Abstract
Objectives: This study aimed to identify the therapeutic target concentration and frequency of anti-drug antibodies (ADAbs) in golimumab-treated patients with inflammatory joint disease (IJD).
Method: Associations between golimumab concentration, ADAbs, and treatment response were examined in 91 patients with IJD [41 axial spondyloarthritis (axSpA), 20 rheumatoid arthritis (RA), and 30 psoriatic arthritis (PsA)] included in the NOR-DMARD study. Treatment response was defined by Ankylosing Spondylitis Disease Activity Score (ASDAS) clinically important improvement in axSpA, European League Against Rheumatism (EULAR) good/moderate response in RA, and improvement of ≥ 50% in modified Disease Activity index for PSoriatic Arthritis (DAPSA) (28 swollen/tender joint counts) in PsA. Serum drug concentrations and ADAbs were analysed using automated in-house assays.
Results: At inclusion, 42% were biological disease-modifying anti-rheumatic drug naïve and 42% used concomitant synthetic disease-modifying anti-rheumatic drug. The median golimumab concentration was 2.2 (interquartile range 1.0–3.5) mg/L. The proportions of responders after 3 months among patients with golimumab concentration < 1.0, 1.0–3.9, and ≥ 4.0 mg/L were 19%, 49%, and 74%, respectively. A higher rate of treatment discontinuation was seen in patients with serum golimumab concentration < 1.0 compared to ≥ 1.0 mg/L (hazard ratio 3.3, 95% confidence interval 1.8–6.0, p < 0.05). ADAbs were detected in 6%, and were associated with lower drug concentrations and both reduced treatment response and drug survival.
Conclusions: Golimumab concentrations ≥ 1.0 mg/L were associated with improved treatment response and better drug survival, although some patients may benefit from higher concentrations. This study suggests a rationale for dosing guided by therapeutic drug monitoring in golimumab-treated patients with IJD. The results should be confirmed in larger studies including trough samples, and the efficacy of such a strategy must be examined in randomized controlled trials.
Golimumab, a human immunoglobulin G1-kappa (IgG1-κ) monoclonal antibody against tumour necrosis factor-alpha (TNF-α), has proven efficacious in the treatment of rheumatoid arthritis (RA), psoriatic arthritis (PsA), and axial spondyloarthritis (axSpA) (Citation1–3). However, not all patients respond adequately to treatment. Lack or loss of response to TNF inhibitors (TNFi) can be caused by subtherapeutic drug concentrations (pharmacokinetic failure), sometimes associated with the formation of anti-drug antibodies (ADAbs), or a mismatch between drug target and key disease mediators (pharmacodynamic failure) (Citation4–12).
As a supplement to standard clinical care, therapeutic drug monitoring (TDM) has the potential to improve the effectiveness, safety, and cost-effectiveness of treatment with biological drugs in rheumatology (Citation13–15). For TDM to be validated as a clinical tool in golimumab-treated patients with inflammatory joint disease (IJD), the therapeutic target concentration must be identified. Associations between serum golimumab concentrations and treatment responses have been suggested in relatively small observational studies on patients with RA and axSpA (Citation3, Citation16–18). The occurrence of ADAbs against golimumab varies between 0% and 15% (Citation16, Citation18, Citation19).
A therapeutic target concentration of 0.7–1.4 mg/L for golimumab has been suggested in axSpA (Citation17). Therapeutic target concentrations for treatment of RA and PsA remain to be identified, and the suggested target concentration in axSpA should be confirmed. Furthermore, there are few reports on the clinical relevance of ADAbs against golimumab in IJD patients (Citation16, Citation18).
The main objective of this study was to identify a therapeutic target concentration for non-trough serum samples in golimumab-treated patients with IJD by examining the association between golimumab concentrations and treatment response and drug survival. In addition, we wanted to assess the clinical significance of early ADAb development.
Method
The NOR-DMARD study and patient selection
The Norwegian DMARD study (NOR-DMARD; clinicaltrials.gov: NCT01581294) is a longitudinal observational study of adult IJD patients initiating treatment with a biological disease-modifying anti-rheumatic drug (bDMARD) (Citation20). Clinical data are registered at baseline, 3, 6, 9, and 12 months, and every 6 months thereafter. Biobank samples are collected at baseline and at the 3 month follow-up visit.
For the current analyses, we included consecutive patients enrolled in the study between January 2013 and June 2017, with a clinical diagnosis of axSpA, RA, or PsA, who had started golimumab treatment and had available biobank samples from the 3 month visit. Serum samples analysed in this study were non-trough samples, collected at the 3 month visit and stored at −80°C. Clinical data from baseline and the 3 and 6 month follow-up visits were used in the analyses.
The study was approved by the Regional Ethics Committee of Eastern Norway (ref. 2011/1339). All patients provided written, informed consent before inclusion.
