Abstract
Objective: To determine whether a family history of spondyloarthritis (SpA) is associated with clinical presentation at the start of tumour necrosis factor inhibitor (TNFi) treatment, or predictive of TNFi drug survival and treatment response in patients with SpA.
Method: Family history of SpA in patients with ankylosing spondylitis (AS), psoriatic arthritis (PsA), and undifferentiated SpA (uSpA) from the Swedish Rheumatology Quality register starting a TNFi as their first biologic in 2006–2018 was assessed through national registers. Clinical characteristics at treatment start were compared by family history status. We used Cox regression to estimate hazard ratios for drug discontinuation, and analysed treatment response at 3 and 12 months with linear regression. Multiple imputation was used to address missing data.
Results: We included 9608 patients. Patients with family history had an earlier age at onset and longer disease duration at TNFi treatment start, but did not differ regarding disease activity and presence of SpA manifestations. Hazard ratios for drug discontinuation were 1.08 [95% confidence interval (CI) 0.89–1.31] for AS patients with a family history of AS, 1.02 (95% CI 0.89–1.18) for PsA patients with a family history of PsA, and 1.11 (95% CI 0.85–1.45) for uSpA patients with a family history of uSpA, after adjusting for demographic, socioeconomic, and SpA-related factors. Treatment response at 3 and 12 months was similar between groups.
Conclusion: Family history of SpA was not found to be associated with clinical presentation at the start of TNFi treatment, nor was it associated with drug survival or treatment response in SpA patients starting a first TNFi.
Tumour necrosis factor inhibitors (TNFi) are highly effective, though costly, treatments for spondyloarthritis (SpA) (Citation1, Citation2). Not all patients respond to TNFi treatment, however. Within one year, 20–25% of patients will have discontinued therapy (Citation3, Citation4). If patients with a low chance of responding could be identified and offered alternative treatment, this would potentially reduce patient suffering, risk of disease progression, and healthcare costs. It is thus important to identify patient characteristics predictive of TNFi treatment response. In SpA, predictors of good response include male gender, high baseline inflammation, and good functional status (Citation5–13), while smoking and obesity predict poorer treatment outcomes (Citation7, Citation14–16). Despite these advances, it is currently not possible to predict response in an individual patient.
A distinctive feature of SpA is the high familial aggregation. We found a 20-fold increased risk of ankylosing spondylitis (AS) in first-degree relatives of AS patients (Citation17), and even higher estimates have been reported (Citation18–20). In psoriatic arthritis (PsA), 30–55 times higher risks are reported in first-degree relatives (Citation21–24). Consequently, family history is essential in clinical evaluation of patients with suspicion of SpA, and is integrated in both the Assessment of SpondyloArthritis international Society (ASAS) classification criteria (Citation25, Citation26) and the ClASsification criteria for Psoriatic ARthritis (CASPAR) (Citation27).
Despite its strong association with disease development, less is known about the role of family history in disease prognosis. Existing studies are mainly cross-sectional, comparing prevalent cases with and without a family history of AS, PsA, psoriasis, or SpA overall. Some have found lower age at onset (Citation28–31), increased prevalence of extra-articular manifestations or deformities (Citation28, Citation31), and increased disease activity in familial versus sporadic cases (Citation28), while others found no such differences (Citation32, Citation33), or even milder disease in patients with familial SpA (Citation29, Citation34, Citation35). The impact of family history on TNFi response at 6 months in axial SpA was investigated along with other potential predictors in two studies (Citation16, Citation36), where no association was found, but with family history data for only 349 and 249 patients, respectively, the power to detect clinically meaningful predictions was low. Thus, the impact of a family history of SpA on disease presentation and clinical outcomes, especially in relation to TNFi treatment, remains unclear.
We aimed to assess whether a family history of SpA overall, or of specific SpA diagnoses, is associated with a different clinical presentation at TNFi treatment start. We further assessed whether a family history is predictive of drug survival and treatment response in SpA patients starting a TNFi as their first biological therapy. To do this, we took advantage of Swedish clinical and national registers, including unique information on relatedness in the Swedish Multi-Generation Register (MGR).
Method
Study cohort
We conducted a nationwide cohort study by linking prospectively collected data on treatment and clinical outcomes from the Swedish Rheumatology Quality Register (SRQ) to national health registers. The SRQ was originally initiated for monitoring of biological therapy, and collects longitudinal data on disease activity and treatment at patient visits (Citation37). Diagnoses in SRQ are rheumatologist based, with 86% coverage of biological treatment in Swedish SpA patients (Citation38). We included patients in SRQ diagnosed with AS [International Classification of Diseases, 10th Revision (ICD-10) codes M45, M08.1], PsA (L40.5, M07.3, M09.0), and undifferentiated SpA (uSpA; M46.8, M46.9, M07.2) starting treatment with a TNFi as their first ever biological therapy between 1 January 2006 and 31 October 2018. To increase validity, the patient’s inclusion date in the SRQ had to be less than 90 days after the treatment start date in the SRQ. Subjects were excluded by cross-reference to the Prescribed Drug Register (PDR) if they had collected a TNFi prescription more than 60 days before treatment start in SRQ, or (non-infliximab only) had no filled TNFi prescription in the 12 months after treatment start. The PDR was established in 2005 and has near complete coverage of prescriptions claimed at pharmacies in Sweden (Citation39).
