Abstract
Objective
To evaluate radiological damage and to explore characteristics associated with radiological progression in rheumatoid arthritis (RA) treated to the target of remission in a real-world setting.
Methods
Baseline to 6 year follow-up data were used from an observational early RA cohort. Radiographs of hands and feet at baseline, 6 months, and 1, 3, and 6 years were scored using the modified Sharp/van der Heijde score (SHS). The threshold for rapid radiological progression (RRP) after 6 months was based on the calculated smallest detectable change of 3.95. Negative binomial generalized linear mixed model and logistic regression analyses were performed to examine which variables were associated with RRP and 6 year radiological progression.
Results
Most radiological damage occurred in the first year of treatment [median 2.0 interquartile range (IQR) 1.0–4.0 SHS points] compared to the subsequent 5 years of follow-up (median 3.0 IQR 1.0–5.0 SHS points). While low disease activity was achieved within 6 months on average, 18.8% of the patients developed RRP. Anti-cyclic citrullinated peptide (anti-CCP) positivity [incidence rate ratio (IRR) 1.42, p = 0.03], baseline erosive disease (IRR 1.60, p = 0.02), and RRP (IRR 3.28, p < 0.001) were associated with 6 year radiological progression. Erosive disease was the strongest predictor of RRP (odds ratio 8.8, p < 0.001).
Conclusion
Long-term radiological outcome is limited in most real-world RA patients treated to the target of remission, but RRP still occurs. Anti-CCP positivity, baseline erosive disease, and RRP remain associated with long-term radiological outcome.
Rheumatoid arthritis (RA) is characterized by chronic polyarticular joint inflammation. When left untreated, the disease may lead to severe permanent disability (Citation1). Functional capacity in patients with RA is mainly determined by inflammatory disease activity early in the disease and by joint destruction later in the disease (Citation2). Therefore, treatment goals for patients with RA are to both quickly and adequately suppress disease activity and thereby to prevent long-term joint damage. Treat-to-target (T2T) treatment strategies, using (combinations of) conventional synthetic disease-modifying anti-rheumatic drugs and biological disease-modifying anti-rheumatic drugs (bDMARDs) in combination with close clinical monitoring of the course of the disease activity, have been shown to result in improved suppression of inflammation and reduced progression of radiological damage (Citation3–7).
However, despite intensive treatment, progression of radiological damage may still occur in some patients (Citation8, Citation9). Even in well-managed patients in randomized controlled trials, rapid radiological progression (RRP) in the first year of treatment is still associated with unfavourable radiological outcome and future functional disability (Citation10). Although early RRP occurs in only a minority of patients, it is probably important to identify these patients and treat them more intensively to reduce accumulation of damage. Over the past few decades, many predictive factors for radiological damage have been identified, such as smoking, rheumatoid factor (RF) seropositivity, the presence of anti-citrullinated peptide (anti-CCP) antibodies, the number of swollen joints, elevated inflammatory markers [erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels], and higher numbers of erosions at baseline (Citation11–17). However, more recent studies show that these identified risk factors lose their impact when RA is diagnosed and treated early and consistently according to T2T principles (Citation18, Citation19). Consequently, knowledge is lacking on the risk factors for unfortunate radiological outcome in real-world RA patients in the current era of T2T.
Observational studies of unselected real-world patients are most appropriate to identify risk factors for the course of a disease. The Dutch Rheumatoid Arthritis Monitoring (DREAM) remission induction study is a multicentre cohort study that aims to evaluate the effectiveness of modern T2T treatment strategies in RA patients in the real world (Citation6, Citation20). This population-based inception cohort provides useful information about the course of T2T in early RA. In this study, we aim to examine the development and predictors of radiological damage over 6 years of T2T treatment.
Method
Patients
This study used data from the observational DREAM remission induction cohort, in which all consecutive newly diagnosed RA patients from two participating hospitals in the Netherlands (Medisch Spectrum Twente Hospital Enschede and Isala Hospital Zwolle) between January 2006 and March 2012 were invited to participate. Inclusion criteria were age > 18 years, clinical diagnosis of RA, symptom duration (defined as the time from the first reported symptoms to the diagnosis of RA) < 1 year, and a Disease Activity Score based on 28-joint count (DAS28; calculated using the ESR (Citation21)] > 2.6. Ethical approval for this observational study was waived by the Medical Ethics Committees of both hospitals (Dutch trial register: NTR578). Informed consent was obtained from all patients. Patients were treated according to a protocolized T2T strategy aiming at remission, defined as DAS28 < 2.6. The treatment strategy is published elsewhere (Citation20).