Clinical response
The disease activity measures used in this study were the Ankylosing Spondylitis Disease Activity Score–C-reactive protein (ASDAS-CRP) for axSpA (Citation21), the 28-joint Disease Activity Score–erythrocyte sedimentation rate (DAS28-ESR) (Citation22) for RA, and a modified version of the Disease Activity index for PSoriatic Arthritis, using 28 swollen/tender joint counts (DAPSA28) for PsA (Citation23–25). Modified DAPSA28 was calculated as DAPSA28 = (28TJC × 1.6) + (28SJC × 1.6) + Patient global (0–10VAS) + Pain (0–10VAS) + CRP (mg/dL) (Citation23). Treatment response was defined by ASDAS Clinically important improvement (CII) (an ASDAS-CRP reduction of ≥ 1.1 units) in axSpA (Citation21), European League Against Rheumatism (EULAR) good or moderate response in RA (Citation26), and DAPSA28 improvement ≥ 50% in PsA (Citation24). Sensitivity analyses, using DAPSA32 (Citation27) improvement ≥ 50% and DAS28 improvement of ≥ 0.6 (Citation28) to define treatment response, were performed in PsA.
Measurement of golimumab drug concentrations and ADAbs
Drug concentrations were measured using an in-house, European In-Vitro Diagnostic Devices Directive-compliant, time-resolved fluorometric assay automated on the AutoDELFIA (PerkinElmer, Waltham, MA, USA) immunoassay platform. The assay is a minor modification of our previously described method for serum belatacept (Citation29) and uses human recombinant TNF-α as the capture reagent. Golimumab binding to the TNF-α solid phase is detected using a europium-labelled protein-A tracer reagent (Citation29–31). ADAbs were detected by an in-house assay measuring neutralizing ADAb. In this assay, the amount of Eu-labelled golimumab (Fab´)2 binding to the TNF-α solid phase is inversely proportional to the amount of ADAb present in the sample. As are most ADAb assays, our assay is drug sensitive. In brief, europium-labelled golimumab (Fab´)2 was dispensed into streptavidin-coated 96-well plates, followed by patient serum. After 1 h incubation, biotinylated recombinant TNF-α was added to the wells. After 30 min incubation, the plates were washed and time-resolved fluorescence was measured. The calibrator in our ADAb assay, which was developed in house, was a high-affinity murine anti-idiotype IgG1 monoclonal antibody (mAb), S17.1, against golimumab. Blank serum was used to prepare a blank calibrator and dilutions of purified mAb, S17.1, to 15, 30, 50, 80, and 100 µg/L. Samples were defined as positive if the ADAb level was ≥ 20 µg/L in combination with golimumab concentration < 5.0 mg/L. All samples were also tested for ADAbs using a more drug-tolerant pH-shift assay, which dissociates complexes of ADAb bound to golimumab. The pH-shift assay was equivalent to the main (drug-sensitive) ADAb assay, but included a predilution of the serum samples in 0.05 M glycine–HCl buffer to pH 2.4 to dissociate the ADAb–drug complexes. Europium-labelled golimumab (Fab´)2, in 0.4 M Tris buffer (pH 7.8), was dispensed onto streptavidin-coated wells before adding the prediluted serum samples (pH 7.0). After incubation, biotinylated TNF-α in 0.4 M Tris buffer was added (pH 7.4).
Statistical analyses
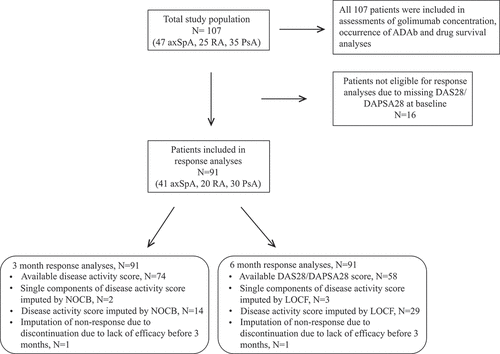
For differences in baseline characteristics, independent samples t-test, Mann–Whitney U test, or chi-squared tests were used. Statistical tests were two sided with the level of significance set at 0.05. Associations between golimumab concentrations and response were assessed by multivariate logistic regression, adjusting for age, gender, and prior use of bDMARDs (Yes/No). For missing 3 and 6 month disease activity data, the next observation carried backwards or last observation carried forward approach was used, respectively ().
Figure 1. Overview of the study population. axSpA, axial spondyloarthritis; RA, rheumatoid arthritis; PsA, psoriatic arthritis; ADAb, anti-drug antibodies; DAS28, 28-joint Disease Activity Score; DAPSA28, 28-joint Disease Activity index for PSoriatic Arthritis; NOCB, next observation carried backwards; LOCF, last observation carried forward
Drug survival was assessed with Kaplan–Meier curves and Cox proportional hazard regression analysis, adjusting for age, gender, and prior use of bDMARD (Yes/No). Patients without data on discontinuation and those who discontinued treatment owing to pregnancy or remission, were censored at the last registered visit. Statistical analyses were performed using IBM SPSS Statistics, version 25 (IBM Corp, Armonk, NY, USA).
Results
Study population and baseline characteristics
An overview of the study population is shown in . Baseline characteristics stratified for diagnosis and golimumab serum concentration < 1.0 mg/L versus ≥ 1.0 mg/L in the 3 month sample are shown in . Among RA patients, those with golimumab concentration < 1.0 mg/L had a significantly lower mean age at inclusion compared to patients with golimumab concentration ≥ 1.0 mg/L. Other baseline characteristics did not differ significantly between patients with golimumab concentration < 1.0 vs ≥ 1.0 mg/L.