The study was performed in accordance with the Helsinki Declaration and approved by the Regional Ethics Review Board in Stockholm. Participants’ consent was not needed because of the register-based study design.
Family history
First-degree relatives (parents, siblings, and children) of index patients were identified via the MGR, a register recording parents of Swedish residents since 1961. Index patients were required to have both parents recorded, to allow for identification of full siblings. Relatives’ medical history from 1987 onwards was ascertained from the National Patient Register (NPR), containing diagnoses from inpatient care since 1964 and specialist outpatient (i.e. non-primary) care since 2001. Family history was defined as having at least one first-degree relative diagnosed prior to the index patient’s start of TNFi treatment. Diagnoses of SpA in the NPR have been shown to have high validity. The positive predictive value (PPV) for an AS diagnosis to fulfil the modified New York criteria is reported to be 80%. For uSpA, a PPV of 89% for fulfilling any of the commonly used SpA criteria has been reported (Citation40). The PPV for PsA has been estimated to be 63–92% (Citation41). We separately considered family history of the index patient’s SpA type, another SpA diagnosis, inflammatory bowel disease (IBD), psoriasis, anterior uveitis, rheumatoid arthritis (RA), other inflammatory arthritis, and the non-inflammatory conditions osteoarthritis, spondylosis, dorsalgia, and fibromyalgia/chronic pain (full definition in Supplementary table S1). With the exception of non-inflammatory conditions, diagnoses had to be given at a clinic with an appropriate speciality. For psoriasis, filled prescriptions in the PDR of anti-psoriatic drugs were also counted.
Covariates
Data on disease activity and patient-reported outcomes were collected from the SRQ at visits closest to treatment start (−60 to +7 days), and 3 months (2–6 months) and 12 months (9–18 months) after treatment start. Measures included were the Health Assessment Questionnaire (HAQ), Patient Global Assessment (PtGA), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Ankylosing Spondylitis Disease Activity Score with C-reactive protein (ASDAS-CRP), Disease Activity Score in 28 joints with CRP (DAS28-CRP), Disease Activity in Psoriatic Arthritis (DAPSA), 28-joint swollen and tender joint counts (SJC and TJC), CRP, erythrocyte sedimentation rate (ESR), and patient-reported pain.
Other baseline characteristics included age, gender, disease duration, TNFi compound (adalimumab, certolizumab-pegol, etanercept, golimumab, infliximab), healthcare region, and country of birth (Sweden, Scandinavia, other). The presence of SpA manifestations (anterior uveitis, psoriasis, peripheral arthritis, and IBD), co-medications (conventional systemic disease-modifying anti-rheumatic drugs, oral steroids, and non-steroidal anti-inflammatory drugs), and medical history (of malignancy, cardiovascular disease, diabetes mellitus, chronic lung disease, chronic kidney disease, receiving knee/hip prosthesis, depression, and serious infection) at treatment start were recorded as binary variables. Data on socioeconomic factors (smoking, disposable income, and educational level) in the year before TNFi start were collected. Detailed definitions are provided in Supplementary table S2.
Drug survival
Drug survival was defined as the number of days for which a patient continued treatment, based on treatment start and end dates in the SRQ. Treatment cessations shorter than 90 days, or switches to a biosimilar of the same compound after a treatment cessation shorter than 90 days, were not considered discontinuations. Censoring occurred if the reason for ending therapy was listed as pregnancy or remission, or at death, emigration, or end of follow-up (31 January 2020).
Treatment response
Response after 3 months of treatment was evaluated as the change in disease activity or functional status compared to baseline. For AS/uSpA, the measures used were the BASDAI, ASDAS-CRP, HAQ, and PtGA. For PsA, response was measured with the DAS28-CRP, DAPSA, HAQ, and PtGA. Because of the expected high proportion of missing data, and the assumption that about 25% of patients would have discontinued treatment after 1 year, we defined treatment response at 12 months as a series of composite outcomes of remaining on treatment and reaching any of the following response criteria: low disease activity (LDA) with BASDAI (< 4) or ASDAS-CRP (< 2.1) in AS/uSpA, DAS28-CRP (< 2.6) or DAPSA (< 4) in PsA, and an HAQ improvement of > 0.2 or PtGA < 20 for all SpA types. Patients who stopped treatment before their 12 month visit, or before 365 days if no visit was registered, were considered non-responders.
Statistical analysis
Comparisons of baseline factors between patients with and without family history were made with chi-squared and Mann–Whitney tests.
Drug survival in patients with and without family history was assessed with Kaplan–Meier plots stratified by SpA type in R version 3.6.3, and hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated with Cox regression. Differences in change in disease activity at 3 months and differences in proportions of patients on therapy and reaching response criteria at 12 months were estimated using linear regression with robust standard errors.
