNA was detected in SF of 13/32 (41%) and 14/23 (61%) of RA patients, respectively. HCMV-IE and pp150 DNA was also found in 24/33 (73%) and in 16/24 (67%) of PB samples obtained from RA patients, respectively. HCMV IgG seroprevalence was 76% in RA patients as well as in healthy controls, while only one RA patient was positive for specific IgM.
Conclusions: HCMV pp65 antigen was found in PMNLs from SF of RA patients, indicating an active infection in the affected joint. Future studies are needed to determine whether HCMV infection can aggravate the inflammatory process in these patients.
Rheumatoid arthritis (RA) is a complex, multifactorial chronic inflammatory disease with characteristic overactivation of both innate and adaptive immunity, leading to, for example, dysregulation of cytokine networks, and osteoclast and chondrocyte activation affecting peripheral joints (Citation1). Latent or active systemic viral infections, such as Epstein–Barr virus and human cytomegalovirus (HCMV) infections, have been suggested to contribute to RA pathogenesis via different mechanisms (Citation2).
HCMV is a betaherpesvirus with a global prevalence of 70–90% (Citation3). HCMV infection is often acquired in childhood, and remains latent in cells of the myeloid lineage after primary infection. Many HCMV-encoded proteins affect cellular and immunological functions, enabling coexistence of the virus with its host. HCMV has a wide tissue tropism, although not all cell types that are susceptible to infection support the production of new virus particles. Reactivation of the latent infection occurs via inflammatory-mediated differentiation of infected cells into macrophages or dendritic cells, and can cause serious disease in immunocompromised individuals. Effects of active or latent HCMV infection have been proposed to contribute to the inflammatory process in patients with different autoimmune diseases, such as dermatomyositis, RA, and systemic sclerosis (Citation4).
If HCMV reactivates in the inflamed joints of RA patients, polymorphonuclear leucocytes (PMNLs) in the synovial fluid (SF) may become infected and express viral proteins. The viral immediate early (IE) gene products are essential for HCMV replication (Citation5). HCMV pp65 and pp150 are tegument phosphoproteins. pp65 is involved in the HCMV immune evasion mechanisms, and suppresses the interferon response, whereas pp150 is highly immunogenic (Citation3) and is involved in the last steps of viral maturation and release.
While not permissive for HCMV replication, PMNLs contribute to haematogenous spread of HCMV by taking up the virus particles, thus acting as a vehicle for virus transport (Citation3). In RA patients, the synovial lining consists mainly of macrophages and fibroblasts, and the subintimal area of the synovium is infiltrated by inflammatory cells, including T and B lymphocytes, macrophages, mast cells, and mononuclear cells that differentiate into multinucleated osteoclasts. In contrast, the SF is dominated by PMNLs. In a previous study, HCMV DNA was shown by in situ hybridization in 31% of 61 synovial tissue samples from RA patients (Citation6), demonstrating its presence in the synovial tissue, but not whether the virus was active or not. Here, we aimed to investigate whether PMNLs were positive for HCMV proteins, thereby distinguishing active from latent infection.
Method
Samples
Peripheral blood (PB) and SF samples were collected from a total of 34 RA patients (female:male ratio = 3.9:1) at the Rheumatology Clinic, Karolinska University Hospital in Stockholm (ethical permission: 03-138, 00-123, 2008/518-31). Samples from eight patients with inflammatory conditions other than RA were also analysed (Supplementary information and Supplementary table S1). Plasma from the patients and 71 gender-matched healthy controls were analysed for the presence of HCMV immunoglobulin G (IgG) and immunoglobulin M (IgM).
Isolation of blood and synovial PMNLs and HCMV analyses
PMNLs were isolated from blood by gradient centrifugation as described before (Citation7). As a positive control, PMNLs from a healthy donor were infected in vitro through co-culturing with HCMV-infected human umbilical vein endothelial cells (Citation7). For the preparation and immunofluorescence (IF) staining of PMNL cytospin slides and the polymerase chain reaction (PCR) detection method, see Supplementary information and Supplementary figure S1. Plasma samples were analysed for HCMV IgM and IgG with Enzygnost Anti-HCMV/IgM and IgG enzyme-linked immunosorbent assay (ELISA) (Dade Behring Holding, Liederbach, Germany), respectively, according to the manufacturer’s instructions.