Table 1. Comparison of baseline characteristics in patients with golimumab serum concentration < 1.0 mg/L vs ≥ 1.0 mg/L at 3 month follow-up
Distribution of golimumab serum concentrations
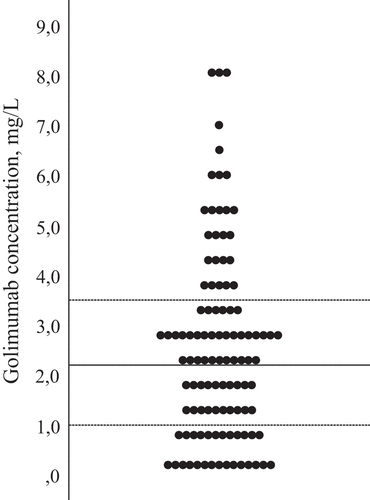
Golimumab concentrations in the 3 month samples varied from 0.0 to 8.2 mg/L (). For the total IJD population, median [interquartile range (IQR)] golimumab concentration was 2.2 (1.0–3.5) mg/L (coefficient of variation 76%). For individual diagnoses, the median (IQR) concentrations were 2.7 (1.2–4.8) in axSpA, 1.6 (0.5–2.8) in RA, and 2.3 (1.2–3.2) mg/L in PsA. The golimumab concentrations were < 1 mg/L in 24 patients. Among these, the concentrations varied from 0.0 to 0.9 mg/L, median (IQR) 0.3 (0.0–0.6) mg/L. Seven patients had undetectable levels.
Figure 2. Distribution of golimumab serum concentrations in mg/L at 3 months (total inflammatory joint disease population). Median (interquartile range) = 2.2 (1.0–3.5) mg/L
Most patients were being given the standard dose, 50 mg every fourth week, at the 3 month visit. Among the three patients who were not being given the standard dose at 3 months, two patients received 100 mg every fourth week and one patient on the standard dose had paused treatment at the 3 month visit.
Association between golimumab concentrations and treatment response
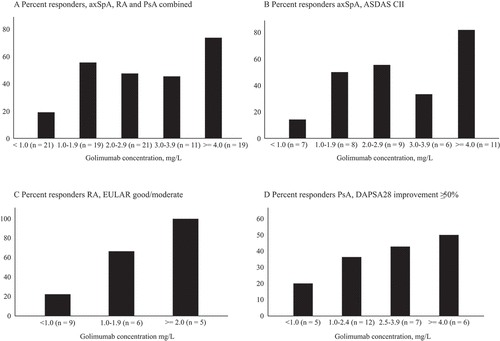
The associations between serum golimumab concentration and treatment response, defined as ASDAS CII in axSpA, EULAR good/moderate response in RA, and improvement of ≥ 50% in DAPSA28 score in PsA, were examined. The proportions of responders after 3 and 6 months, stratified by golimumab serum concentration at 3 months, for the total IJD population and for axSpA, RA, and PsA separately, are shown in and –). Based on the explorative concentration–effect analyses (depicted in ), we wanted to assess the association between golimumab concentration ≥ 1.0 mg/L and treatment response. To this end, we compared responses among patients with golimumab ≥ 1.0 versus < 1.0 mg/L. Furthermore, the concentration–effect analyses suggested an additional effect of having a golimumab concentration ≥ 4.0 mg/L; hence, we compared responses among patients with golimumab ≥ 4.0 versus 1.0–3.9 mg/L. For the total population of IJD, the likelihood of response after 3 months of treatment was significantly higher in patients with serum golimumab concentrations ≥ 1.0 mg/L than in those with golimumab < 1.0 mg/L [odds ratio (OR) 5.8, 95% confidence interval (CI) 1.7–19.7, p = 0.005]. The association between serum golimumab concentration ≥ 1 mg/L and response after 6 months showed a similar tendency but was not statistically significant (OR 2.7, 95% CI 0.9–7.8, p = 0.08). Across the total IJD population, the proportion of responders was highest among patients with golimumab concentration ≥ 4.0 mg/L (–), although the difference in response between the group with golimumab concentration ≥ 4.0 mg/L compared to 1.0–4.0 mg/L was not statistically significant (ORs for response for golimumab concentration ≥ 4.0 mg/L vs 1.0–4.0 mg/L at 3 and 6 months: OR 2.1, 95% CI 0.6–7.1, p = 0.24, and OR 1.5, 95% CI 0.4–5.1, p = 0.54, respectively). The results were confirmed by sensitivity analyses using ≥ 50% improvement in DAPSA32 and improvement of ≥ 0.6 in DAS28 as response criteria in PsA (results not shown).