To evaluate whether potential differences could be explained by other known patient characteristics, three multivariable models were applied. Model A adjusted for basic characteristics: age, gender, TNFi compound, country of birth, and healthcare region. Model B further adjusted for SpA-specific clinical factors: symptom duration, baseline disease activity, SpA manifestations, and co-medications. Model C, in addition to covariates from models A and B, included socioeconomic factors and medical history. In addition, all models of drug survival were adjusted for family history of the other inflammatory and non-inflammatory conditions studied. Continuous covariates were modelled with linear and quadratic terms.
Multiple imputation of missing covariates and outcome variables was used. Twenty-five imputed data sets were created by fully conditional specification in SAS, with models including all exposures, outcomes, and covariates. Continuous variables were modelled with linear and quadratic terms and imputed with predictive mean matching.
Confidence intervals were not adjusted for multiple testing. All analyses, unless otherwise stated, were conducted in SAS version 9.4.
Sensitivity analyses
While diagnosis is registered in the SRQ, the main indication for starting TNFi treatment is not specified, making it unclear in which domain to evaluate response in specific patients. To reduce heterogeneity, we analysed the 12 month response among patients with active disease at baseline (defined as BASDAI ≥ 4 or ASDAS-CRP ≥ 2.1 in AS/uSpA or at least one swollen or tender joint in PsA), and drug survival among patients with systemic inflammation (baseline CRP > 10 mg/L or ESR > 20 mm/h).
We also analysed drug survival excluding patients on etanercept. Since this drug is reported as being less effective than other TNFi in decreasing the risk of anterior uveitis (Citation42), patients developing anterior uveitis on etanercept may switch therapies despite overall good treatment effect.
Finally, to investigate whether the introduction of new classification criteria and alternative treatments over the study period may have affected the results, we analysed drug survival stratified by calendar period of treatment start: 2006–2009, 2010–2014, and 2015–2018.
Results
We identified 12 936 SpA patients starting their first TNFi treatment between 2006 and 2018. After excluding patients without both parents in the MGR (n = 1466), with a treatment start date more than 90 days before inclusion in the SRQ (n = 1045), patients who had filled a TNFi prescription more than 60 days before treatment start in the SRQ (n = 715), and patients with no filled TNFi prescriptions in the 12 months following their treatment start date (n = 102), 9608 patients were included in the study. Among 2568 patients with AS, 292 (11%) had a family history of AS and 440 (17%) a family history of any SpA. Similarly, 456 (11%) of 4282 patients with PsA had a family history of PsA, while 583 (14%) had a family history of any SpA. Among 2758 patients with uSpA, 137 (5%) had a family history of uSpA and 405 (15%) a family history of any SpA.
Clinical presentation at treatment start
For all three SpA types, patients with a positive family history of the same diagnosis were on average younger at disease onset, but of similar age when starting treatment, yielding a longer disease duration at treatment start (; additional variables in Supplementary table S3). In PsA, family history was more common among females. There were no significant differences by family history status in disease activity at TNFi start, or prevalence of SpA manifestations. Moreover, no notable differences were seen in co-medication use, medical history, or socioeconomic factors, except for a lower proportion of oral steroid users among uSpA patients with a family history of uSpA (Supplementary table S3).
Table 1. Patient characteristics at start of first tumour necrosis factor inhibitor treatment in Swedish spondyloarthritis (SpA) patients diagnosed with ankylosing spondylitis (AS), psoriatic arthritis (PsA), and undifferentiated spondyloarthritis (uSpA), by family history status
Drug survival
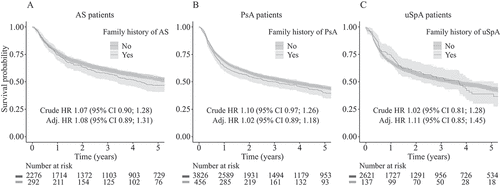
Overall drug survival differed by SpA type (–C), with better drug survival in AS compared to PsA and uSpA. Family history of the same SpA type was not associated with drug survival in either condition. This lack of difference was not meaningfully altered by adjustments, e.g. in AS, where the crude HR of family history of AS predicting drug discontinuation was 1.07 (95% CI 0.90–1.28) and the fully adjusted HR was 1.08 (95% CI 0.89–1.31) (; intermediately adjusted models in Supplementary table S4). This lack of association also held for a family history of any SpA. After adjustments for basic characteristics, SpA-specific clinical factors, socioeconomic factors, and medical history, the HR for drug discontinuation for AS was 1.05 (95% CI 0.90–1.22), for PsA 1.02 (95% CI 0.91–1.16), and for uSpA 1.02 (95% CI 0.88–1.19).