Results
The isolated PMNLs from SF and PB of 17 RA patients were tested for the presence of HCMV-IE and pp65 antigens by direct IF. Of these, 11/17 (65%) were positive for pp65 in SF (), while none was positive for HCMV-immediate early antigen (IEA). HCMV-infected PMNLs in vitro served as a positive control (). Three of 17 (18%) PMNL samples from PB were pp65 positive, and none was IEA positive ().
Table 1. Results from polymerase chain reaction (PCR), direct immunofluorescence (IF), and serology analyses of samples from rheumatoid arthritis (RA) patients, and healthy controls
Figure 1. (A) Human cytomegalovirus (HCMV) pp65 in polymorphonuclear leucocytes (PMNLs) (ethanol fixed) from synovial fluid (SF) obtained from the knee of a patient with rheumatoid arthritis (RA). (B) HCMV-pp65 in in-vitro HCMV-infected PMNLs obtained from a healthy donor. (C) Distribution of positive samples for immediate early (IE) and pp150 DNA in PMNLs from peripheral blood (PB) and SF, respectively, in patients with RA. Total number of analysed samples was, for PB IE, n = 33; pp150, n = 24; SF IE, n = 32; SF pp150, n = 23. (D) Delta Ct values (mean and range, calculated by subtracting mean Ct of housekeeping gene from mean Ct of target gene) in samples positive for HCMV IE and/or pp150 DNA (mean Ct ≤ 40, at least one out of two or three reactions positive), in PB and SF, for HCMV IE DNA and pp150 DNA, respectively. PB IE, n = 24; SF IE, n = 13; PB pp150, n = 16; SF pp150, n = 13
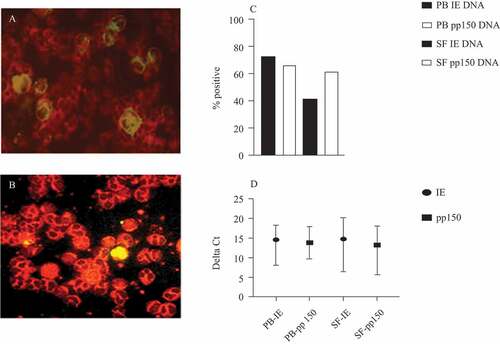
We performed PCR on PMNL DNA samples from an additional 17 RA patients (n = 34). Samples with no detectable housekeeping gene (RNAse P) were excluded from the analysis.
HCMV-IE DNA was detected in 13/32 (41%) of synovial PMNLs and in 24/33 (73%) of PMNLs from PB of RA patients (, ). HCMV-pp150 DNA was detected in PMNLs from SF and PB in 14/23 (61%) and 16/24 (67%) of RA patients, respectively (, ). Mean Ct values for target genes were 22.1–39.7, while mean Ct values for RNAse P were 16–39.7, resulting in delta Ct values (Ct value of target gene subtracted from Ct value of housekeeping gene) of 5.6–20.2 (). In total, 30/34 RA patients (88%) were positive for HCMV DNA (IE and/or pp150) in either PB or SF ().
Table 2. Results from polymerase chain reaction, enzyme-linked immunosorbent assay, and direct immunofluorescence analyses of polymorphonuclear leucocytes from peripheral blood (PB) and synovial fluid (SF) of rheumatoid arthritis patients, including data on anti-citrullinated protein antibody (ACPA) positivity, gender, and age at sampling
There was no difference in HCMV IgG positivity among RA patients compared to healthy controls (76% in both groups). One RA patient was positive for HCMV-IgM; IE DNA was detected in PB of this patient, but not in SF. In five RA patients with positive HCMV IgG serostatus, the HCMV IgM results were equivocal ().
In eight patients with other arthritic diagnoses, HCMV IE DNA was detected in PMNLs from PB and SF in 4/8 (50%) and 3/7 (43%), respectively, while corresponding results for pp150 DNA were 2/7 (29%) and 4/6 (67%), respectively (Supplementary table 1). In this group, 75% were HCMV IgG positive, and one of the two serology-negative individuals was positive for HCMV DNA in PMNLs from PB.