Table 2. Response* (%) at 3 and 6 months, stratified by golimumab concentration at 3 months
Figure 3. Proportion of responders at 3 months, stratified by golimumab concentration (mg/L). (A) Total inflammatory joint disease population; (B) Ankylosing Spondylitis Disease Activity Score (ASDAS) clinically important improvement (CII) responders in axial spondyloarthritis (AxSpA); (C) European League Against Rheumatism (EULAR) good/moderate response in rheumatoid arthritis (RA); (D) 28-joint Disease Activity Index for PSoriatic Arthritis (DAPSA28) ≥ 50% improvement in psoriatic arthritis (PsA)
As shown in , the association between concentration and response was strongest in bDMARD-naïve patients [OR 21.6, 95% CI 2.0–233.5, p = 0.01 for response among patients with golimumab ≥ 1.0 mg/L compared to golimumab < 1.0 mg/L (all diagnoses)]. bDMARD-naïve patients were responders after 3 months in 60% of cases, compared to 38% among those with prior use of bDMARD (p = 0.04). The results were consistent after 6 months. The median (IQR) golimumab serum concentration was similar in patients with prior use of bDMARD, compared to bDMARD-naïve, 2.1 (1.0–3.0) mg/L versus 2.8 (1.7–4.5) mg/L, respectively (p = 0.06). However, the proportion of patients with high golimumab concentrations, ≥ 4 mg/L, was higher among bDMARD-naïve patients than those with prior use of bDMARDs, 14 (32%) versus eight (13%), respectively (p = 0.02).
Drug survival
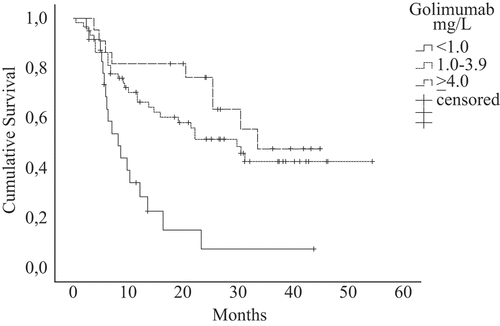
Data for discontinuation were registered in 61 of 107 patients within the first 36 months of golimumab treatment. The discontinuation rates differed between groups stratified by golimumab concentration (). The hazard ratio (HR) for discontinuation was 3.3 (95% CI 1.8–6.0) (p < 0.001), for patients with golimumab concentration < 1.0 mg/L versus ≥ 1.0 mg/L. We found a trend, although not statistically significant, towards a higher discontinuation rate in the 1.0–3.9 mg/L group compared to the ≥ 4.0 mg/L group (HR 1.5, 95% CI 0.7–3.3, p = 0.32).
Among patients with golimumab concentration < 1.0 mg/L, 20 of 24 (83%) discontinued within 36 months of treatment initiation, compared to 32 of 61 (53%) of those with golimumab concentration 1.0–3.9 mg/L and nine of 22 (41%) with ≥ 4.0 mg/L.
Reasons for discontinuation were lack of efficacy (LOE) in 37 patients (61%), adverse events (AEs) in 16 (26%), remission in one (2%), pregnancy in three (5%), other reasons in three patients (5%), and unknown in one patient (2%). Stratified by golimumab concentration, LOE was the discontinuation cause in 10 of 24 (42%), 22 of 61 (36%), and five of 22 (23%), and AEs were the cause in seven of 24 (29%), seven of 61 (12%), and two of 22 patients (9%) among patients with golimumab concentrations of < 1 mg/L, 1–3.9 mg/L, and ≥ 4 mg/L, respectively.
Frequency and clinical significance of ADAbs at 3 month sampling
Out of 107 patients, six (6%) had developed ADAbs after 3 months of treatment (two axSpA, two RA, and two PsA). Golimumab serum concentrations were significantly lower in ADAb-positive patients compared to ADAb-negative individuals [median 0.1 (IQR 0.0–1.0) mg/L vs 2.4 (1.2–3.6) mg/L, p = 0.001].
Out of 91 patients with available response data, only one out of five ADAb-positive patients was a responder at 3 and 6 months. Conversely, 42 and 43 out of 86 ADAb-negative patients were responders after 3 and 6 months, respectively. Using the pH-shift assay, an additional two samples (both PsA) were ADAb positive. Of these, one was a responder at 3 months while neither was a responder at 6 months.
Out of the six ADAb-positive patients, four discontinued treatment owing to LOE and one to AEs within the first 14 months. One patient discontinued after 5 months because of pregnancy.
Mean drug survival time was 7 (95% CI 2–11) months in patients who were ADAb positive at 3 months, compared to 30 (95% CI 25–34) months in the ADAb-negative group. Both of the additional two patients who were positive in the pH-shift assay had discontinued treatment within 12 months.
Among ADAb-positive patients with data on the use of concomitant synthetic DMARDs, two out of five used a concomitant synthetic DMARD (methotrexate), and among ADAb-negative patients the proportion was 43 out of 101 (37 methotrexate, six other synthetic DMARDs).
Discussion
Our results demonstrate a concentration–effect relationship in non-trough samples from golimumab-treated patients with IJD. We found a higher proportion of responders among patients with serum concentration ≥ 1.0 mg/L compared to < 1.0 mg/L, across the total IJD population as well as for the individual diagnoses. Furthermore, drug survival was better among patients with serum concentration ≥ 1.0 mg/L. A trend towards even better response rates and drug survival in patients with drug concentrations ≥ 4.0 mg/L was found across all diagnoses.