Figure 1. Kaplan–Meier plots for time to drug discontinuation during the first 5 years of tumour necrosis factor inhibitor (TNFi) treatment in (A) ankylosing spondylitis (AS) patients with and without a family history of AS, (B) psoriatic arthritis (PsA) patients with and without a family history of PsA, and (C) undifferentiated spondyloarthritis (uSpA) patients with and without a family history of uSpA. Ninety-five per cent confidence intervals (95% CIs) are displayed as shaded bands. Hazard ratios (HRs) from Cox proportional hazards models, unadjusted and adjusted for age, gender, TNFi compound, country of birth, healthcare region, family history of other inflammatory and non-inflammatory conditions, disease duration, SpA manifestations, baseline disease activity, co-medication, medical history, and socioeconomic factors
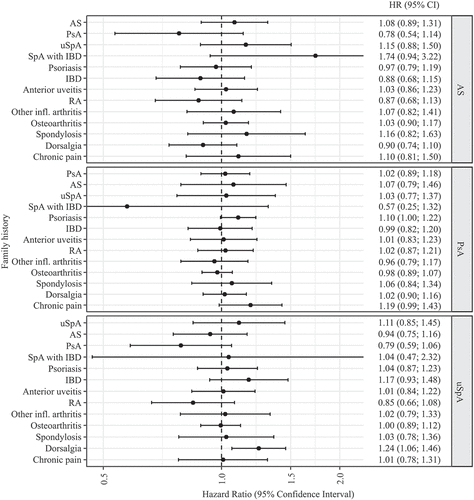
With regard to family history of other inflammatory and non-inflammatory conditions, most showed no association with drug survival (). Borderline significant associations were seen for family history of psoriasis in PsA patients (adjusted HR 1.10, 95% CI 1.00–1.22) and family history of dorsalgia in uSpA (adjusted HR 1.24, 95% CI 1.06–1.46). Family history of SpA with IBD in AS patients and of fibromyalgia/chronic pain in PsA was borderline significant before, but not after, adjustments (Supplementary table S4). Confidence intervals were not corrected for multiple testing. Several false-positive findings may be expected given the number of tests.
Figure 2. Hazard ratios (with 95% confidence intervals) for tumour necrosis factor inhibitor (TNFi) discontinuation in subgroups of spondyloarthritis (SpA) patients, by family history status. Results are from a Cox proportional hazards model adjusted for age, gender, TNFi compound, country of birth, healthcare region, family history of the other diseases in the figure, disease duration, SpA manifestations, baseline disease activity, co-medication, medical history, and socioeconomic factors. AS, ankylosing spondylitis; PsA, psoriatic arthritis; uSpA, undifferentiated spondyloarthritis; IBD, inflammatory bowel disease; RA, rheumatoid arthritis
Treatment response at 3 and 12 months
Patients with a positive family history did not have a different crude treatment response at 3 months compared to patients without a family history (), except for PsA patients with a family history of PsA, who had a slightly lower, although perhaps not clinically relevant, decrease in HAQ score compared to patients without a family history (crude difference 0.06 units, 95% CI 0.01–0.12). Adjustments did not substantially alter the results (; additional models in Supplementary table S5).
Table 2. Differences in treatment response at 3 months of tumour necrosis factor inhibitor (TNFi) treatment for spondylitis (SpA) patients with and without family history
At 12 months, the proportion of patients on treatment and reaching the evaluated response criteria did not significantly differ by family history status (), except for a lower proportion of AS patients with a family history remaining on treatment and reaching PtGA < 20 (Supplementary table S6). However, differences were not significant in the multivariable models.
Figure 3. Proportions of patients with spondyloarthritis (SpA) remaining on tumour necrosis factor inhibitor (TNFi) treatment at 12 months and reaching response criteria, by family history status. Numbers on bars are proportions of patients; numbers outside bars are differences and 95% confidence intervals from multivariable linear regression, adjusted for age, gender, TNFi compound, country of birth, healthcare region, disease duration, SpA manifestations, baseline disease activity, co-medication, medical history, and socioeconomic factors. AS, ankylosing spondylitis; PsA, psoriatic arthritis; uSpA, undifferentiated spondyloarthritis; HAQ, Health Assessment Questionnaire; PtGA, Patient Global Assessment; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; ASDAS-CRP, Ankylosing Spondylitis Disease Activity Score with C-reactive protein; DAS28-CRP, Disease Activity Score in 28 joints with CRP; DAPSA, Disease Activity in Psoriatic Arthritis Score; LDA, low disease activity
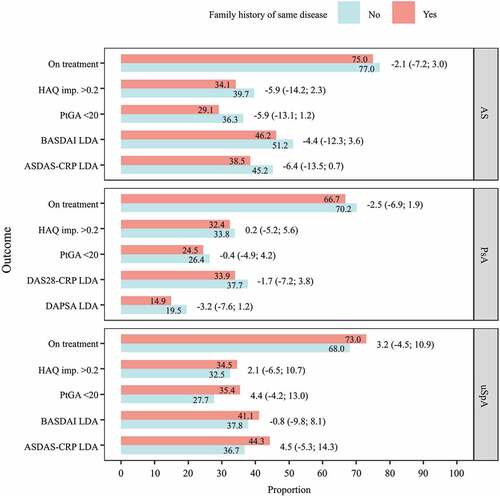
Having a family history of any SpA was not associated with a different treatment response at 12 months, with some exceptions. The proportion of AS patients remaining on treatment and reaching PtGA < 20 and ASDAS-CRP LDA was lower among those with a family history. Only for ASDAS-CRP did the difference remain significant after adjustments for basic characteristics, SpA-specific clinical factors, socioeconomic factors, and medical history. The proportion of patients with PsA remaining on treatment and reaching DAPSA LDA was lower among those with a family history of any SpA, but differences were non-significant after adjustments (Supplementary table S7). Again, no correction of confidence intervals for multiple testing was made.