Assessments
Patients were assessed for various clinical and patient-reported outcome measures at the time of study entry and at every follow-up visit at weeks 8, 12, 20, and 24, and every 3 months thereafter. Radiographs of the hands and feet were obtained at baseline, after 6 and 12 months, and annually thereafter. Radiographs were consensus scored in chronological order by two observers together (GAV and LMMS) according to the original methodology developed and published as the modified Sharp/van der Heijde method (Citation22). The threshold for RRP after 6 months was based on the calculated smallest detectable change (SDC) of 5.38 using the standard deviation (sd) of the change scores between baseline and 6 months described by Bruynesteyn et al (Citation23). Because the sd (and therefore the SDC) was highly influenced by one outlier with a progression of 39 units on the Sharp/van der Heijde score (SHS) after 6 months, we also calculated the SDC after excluding this outlier, which resulted in an SDC of 3.95. Erosive disease was defined – using the current European League Against Rheumatism (EULAR) definition accompanying the 2010 RA criteria – as at least three erosions in separate joints (Citation24). Data collection, including DAS28 assessment, was performed by well-trained rheumatology nurses and was facilitated by a web-based monitoring application which was accessible for the healthcare providers as well as for the patients.
Analysis
Mean disease activity scores according to the DAS28 and mean SHS over the first 6 years of follow-up were estimated using a linear mixed model with an unstructured covariance matrix for repeated measures, which accounts for missing values. Changes in median SHS scores over time, both the total score and the erosion score and joint space narrowing (JSN) score separately, were analysed at group level using non-parametric Wilcoxon signed-rank tests. Individual progression scores of radiological damage after 6 years were visualized as cumulative probability plots (Citation25). To examine which patient and clinical characteristics were associated with 6 year radiological progression, negative binomial generalized linear mixed model (GLMM) analyses were performed to take into account missing values and the zero-inflated and positively skewed distribution of the progression data (Citation26). As dependent variables, all four change scores at the different measurement time-points (6 months, and 1, 3, and 5 years) were used. The models were fitted with a first order autoregressive covariance structure for the repeated measurements as this structure provided the best fit to the data according to the Akaike and Bayesian information criteria and likelihood ratio tests. Variables marginally associated with progression (p < 0.10) in univariate analyses were entered simultaneously into the multivariable GLMM regression analysis model. In case of multicollinearity (r > 0.5), the variable with the strongest univariate association with progression was entered in the multivariate model. Results were expressed as incidence rate ratios (IRRs) with 95% confidence intervals (CIs). Next, logistic regression analyses were performed to explore which patient characteristics were related to RRP. Continuous predictors were evaluated for the suitability of the linearity assumption by plotting each predictor variable against the logit of the outcome. Variables found to be related marginally (p < 0.10) in the univariate analysis were entered into a multivariable logistic regression analysis as independent variables. Results were expressed as odds ratios (ORs) with 95% CIs. The final logistic model was additionally tested for goodness of fit using the Hosmer and Lemeshow test, where a non-significant result indicates support for the model. The explained variance of the model was examined using Nagelkerke’s pseudo-R2. Analyses were performed with both RRP of at least 4 units (based on the SDC without the outlier) and RRP of at least 6 units (based on SDC with the outlier) as the dependent variable. All statistical calculations were performed using version 25 of the SPSS statistical package for Windows.
Results
Patient characteristics
For this study, we were able to use the data of 219 patients from whom radiographs of hands and feet were available at baseline and who had at least one additional pair of radiographs during 6 years of follow-up. The baseline characteristics of the study population are shown in , representing a typical early RA population with active disease. Patients without available DAS28 or SHS data after a certain time-point were considered as lost to follow-up (77/219, 35.2%). Specified reasons for loss to follow-up after 6 years were unknown (43/77), drug-free remission (16/77), death (11/77), patient’s own decision (4/77), other diagnosis (2/77), and moving out of the region (1/77). The patients lost to follow-up were more often men (48.% vs 30.3%, p = 0.009), had a higher age at the moment of diagnosis (mean ± sd 63.0 ± 15.7 vs 54.3 ± 12.8, p < 0.001), and were less often anti-CCP positive (46.7% vs 65.9%, p = 0.006) or RF positive (51.9% vs 66.2%, p = 0.039) than the patients who continued in the cohort.