Golimumab serum concentrations varied significantly between individuals, which might suggest both undertreatment and overtreatment. We aimed to identify a therapeutic target concentration applicable to clinical practice. Our results suggest that a non-trough golimumab concentration ≥ 1.0 mg/L increases the likelihood of achieving a response to golimumab treatment. This is supported by drug survival analyses and is consistent with previous results from small observational studies in RA and axSpA (Citation16–18). We were not able to identify an upper limit of the target concentration and our results may suggest an additional benefit of higher drug concentrations (≥ 4.0 mg/L) in some patients. The authors of a previous dose-finding study did not find a clear difference in response between different dosing groups, although they did report a higher proportion of American College of Rheumatology 20% improvement responders in the group receiving 100 mg every 2 weeks (Citation32). In a study on patients with RA, a better treatment response was found among patients with golimumab concentration ≥ 1.4 mg/L, compared to < 1.4 mg/L (Citation18). A study on axSpA patients suggested 0.7–1.4 mg/L as the therapeutic range of golimumab trough concentrations (Citation17). Response among patients with higher drug concentrations was not assessed in these studies, and results from these studies may not be directly comparable to our results owing to different sampling times. The discrepancy between the therapeutic levels suggested by these previous studies could be a consequence of different patient populations with regard to diagnosis, age, and gender, as well as differences in study design, disease-specific outcome measures, and assays to measure drug concentration.
The random timing of serum sampling during an injection cycle could contribute to the large variation in golimumab concentrations seen between individuals in this study and could potentially influence the results of the concentration–effect analyses. However, in clinical practice it is rarely feasible or cost-effective to measure trough concentration for TNFi that are subcutaneous and self-administered. Hence, knowledge about therapeutic concentrations for non-trough samples is clinically useful. By assessing samples collected at random times, we risk underestimating the incidence of low drug levels, but we expect the specificity of a low concentration to be high. Importantly, a low random-sampling drug measurement will alert the clinician to a patient who may benefit from specific evaluation of whether the golimumab dose may be adjusted. In that case, a trough sample may be taken from that one specific patient to ensure the best evaluation possible of eligible patients, without the challenging logistics of trough measurements in all patients. Previous studies have demonstrated a benefit of pharmacological testing of non-trough samples from patients treated with subcutaneous TNFi (Citation9, Citation10, Citation33). In a pharmacokinetic study assessing golimumab serum concentrations at different time points during an injection cycle in PsA patients, a relatively small variation was illustrated in observed concentrations, on a group level, for patients treated with golimumab 50 mg every 4 weeks (Citation34). Furthermore, pharmacokinetic testing and simulation for other subcutaneous TNFi have suggested that the intraindividual variation in serum concentrations is moderate between injections (Citation35, Citation36).
The association between golimumab serum concentrations and response after 3 months was most consistent for axSpA and RA. Our study was not powered to evaluate axSpA, RA, and PsA separately, but we considered it relevant to assess whether there were considerable differences between individual diagnoses. Stratification of patients in groups based on drug concentrations was tailored to the individual diagnoses (–), based on differences in numbers of patients and the distribution of drug concentrations between diagnoses.
We found a relatively low proportion of responders among PsA patients in our cohort. This may be related to the response criteria used. PsA is a heterogeneous disease with diverse manifestations, making appropriate response measures a challenging issue. DAPSA28 was used as a disease activity measure in PsA because DAPSA based on the 28 swollen/tender joint count has been shown to be a valid disease activity measure in PsA, if the more extensive 66/68 joint count is not available (Citation23). DAPSA reductions of 50/75/85% have previously been validated as cut-off points for DAPSA response (Citation24). Sensitivity analyses using improvement of ≥ 50% in DAPSA32 (Citation27) and of ≥ 0.6 in DAS28 (Citation28) as response criteria were performed in PsA and showed similar results (results not shown).
Our results demonstrated a higher rate of premature discontinuation of treatment in patients with serum golimumab concentration < 1.0 mg/L compared to ≥ 1.0 mg/L. Low serum concentrations of golimumab and ADAbs have been shown to be associated with LOE, and ADAbs may theoretically be a risk factor for AE (Citation16–18, Citation37). Pregnancy and remission as reasons for discontinuation are unlikely to be associated with low drug concentration; thus, these patients were censored in the survival analyses.
The incidence of ADAbs against golimumab detected in our study is consistent with previous studies (Citation16, Citation18, Citation19). ADAbs were associated with LOE in two small cohorts of golimumab-treated RA patients (Citation16, Citation18). We found a lower proportion of responders and an increased incidence of premature treatment discontinuation among ADAb-positive patients, compared to ADAb-negative, although the number of ADAb-positive patients was relatively small. Detection of ADAbs early after treatment initiation is potentially clinically relevant, as anti-infliximab antibodies have been shown to appear early after treatment initiation and precede loss of response to treatment as well as hypersensitivity reactions (Citation38). In line with these results, a study by Siljehult et al showed similar incidences of anti-infliximab antibodies at weeks 14 and 52 (Citation11). We detected ADAbs in an additional two samples with the more drug-tolerant pH-shift assay. Acid pretreatment may improve detection of ADAbs, particularly in non-trough samples (Citation39–41), but the clinical utility remains unclear (Citation42, Citation43).