Sensitivity analyses
Restricting the analysis to patients with active disease at baseline, measured by BASDAI or ASDAS-CRP for AS and uSpA and any swollen or tender joint for PsA, did not result in significant differences in treatment outcomes at 12 months, although the power was reduced (Supplementary table S8). Neither did restricting the drug survival analysis to patients with elevated inflammatory markers at baseline reveal any significant differences (Supplementary table S9). Crude HRs when excluding patients on etanercept from drug survival analysis (Supplementary table S10) were similar to the results from the full cohort. When stratifying on calendar period of treatment start, drug survival was not significantly different between calendar periods (Supplementary table S11).
Missing data
Missingness varied from zero for certain covariates retrieved from national registers, including medical history and disposable income, to substantial for clinical scales in the SRQ (Supplementary table S12). As an example, 20–27% of patients were missing a CRP measure at treatment start, and 65% of AS patients did not have a value for BASDAI at this time. After multiple imputation, distributions of imputed variables were similar to those in the original data set, and conclusions of analyses at 3 and 12 months were equivalent when using imputed data compared to a complete-case analysis, although the power was improved.
Discussion
In this nationwide study with prospectively collected data, a family history of SpA was generally not associated with a different clinical presentation at the start of first TNFi treatment. Nor did a family history of SpA predict drug survival or response to TNFi treatment in the SpA diseases under study.
To our knowledge, this is the first study to address the association between family history and clinical presentation at the start of TNFi treatment in patients with SpA. Our findings imply that at the time of starting TNFi, a family history did not affect the clinicians’ treatment decisions. We did observe that patients with a family history reported an earlier age at onset and had, on average, longer disease duration at treatment start. Younger age at onset is also found in other studies comparing the clinical characteristics of familial and sporadic disease (Citation28–30). One possible explanation for this is that individuals with familial SpA are more observant of early symptoms. A larger proportion of females in familial PsA has also been found in other populations (Citation43), but the reasons for this are unclear. This study was not designed to answer the question of whether a family history of SpA is associated with disease severity overall, as it only included patients on TNFi treatment.
Few previous studies have evaluated family history as a predictor for TNFi treatment response in SpA. Yahya et al (Citation16) found no association between family history and 6 month BASDAI LDA or remission in British patients with axial SpA. Similarly, Alazmi et al (Citation36) found no association between family history of SpA and 6 month BASDAI50 response in axial SpA patients in Canada. In RA, a related but less familial disease, family history was not associated with TNFi treatment outcome among Swedish patients (Citation44). These findings are in line with ours, showing a lack of association of family history with both drug survival and treatment response. The few results in our study that reached significance must be seen in light of the number of tests performed. For drug survival, with 14 types of family history in three different diseases, a Bonferroni correction would change the significance level from 0.05 to 0.001. The significant associations shown in for family history of psoriasis in PsA (p = 0.061) and family history of dorsalgia in uSpA (p = 0.0064) may thus very well be chance findings. The same goes for differences in HAQ at 3 months in PsA (p = 0.013 in the fully adjusted model) and in ASDAS-CRP at 12 months between AS patients with and without a family history of any SpA (p = 0.017).
Although a chance finding cannot be ruled out, the association between family history of dorsalgia with worse drug survival in uSpA may deserve further attention. A family history of non-inflammatory back pain could indicate that the patient also suffers from non-inflammatory pain, and thus will not respond to TNFi treatment. Even if disease activity is high according to validated outcome measures such as BASDAI, patients with the highest BASDAI at baseline, irrespective of CRP level, have been shown to have worse treatment responses, implying that high BASDAI can be triggered by causes other than inflammatory SpA disease (Citation45). If the type of back pain in the family is unknown to the patient, or mistaken as SpA related, there is a risk that this family history will contribute to an incorrect SpA diagnosis.
One major strength in this study is that identification of disease in relatives was based on national registers, and thus independent of registration on treatment and disease characteristics/activity of index patients in SRQ. This minimizes recall bias or misclassification from patients’ lack of awareness of their relatives’ medical history. We investigated the impact of family history of several SpA diagnoses separately and combined, without finding strong associations with drug survival and treatment response for any of them.