Discussion
Latent HCMV can be reactivated in inflamed tissues and aggravate inflammatory processes, and therefore HCMV is implicated in the pathogenesis of inflammatory diseases. HCMV DNA has been detected in RA synovial tissue (Citation8), but the pathogenic role of HCMV in RA is unclear. In the 17 patient samples analysed with IF, 65% tested positive for HCMV pp65 protein in PMNLs obtained from SF, but none was positive for IE protein. HCMV replicates abortively in PMNLs, and transfer of virus may be conferred by microfusion events and endocytosis (Citation8). Thus, our finding suggests that viral particles produced by, for example, infected macrophages, epithelial cells, or endothelial cells, in the joints of RA patients, are taken up by PMNLs, which themselves do not support virus replication. This may explain why RA synovial PMNLs did not express HCMV-IE antigen.
Both endothelial and epithelial cells have the capacity to transfer virus to neutrophils, which take up the virus in endosomes and carry it to infect other cells. In vitro experiments have shown that HCMV-infected PMNLs become anti-apoptotic, enhance their phagocytosing capacity, and produce high levels of extracellular oxidative metabolites (Citation7). Considering these findings, we hypothesize that HCMV infection of synovial PMNLs may contribute to the damage of surrounding tissue by the production of toxic metabolites. Although the method employed for isolation of PMNLs in PB should give a pure population of PMNLs, it cannot be excluded that monocytes or dendritic cells may have contaminated the samples. However, these cells support HCMV productive infection, and infected monocytes/dendritic cells would not have shown the presence of pp65 protein without IE protein expression. HCMV DNA was detected in all of the HCMV-seronegative RA patients, confirming existing data indicating that some HCMV-infected patients do not develop antibodies detectable by ELISA despite exhibiting circulating cytomegalovirus (CMV) DNA and/or having CMV-specific T cells (Citation9, Citation10). HCMV DNA was found in PMNLs from patients with other types of arthritis as well, which fits well with our hypothesis of a synergic relationship between inflammation and HCMV reactivation. Since HCMV DNA has been detected in HCMV-seronegative individuals in studies of blood donors, it is possible that some of the seronegative blood donors in our control group would also be DNA positive, if we had a possibility to analyse them.
Kaneshiro et al previously reported increased proliferation of RA fibroblast-like synovial cells by induction of interleukin-6 (IL-6) and tumour necrosis factor-α (TNF-α) (Citation11). Active HCMV infection in the arthritic joint could modulate the production of and response to different cytokines and chemokines; contribute to the inflammation by induction of potent inflammatory factors such as IL-1β, IL-6, IL-18, and TNF-α; induce 5-lipoxygenase and production of leukotrienes; and induce cyclooxygenase-2 (COX-2) (Citation12, Citation13), which upregulates HCMV replication. Drugs used in RA treatment may affect the risk of HCMV infection; COX-2 inhibitors inhibit HCMV replication (Citation12, Citation13), while corticosteroid treatment increases the risk of HCMV reactivation (Citation14). The HCMV IE gene has TNF-α-responsive elements in the HCMV major IE promoter, and can be activated by TNF-α, suggesting that the activity of HCMV should be expected to decrease under TNF-α blocking therapy. Clinically, treatment with TNF inhibitors instead may pose an increased risk for HCMV infection (Citation15). However, since these patients were also treated with other, concomitant immunosuppressive/immunomodulatory drugs (Citation15), additional studies are needed to clarify a potential risk association between TNF-α inhibitors and HCMV reactivation.
Conclusion
HCMV-pp65 protein was detected in 65% of SF samples from RA patients, suggesting active infection in the affected joints of these patients. Reactivation and replication of HCMV in RA patients should be limited by anti-inflammatory and immune-modulatory treatment and resolve the inflammatory process in these patients. These insights will help us to further understand the pathogenesis of RA.
Supporting information
Additional supporting information may be found in the online version of this article.
Supplementary information. Methods.