Our study shows associations between serum golimumab concentration, ADAbs, and clinical efficacy on a group level. The findings that some patients were responders despite low drug concentration, and others did not respond in the presence of high drug concentrations, may appear counterintuitive. However, these scenarios illustrate the potential utility of TDM. It is relevant to identify patients likely to be in spontaneous remission despite low or unmeasurable concentrations of active drug, as these patients may potentially taper or discontinue treatment without increasing the risk of a disease worsening (Citation44). Conversely, non-response in the presence of high drug levels could indicate a pharmacodynamic failure and these patients could probably benefit from switching to a bDMARD with another mechanism of action or a targeted synthetic DMARD (Citation14, Citation44). These strategies remain to be examined in randomized controlled strategy trials.
The main strength of this study is the applicability of the results to regular clinical care, including feasible sample collection. Data are from a real-life cohort using non-trough biobank samples collected at 3 months after treatment initiation. A clinical tool to aid in decision making at the 3 month evaluation of treatment efficacy is useful and in line with the early treat-to-target strategy recommended by EULAR and EULAR-ASAS (Citation45–47).
Limitations of our study include the small sample sizes and subsequent lack of statistical power when studying individual diagnoses and higher drug concentrations across diagnoses. Patients with incomplete data on follow-up, and the subsequent use of imputation of data, could represent a bias. Furthermore, the lack of data regarding the timing of sample collection in relation to the last administered dose of golimumab is a weakness. These limitations should be kept in mind when interpreting the results of our study, and the results should be confirmed in larger studies including trough samples. The lack of more extensive joint counts (discussed above) and extra-articular manifestations in PsA patients is a weakness, as well as the lack of data for body weight or body mass index, which are relevant when assessing serum drug concentrations. These parameters were not recorded in the NOR-DMARD study. Our study included relatively few bDMARD-naïve patients, especially among RA patients. Previous results from the NOR-DMARD cohort have shown similar drug survival, but better treatment responses, in bDMARD-naïve compared to non-naïve golimumab-treated patients with IJD (Citation48).
Conclusion
Our results suggest that a golimumab serum concentration ≥ 1.0 mg/L increases the likelihood of achieving a clinical response in IJD, although we cannot exclude that higher concentrations are beneficial in subsets of patients or in certain clinical settings. A significant proportion of patients had already developed ADAbs after 3 months of treatment. These patients had lower drug concentrations, reduced response, and reduced drug survival rates compared to ADAb-negative patients. We observed large variations in golimumab concentrations between individuals on a standard dose, and consistent relationships between drug concentration and both effectiveness and drug survival. Taken together, our results suggest a rationale for personalized dosing guided by TDM in golimumab-treated patients with IJD, but the efficacy of such a strategy must be examined in randomized controlled trials.
Acknowledgements
We thank the patients who participated in the study, the staff at the Departments of Rheumatology at Diakonhjemmet Hospital, Lillehammer Hospital for Rheumatic Diseases, and Vestre Viken Hospital Trust for collection of data and biobank samples, and Åge Winje Brustad, Sigurd Leinæs Bøe, Miriam Øijordsbakken, and Elisabeth Paus at the Department of Medical Biochemistry at Oslo University Hospital for help with producing the murine anti-golimumab monoclonal antibody.
The NOR-DMARD study has been financially supported by pharmaceutical companies but the sponsors had no influence on study design, analyses, and presentation of data.
Disclosure statement
JG has received speaking fee from Roche. SWS has received speaking fee from Roche and Thermo Fisher. TKK has received fees for speaking and/or consulting AbbVie, Biogen, BMS, Celltrion, Egis, Eli Lilly, Hikma, MSD, Mylan, Novartis, Oktal, Orion Pharma, Hospira/Pfizer, Roche, Sandoz, Sanofi and UCB. NB has received fees for speaking and/or consulting Pfizer, Orion Pharma, Napp pharmaceuticals, Takeda, Roche, Janssen and Novartis. GLG has received fees for speaking and/or consulting Abbvie, Biogen, Boehringer Ingelheim, Orion Pharma, Eli Lilly, Novartis, Pfizer, MSD, Roche and UCB. The remaining authors declare no conflicts of interest.
References
- Keystone EC, Genovese MC, Klareskog L, Hsia EC, Hall ST, Miranda PC, et al. Golimumab, a human antibody to tumour necrosis factor {alpha} given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO-FORWARD study. Ann Rheum Dis 2009;68:789–96.
- Kavanaugh A, van der Heijde D, McInnes IB, Mease P, Krueger GG, Gladman DD, et al. Golimumab in psoriatic arthritis: one-year clinical efficacy, radiographic, and safety results from a phase III, randomized, placebo-controlled trial. Arthritis Rheum 2012;64:2504–17.
- Inman RD, Davis JC Jr., Heijde D, Diekman L, Sieper J, Kim SI, et al. Efficacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum 2008;58:3402–12.
- St Clair EW, Wagner CL, Fasanmade AA, Wang B, Schaible T, Kavanaugh A, et al. The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2002;46:1451–9.
- Wolbink GJ, Voskuyl AE, Lems WF, de Groot E, Nurmohamed MT, Tak PP, et al. Relationship between serum trough infliximab levels, pretreatment C reactive protein levels, and clinical response to infliximab treatment in patients with rheumatoid arthritis. Ann Rheum Dis 2005;64:704–7.
- Pouw MF, Krieckaert CL, Nurmohamed MT, van der Kleij D, Aarden L, Rispens T, et al. Key findings towards optimising adalimumab treatment: the concentration-effect curve. Ann Rheum Dis 2015;74:513–18.