The study also has limitations. As diagnoses were based on ICD codes in the NPR and on clinical diagnoses set by rheumatologists in SRQ, we could not classify patients according to the ASAS classification criteria as axial or peripheral SpA (Citation25, Citation26). We also lacked information on, for example, human leucocyte antigen (HLA)-B27 status or specific disease phenotypes. The accuracy of diagnoses in relatives is dependent on the validity of these diagnoses in the NPR, which is high for several of the studied diseases (Citation40, Citation41, Citation46, Citation47), but validation studies have not been performed for all diagnoses included in this study. We probably missed diagnoses mainly set in primary care, such as psoriasis and non-inflammatory diseases. Although we included filled prescriptions of anti-psoriatic drugs in the definition of psoriasis, the proportion of psoriasis in PsA index patients remained low. However, this measurement error should be independent of treatment outcomes, and should not generate differential misclassification. Another limitation is the high proportion of missing data for certain outcome variables and covariates, which we tried to overcome with multiple imputation. For drug survival, there were no missing data in the crude analysis or when adjusting for basic characteristics. In the two following models, which included SpA-specific clinical factors where there was a substantial amount of missing data, using multiply imputed data did not lead to major changes, which is reassuring. At 3 and 12 months, where there were missing data also in outcome variables, results from the multiple imputation were similar to complete-case analysis.
Conclusion
At the start of their first TNFi treatment, patients with SpA did not differ in clinical presentation depending on whether they had a family history of SpA or not, which implies that family history did not affect the treatment decision of the clinicians. In addition, family history was not predictive of drug survival and treatment response, which, although unfortunate in the sense that it does not add to clinical prediction, should be comforting to the substantial group of patients with SpA who have a family history of these conditions.
Supporting Information
Additional supporting information may be found in the online version of this article.
Supplementary table S1. Disease definitions in first-degree relatives used to assess family history of each disease in index patients.
Supplementary table S2. Sources and definitions of covariates for Swedish SpA patients starting a first TNFi.
Supplementary table S3. Additional patient characteristics at start of TNFi treatment.
Supplementary table S4. Hazard ratios for time to drug discontinuation by family history of disease, with all adjustments.
Supplementary table S5. Differences in treatment response at three months of TNFi treatment for SpA patients with and without family history, with three levels of adjustments.
Supplementary table S6. Differences in treatment response at 12 months of TNFi treatment for SpA patients with and without family history, with three levels of adjustments.
Supplementary table S7. Differences in treatment response at 12 months of TNFi treatment for SpA patients with and without family history of any SpA.
Supplementary table S8. Differences in treatment response at 12 months of TNFi treatment only including patients with active disease at treatment start.
Supplementary table S9. Hazard ratios for time to drug discontinuation by family history of disease in SpA patients with elevated inflammatory markers at TNFi treatment start .
Supplementary table S10. Hazard ratios for time to drug discontinuation by family history of disease excluding patients on etanercept.
Supplementary table S11. Hazard ratios for time to drug discontinuation in SpA patients with and without family history of same diagnosis, by calendar period of treatment start.
Supplementary table S12. Proportion of missing data before multiple imputation by diagnosis and family history.
Please note that the editors are not responsible for the content or functionality of any supplementary material supplied by the authors. Any queries should be directed to the corresponding author.
Supplemental Material
Download PDF (327.3 KB)Acknowledgement
This work was supported by the Swedish Research Council [DNR 2016-01355].
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ash Z, Gaujoux-Viala C, Gossec L, Hensor EMA, FitzGerald O, Winthrop K, et al. A systematic literature review of drug therapies for the treatment of psoriatic arthritis: current evidence and meta-analysis informing the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis 2012;71:319–26.
- van der Heijde D, Breban M, Halter D, DiVittorio G, Bratt J, Cantini F, et al. Maintenance of improvement in spinal mobility, physical function and quality of life in patients with ankylosing spondylitis after 5 years in a clinical trial of adalimumab. Rheumatology (Oxford) 2014;54:1210–19.
- Ornbjerg LM, Brahe CH, Askling J, Ciurea A, Mann H, Onen F, et al. Treatment response and drug retention rates in 24 195 biologic-naive patients with axial spondyloarthritis initiating TNFi treatment: routine care data from 12 registries in the EuroSpA collaboration. Ann Rheum Dis 2019;78:1536–44.
- Brahe CH, Ornbjerg LM, Jacobsson L, Nissen MJ, Kristianslund EK, Mann H, et al. Retention and response rates in 14 261 PsA patients starting TNF inhibitor treatment-results from 12 countries in EuroSpA. Rheumatology (Oxford) 2020;59:1640–50.
- Glintborg B, Ostergaard M, Dreyer L, Krogh NS, Tarp U, Hansen MS, et al. Treatment response, drug survival, and predictors thereof in 764 patients with psoriatic arthritis treated with anti-tumor necrosis factor alpha therapy: results from the nationwide Danish DANBIO registry. Arthritis Rheum 2011;63:382–90.