Supplementary figure S1. Preparation and immunofluorescence (IF) staining of PMNL cytospin slides and PCR detection method.
Supplementary table S1. Results from PCR and serology analyses of SF and PB samples from patients with diseases other than RA.
Please note that the editors are not responsible for the content or functionality of any supplementary material supplied by the authors. Any queries should be directed to the corresponding author.
Supplemental Material
Download PDF (198.7 KB)Acknowledgement
We thank Hanneke Vermeulen for excellent technical assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Firestein GS, McInnes IB. Immunopathogenesis of rheumatoid arthritis. Immunity 2017;46:183–96.
- Pierer M, Rothe K, Quandt D, Schulz A, Rossol M, Scholz R, et al. Association of anticytomegalovirus seropositivity with more severe joint destruction and more frequent joint surgery in rheumatoid arthritis. Arthritis Rheum 2012;64:1740–9.
- Mocarski E, Shenk T. In: Field BN, Knipe DM, Howley PM, editors. Fields virology, 5th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2007:2701–72.
- Varani S, Frascaroli G, Landini MP, Soderberg-Naucler C. Human cytomegalovirus targets different subsets of antigen-presenting cells with pathological consequences for host immunity: implications for immunosuppression, chronic inflammation and autoimmunity. Rev Med Virol 2009;69:131–45.
- Stinski MF, Petrik DT. Functional roles of the human cytomegalovirus essential IE86 protein. Curr Top Microbiol Immunol 2008;325:133–52.
- Mehraein Y, Lennerz C, Ehlhardt S, Remberger K, Ojak A, Zang KD. Latent Epstein-Barr virus (EBV) infection and cytomegalovirus (CMV) infection in synovial tissue of autoimmune chronic arthritis determined by RNA- and DNA-in situ hybridization. Mod Pathol 2004;17:781–9.
- Skarman PJ, Rahbar A, Xie X, Soderberg-Naucler C. Induction of polymorphonuclear leukocyte response by human cytomegalovirus. Microbes Infect 2006;8:1592–601.
- Gerna G, Percivalle E, Baldanti F, Sozzani S, Lanzarini P, Genini E, et al. Human cytomegalovirus replicates abortively in polymorphonuclear leukocytes after transfer from infected endothelial cells via transient microfusion events. J Virol 2000;74:5629–38.
- Larsson S, Soderberg-Naucler C, Wang FZ, Cytomegalovirus ME. DNA can be detected in peripheral blood mononuclear cells from all seropositive and most seronegative healthy blood donors over time. Transfusion 1998;38:271–8.
- Litjens NHR, Huang L, Dedeoglu B, Meijers RWJ, Kwekkeboom J, Betjes MGH. Protective cytomegalovirus (CMV)-specific T-cell immunity is frequent in kidney transplant patients without serum anti-CMV antibodies. Front Immunol 2017;8:1137.
- Kaneshiro K, Sakai Y, Suzuki K, Uchida K, Tateishi K, Terashima Y, et al. Interleukin-6 and tumour necrosis factor-alpha cooperatively promote cell cycle regulators and proliferate rheumatoid arthritis fibroblast-like synovial cells. Scand J Rheumatol 2019;48:353–61.
- Gredmark-Russ S, Soderberg-Naucler C. Dendritic cell biology in human cytomegalovirus infection and the clinical consequences for host immunity and pathology. Virulence 2012;3:621–34.
- Maussang D, Langemeijer E, Fitzsimons CP, Stigter-van Walsum M, Dijkman R, Borg MK, et al. The human cytomegalovirus-encoded chemokine receptor US28 promotes angiogenesis and tumor formation via cyclooxygenase-2. Cancer Res 2009;69:2861–9.
- Migita K, Arai T, Ishizuka N, Jiuchi Y, Sasaki Y, Izumi Y, et al. Rates of serious intracellular infections in autoimmune disease patients receiving initial glucocorticoid therapy. PLoS One 2013;8:e78699.
- Kim SY, Solomon DH. Tumor necrosis factor blockade and the risk of viral infection. Nat Rev Rheumatol 2010;6:165–74.