- van Kuijk AW, de Groot M, Stapel SO, Dijkmans BA, Wolbink GJ, Tak PP. Relationship between the clinical response to adalimumab treatment and serum levels of adalimumab and anti-adalimumab antibodies in patients with psoriatic arthritis. Ann Rheum Dis 2010;69:624–5.
- Garces S, Demengeot J, Benito-Garcia E. The immunogenicity of anti-TNF therapy in immune-mediated inflammatory diseases: a systematic review of the literature with a meta-analysis. Ann Rheum Dis 2013;72:1947–55.
- Jani M, Isaacs JD, Morgan AW, Wilson AG, Plant D, Hyrich KL, et al. High frequency of antidrug antibodies and association of random drug levels with efficacy in certolizumab pegol-treated patients with rheumatoid arthritis: results from the BRAGGSS cohort. Ann Rheum Dis 2017;76:208–13.
- Gehin JE, Goll GL, Warren DJ, Syversen SW, Sexton J, Strand EK, et al. Associations between certolizumab pegol serum levels, anti-drug antibodies and treatment response in patients with inflammatory joint diseases: data from the NOR-DMARD study. Arthritis Res Ther 2019;21:256.
- Siljehult F, Ärlestig L, Eriksson C, Rantapää-Dahlqvist S. Concentrations of infliximab and anti-drug antibodies in relation to clinical response in patients with rheumatoid arthritis. Scand J Rheumatol 2018;47:345–50.
- Buch MH. Defining refractory rheumatoid arthritis. Ann Rheum Dis 2018;77:966–9.
- Martin-Lopez M, Carmona L, Balsa A, Calvo-Alen J, Sanmarti R, Tornero J, et al. Serum drug levels of biologic agents in the management of rheumatoid arthritis and spondyloarthritis: a systematic review. Rheumatol Int 2018;38:975–83.
- Krieckaert CL, Nair SC, Nurmohamed MT, van Dongen CJ, Lems WF, Lafeber FP, et al. Personalised treatment using serum drug levels of adalimumab in patients with rheumatoid arthritis: an evaluation of costs and effects. Ann Rheum Dis 2015;74:361–8.
- Mulleman D, Meric JC, Paintaud G, Ducourau E, Magdelaine-Beuzelin C, Valat JP, et al. Infliximab concentration monitoring improves the control of disease activity in rheumatoid arthritis. Arthritis Res Ther 2009;11:R178.
- Chen DY, Chen YM, Hung WT, Chen HH, Hsieh CW, Chen YH, et al. Immunogenicity, drug trough levels and therapeutic response in patients with rheumatoid arthritis or ankylosing spondylitis after 24-week golimumab treatment. Ann Rheum Dis 2015;74:2261–4.
- Martinez-Feito A, Plasencia-Rodriguez C, Navarro-Compan V, Jurado T, Kneepkens EL, Wolbink GJ, et al. Optimal concentration range of golimumab in patients with axial spondyloarthritis. Clin Exp Rheumatol 2018;36:110–14.
- Kneepkens EL, Plasencia C, Krieckaert CL, Pascual-Salcedo D, van der Kleij D, Nurmohamed MT, et al. Golimumab trough levels, antidrug antibodies and clinical response in patients with rheumatoid arthritis treated in daily clinical practice. Ann Rheum Dis 2014;73:2217–19.
- Vincent FB, Morand EF, Murphy K, Mackay F, Mariette X, Marcelli C. Antidrug antibodies (ADAb) to tumour necrosis factor (TNF)-specific neutralising agents in chronic inflammatory diseases: a real issue, a clinical perspective. Ann Rheum Dis 2013;72:165–78.
- Olsen IC, Haavardsholm EA, Moholt E, Kvien TK, Lie E. NOR-DMARD data management: implementation of data capture from electronic health records. Clin Exp Rheumatol 2014;32(Suppl 85):S–158–62.
- Machado P, Landewe R, Lie E, Kvien TK, Braun J, Baker D, et al. Ankylosing Spondylitis Disease Activity Score (ASDAS): defining cut-off values for disease activity states and improvement scores. Ann Rheum Dis 2011;70:47–53.
- Prevoo ML, van ‘T Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8.
- Michelsen B, Sexton J, Smolen JS, Aletaha D, Krogh NS, van der Heijde D, et al. Can disease activity in patients with psoriatic arthritis be adequately assessed by a modified Disease Activity index for PSoriatic Arthritis (DAPSA) based on 28 joints? Ann Rheum Dis 2018;77:1736–41.
- Schoels MM, Aletaha D, Alasti F, Smolen JS. Disease activity in psoriatic arthritis (PsA): defining remission and treatment success using the DAPSA score. Ann Rheum Dis 2016;75:811–18.
- Schoels M, Aletaha D, Funovits J, Kavanaugh A, Baker D, Smolen JS. Application of the DAREA/DAPSA score for assessment of disease activity in psoriatic arthritis. Ann Rheum Dis 2010;69:1441–7.
- van Gestel AM, Haagsma CJ, van Riel PL. Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheum 1998;41:1845–50.