- Kristensen LE, Gulfe A, Saxne T, Geborek P. Efficacy and tolerability of anti-tumour necrosis factor therapy in psoriatic arthritis patients: results from the South Swedish Arthritis Treatment Group register. Ann Rheum Dis 2008;67:364–9.
- Vieira-Sousa E, Eusébio M, Ávila-Ribeiro P, Khmelinskii N, Cruz-Machado R, Rocha TM, et al. Real-world long-term effectiveness of tumor necrosis factor inhibitors in psoriatic arthritis patients from the rheumatic diseases Portuguese register. J Rheumatol 2020;47:690–700.
- Arends S, Brouwer E, van der Veer E, Groen H, Leijsma MK, Houtman PM, et al. Baseline predictors of response and discontinuation of tumor necrosis factor-alpha blocking therapy in ankylosing spondylitis: a prospective longitudinal observational cohort study. Arthritis Res Ther 2011;13:R94.
- Baraliakos X, Szumski A, Koenig AS, Jones H. The role of C-reactive protein as a predictor of treatment response in patients with ankylosing spondylitis. Semin Arthritis Rheum 2019;48:997–1004.
- Davis JC Jr., Van der Heijde DM, Dougados M, Braun J, Cush JJ, Clegg DO, et al. Baseline factors that influence ASAS 20 response in patients with ankylosing spondylitis treated with etanercept. J Rheumatol 2005;32:1751–4.
- Glintborg B, Ostergaard M, Krogh NS, Dreyer L, Kristensen HL, Hetland ML. Predictors of treatment response and drug continuation in 842 patients with ankylosing spondylitis treated with anti-tumour necrosis factor: results from 8 years’ surveillance in the Danish nationwide DANBIO registry. Ann Rheum Dis 2010;69:2002–8.
- Kristensen LE, Karlsson JA, Englund M, Petersson IF, Saxne T, Geborek P. Presence of peripheral arthritis and male sex predicting continuation of anti-tumor necrosis factor therapy in ankylosing spondylitis: an observational prospective cohort study from the South Swedish Arthritis Treatment Group Register. Arthritis Care Res (Hoboken) 2010;62:1362–9.
- Rudwaleit M, Claudepierre P, Wordsworth P, Cortina EL, Sieper J, Kron M, et al. Effectiveness, safety, and predictors of good clinical response in 1250 patients treated with adalimumab for active ankylosing spondylitis. J Rheumatol 2009;36:801–8.
- Glintborg B, Højgaard P, Lund Hetland M, Steen Krogh N, Kollerup G, Jensen J, et al. Impact of tobacco smoking on response to tumour necrosis factor-alpha inhibitor treatment in patients with ankylosing spondylitis: results from the Danish nationwide DANBIO registry. Rheumatology (Oxford) 2015;55:659–68.
- Micheroli R, Hebeisen M, Wildi LM, Exer P, Tamborrini G, Bernhard J, et al. Impact of obesity on the response to tumor necrosis factor inhibitors in axial spondyloarthritis. Arthritis Res Ther 2017;19:164.
- Yahya F, Gaffney K, Hamilton L, Lonsdale E, Leeder J, Brooksby A, et al. Tumour necrosis factor inhibitor survival and predictors of response in axial spondyloarthritis-findings from a United Kingdom cohort. Rheumatology (Oxford) 2018;57:619–24.
- Morin M, Hellgren K, Frisell T. Familial aggregation and heritability of ankylosing spondylitis - a Swedish nested case-control study. Rheumatology (Oxford) 2020;59:1695–702.
- Brown MA, Laval SH, Brophy S, Calin A. Recurrence risk modelling of the genetic susceptibility to ankylosing spondylitis. Ann Rheum Dis 2000;59:883–6.
- Geirsson AJ, Kristjansson K, Gudbjornsson B. A strong familiality of ankylosing spondylitis through several generations. Ann Rheum Dis 2010;69:1346–8.
- Thjodleifsson B, Geirsson AJ, Bjornsson S, Bjarnason I. A common genetic background for inflammatory bowel disease and ankylosing spondylitis: a genealogic study in Iceland. Arthritis Rheum 2007;56:2633–9.
- Chandran V, Schentag CT, Brockbank JE, Pellett FJ, Shanmugarajah S, Toloza SMA, et al. Familial aggregation of psoriatic arthritis. Ann Rheum Dis 2009;68:664–7.
- Karason A, Love TJ, Gudbjornsson B. A strong heritability of psoriatic arthritis over four generations–the Reykjavik Psoriatic Arthritis Study. Rheumatology (Oxford) 2009;48:1424–8.
- Moll JM, Wright V. Familial occurrence of psoriatic arthritis. Ann Rheum Dis 1973;32:181–99.
- Myers A, Kay LJ, Lynch SA, Walker DJ. Recurrence risk for psoriasis and psoriatic arthritis within sibships. Rheumatology (Oxford) 2005;44:773–6.
- Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of SpondyloArthritis international Society classiication criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83.
- Rudwaleit M, van der Heijde D, Landewé R, Akkoc N, Brandt J, Chou CT, et al. The Assessment of SpondyloArthritis international Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis 2011;70:25–31.
- Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–73.
- Almodovar R, Font P, Zarco-Montejo P, Collantes E, Mulero J, Gratacos J, et al. Phenotypic differences between familial versus sporadic ankylosing spondylitis: a cross-sectional Spanish registry of spondyloarthropathies (REGISPONSER). Clin Exp Rheumatol 2011;29:822–7.
- Almodovar R, Navarro-Compan V, Fernandez-Carballido C, Hernandez A, De Miguel E, Zarco P. Differences between familial and sporadic early spondyloarthritis: results from the ESPERANZA cohort. Clin Exp Rheumatol 2016;34:575–80.
- Rahman P, Schentag CT, Beaton M, Gladman DD. Comparison of clinical and immunogenetic features in familial versus sporadic psoriatic arthritis. Clin Exp Rheumatol 2000;18:7–12.
- Solmaz D, Bakirci S, Kimyon G, Gunal EK, Dogru A, Bayindir O, et al. Impact of having family history of psoriasis or psoriatic arthritis on psoriatic disease. Arthritis Care Res (Hoboken) 2020;72:63–8.
- Joshi R, Reveille JD, Brown MA, Weisman MH, Ward MM, Gensler LS, et al. Is there a higher genetic load of susceptibility loci in familial ankylosing spondylitis? Arthritis Care Res (Hoboken) 2012;64:780–4.
- Paardt M, Dijkmans B, Giltay E, van der Horst-bruinsma I. Dutch patients with familial and sporadic ankylosing spondylitis do not differ in disease phenotype. J Rheumatol 2002;29:2583–4.
- Calin A, Gail Kennedy L, Edmunds L, Will R. Familial versus sporadic ankylosing spondylitis. two different diseases? Arthritis Rheum 1993;36:676–81.
- Kim HW, Choe HR, Lee SB, Chang WI, Chae HJ, Moon JY, et al. Phenotype difference between familial and sporadic ankylosing spondylitis in Korean patients. J Korean Med Sci 2014;29:782–7.
- Alazmi M, Sari I, Krishnan B, Inman RD, Haroon N. Profiling response to tumor necrosis factor inhibitor treatment in axial spondyloarthritis. Arthritis Care Res (Hoboken) 2018;70:1393–9.
- Eriksson JK, Askling J, Arkema EV. The Swedish rheumatology quality register: optimisation of rheumatic disease assessments using register-enriched data. Clin Exp Rheumatol 2014;32(Suppl 85):S–147–9.
- Wadstrom H, Eriksson JK, Neovius M, Askling J. How good is the coverage and how accurate are exposure data in the Swedish Biologics Register (ARTIS)? Scand J Rheumatol 2015;44:22–8.
- Wettermark B, Hammar N, Fored CM, Leimanis A, Otterblad Olausson P, Bergman U, et al. The new Swedish prescribed drug register–opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf 2007;16:726–35.
- Lindstrom U, Exarchou S, Sigurdardottir V, Sundstrom B, Askling J, Eriksson JK, et al. Validity of ankylosing spondylitis and undifferentiated spondyloarthritis diagnoses in the Swedish National Patient Register. Scand J Rheumatol 2015;44:369–76.
- Löfvendahl S, Theander E, Svensson Å, Carlsson KS, Englund M, Petersson IF. Validity of diagnostic codes and prevalence of physician-diagnosed psoriasis and psoriatic arthritis in southern Sweden–a population-based register study. PLoS One 2014;9:e98024.
- Lie E, Lindström U, Zverkova-Sandström T, Olsen IC, Forsblad D, Elia H, et al. Tumour necrosis factor inhibitor treatment and occurrence of anterior uveitis in ankylosing spondylitis: results from the Swedish biologics register. Ann Rheum Dis 2017;76:1515–21.
- Eder L, Thavaneswaran A, Chandran V, Gladman DD. Gender difference in disease expression, radiographic damage and disability among patients with psoriatic arthritis. Ann Rheum Dis 2013;72:578–82.
- Frisell T, Saevarsdottir S, Askling J. Does a family history of RA influence the clinical presentation and treatment response in RA? Ann Rheum Dis 2016;75:1120–5.
- Krabbe S, Glintborg B, Østergaard M, Hetland ML. Extremely poor patient-reported outcomes are associated with lack of clinical response and decreased retention rate of tumour necrosis factor inhibitor treatment in patients with axial spondyloarthritis. Scand J Rheumatol 2019;48:128–32.
- Jakobsson GL, Sternegård E, Olén O, Myrelid P, Ljung R, Strid H, et al. Validating inflammatory bowel disease (IBD) in the Swedish National Patient Register and the Swedish Quality Register for IBD (SWIBREG). Scand J Gastroenterol 2017;52:216–21.
- Waldenlind K, Eriksson JK, Grewin B, Askling J. Validation of the rheumatoid arthritis diagnosis in the Swedish National Patient Register: a cohort study from Stockholm County. BMC Musculoskelet Disord 2014;15:432.