- Michelsen B, Kristianslund EK, Hammer HB, Fagerli KM, Lie E, Wierod A, et al. Discordance between tender and swollen joint count as well as patient’s and evaluator’s global assessment may reduce likelihood of remission in patients with rheumatoid arthritis and psoriatic arthritis: data from the prospective multicentre NOR-DMARD study. Ann Rheum Dis 2017;76:708–11.
- Fransen J, Antoni C, Mease PJ, Uter W, Kavanaugh A, Kalden JR, et al. Performance of response criteria for assessing peripheral arthritis in patients with psoriatic arthritis: analysis of data from randomised controlled trials of two tumour necrosis factor inhibitors. Ann Rheum Dis 2006;65:1373–8.
- Klaasen RA, Egeland EJ, Chan J, Midtvedt K, Svensson M, Bolstad N, et al. A fully automated method for the determination of serum belatacept and its application in a pharmacokinetic investigation in renal transplant recipients. Ther Drug Monit 2019;41:11–18.
- Jorgensen KK, Olsen IC, Goll GL, Lorentzen M, Bolstad N, Haavardsholm EA, et al. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet 2017;389:2304–16.
- Bader LI, Solberg SM, Kaada SH, Bolstad N, Warren DJ, Gavasso S, et al. Assays for infliximab drug levels and antibodies: a matter of scales and categories. Scand J Immunol 2017;86:165–70.
- Kay J, Matteson EL, Dasgupta B, Nash P, Durez P, Hall S, et al. Golimumab in patients with active rheumatoid arthritis despite treatment with methotrexate: a randomized, double-blind, placebo-controlled, dose-ranging study. Arthritis Rheum 2008; 58:964–75.
- Jani M, Chinoy H, Warren RB, Griffiths CE, Plant D, Fu B, et al. Clinical utility of random anti-tumor necrosis factor drug-level testing and measurement of antidrug antibodies on the long-term treatment response in rheumatoid arthritis. Arthritis Rheumatol 2015;67:2011–19.
- Xu Z, Vu T, Lee H, Hu C, Ling J, Yan H, et al. Population pharmacokinetics of golimumab, an anti-tumor necrosis factor-alpha human monoclonal antibody, in patients with psoriatic arthritis. J Clin Pharmacol 2009;49:1056–70.
- Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther 2008;117:244–79.
- Ungar B, Engel T, Yablecovitch D, Lahat A, Lang A, Avidan B, et al. Prospective observational evaluation of time-dependency of adalimumab immunogenicity and drug concentrations: the poetic study. Am J Gastroenterol 2018;113:890–8.
- Thomas SS, Borazan N, Barroso N, Duan L, Taroumian S, Kretzmann B, et al. Comparative immunogenicity of TNF inhibitors: impact on clinical efficacy and tolerability in the management of autoimmune diseases. A systematic review and meta-analysis. BioDrugs 2015;29:241–58.
- Nencini F, Vultaggio A, Pratesi S, Cammelli D, Milla M, Fiori G, et al. The kinetics of antidrug antibodies, drug levels, and clinical outcomes in infliximab-exposed patients with immune-mediated disorders. J Allergy Clin Immunol Pract 2018;6:2065–72.e2.
- Lofgren JA, Wala I, Koren E, Swanson SJ, Jing S. Detection of neutralizing anti-therapeutic protein antibodies in serum or plasma samples containing high levels of the therapeutic protein. J Immunol Methods 2006;308:101–8.
- Bourdage JS, Cook CA, Farrington DL, Chain JS, Konrad RJ. An Affinity Capture Elution (ACE) assay for detection of anti-drug antibody to monoclonal antibody therapeutics in the presence of high levels of drug. J Immunol Methods 2007;327:10–17.
- van Schouwenburg PA, Bartelds GM, Hart MH, Aarden L, Wolbink GJ, Wouters D. A novel method for the detection of antibodies to adalimumab in the presence of drug reveals “hidden” immunogenicity in rheumatoid arthritis patients. J Immunol Methods 2010;362:82–8.
- Leu JH, Adedokun OJ, Gargano C, Hsia EC, Xu Z, Shankar G. Immunogenicity of golimumab and its clinical relevance in patients with rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis. Rheumatology (Oxford) 2019;58:441–6.
- Van Stappen T, Vande Casteele N, Van Assche G, Ferrante M, Vermeire S, Gils A. Clinical relevance of detecting anti-infliximab antibodies with a drug-tolerant assay: post hoc analysis of the TAXIT trial. Gut 2018;67:818–26.
- Garcês S, Antunes M, Benito-Garcia E, da Silva JC, Aarden L, Demengeot J. A preliminary algorithm introducing immunogenicity assessment in the management of patients with RA receiving tumour necrosis factor inhibitor therapies. Ann Rheum Dis 2014;73:1138–43.
- Smolen JS, Landewe R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–77.
- van der Heijde D, Ramiro S, Landewe R, Baraliakos X, Van den Bosch F, Sepriano A, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 2017;76:978–91.
- Gossec L, Smolen JS, Ramiro S, de Wit M, Cutolo M, Dougados M, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis 2016;75:499–510.
- Michelsen B, Sexton J, Wierod A, Bakland G, Rodevand E, Kroll F, et al. Four-year follow-up of inflammatory arthropathy patients treated with golimumab: data from the observational multicentre NOR-DMARD study. Semin Arthritis Rheum 2020;50:12–16.