Table 1. Baseline characteristics of the patients (n = 219)
Disease activity
Estimated marginal mean disease activity, according to the DAS28, decreased from 4.91 (95% CI 4.76–5.06) at baseline to 3.29 (95% CI 3.16–3.24) at 3 months (p < 0.001). After 6 months, the mean DAS28 was 2.81 (95% CI 2.67–2.95) and on average reached remission (DAS28 = 2.48, 95% CI 2.33–2.63) at 12 months. Thereafter, mean disease activity remained stable below the threshold of remission throughout the 6 year follow-up [data not shown; further details regarding disease activity and other disease and patient-reported outcomes have been reported elsewhere (Citation27)].
Radiological outcome
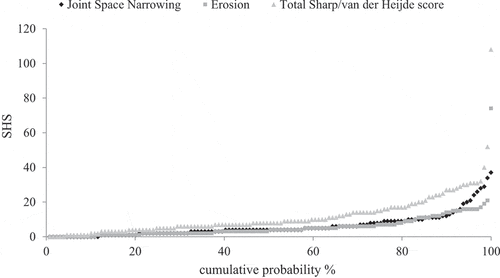
displays the observed median total SHS scores as well as the observed median erosion and JSN scores at the subsequent time-points. Most radiological damage occurred in the first year of treatment [median ΔSHS from baseline to year 1 (IQR) 2.0 (1.0–4.0)] compared to the subsequent 5 years of follow-up [median ΔSHS from year 1 to year 6 (IQR) 3.0 (1.0–5.0] (Δscores not shown in ). There were no substantial differences in median progression rates between erosions and JSN. Estimated marginal mean SHS increased from 4.69 (95% CI 3.78–5.84) at baseline to 14.99 (95% CI 12.10–18.58) at 6 years (main effect of time: p < 0.001). Of the 192 patients with available SHS data after 6 months, 36 (18.8%) developed RRP. The number of patients with erosive disease increased from 35 (16%) at baseline to 126 at 6 years (58%). These erosive patients had a mean age at baseline of 59.1 years; 63.5% were female; and 69.4% were anti-CCP positive and 67.5% RF positive. Their mean DAS28 at baseline was 4.93, and 38 (30.9%) of them reached DAS28 remission at 3 months and 28 (22.2%) reached DAS28 remission at 6 months. represents the cumulative probability plots of the individual SHS progression scores after 6 years’ follow-up. This figure confirms the absent or modest radiological damage in most patients and the nearly equal increase in erosive damage and JSN over time. One patient shows a massive increase in radiological damage of 108 SHS units. This outlier concerned a 66-year-old woman with anti-CCP-positive RA and moderate disease activity (8 swollen joints) and an erosion score of 8 (total SHS = 13) at baseline. This patient was in DAS28 remission during most of the follow-up time (16 of the 22 DAS28 scores < 2.6). She had been treated with methotrexate only, in accordance with the T2T treatment protocol.
Table 2. Radiological outcomes over 6 years of follow-up
Variables associated with 6 year radiological progression
Anti-CCP positivity, higher ESR, CRP, total SHS, SHS erosion score and SHS JSN score, and baseline erosive status and RRP were univariately significantly or marginally associated with radiological progression over 6 years (). Because RRP is also part of the 6 year radiological progression, we also determined the relationship between RRP and the increase in SHS between 6 months and 6 years, which remained significant (IRR = 3.93, 95% CI 2.83–5.46, p < 0.001). Because of multicollinearity between the different components of baseline damage, only baseline erosive disease was included in the multivariable analysis. Anti-CCP positivity, baseline erosive disease, and RRP remained independently associated with radiological progression over 6 years. Of these, RRP turned out to be the strongest predictor, with an IRR of 3.28 (). This means that patients with RRP had a more than three-fold increase in radiological progression over 6 years compared with those without RRP.
Table 3. Univariate negative binomial generalized linear mixed model (GLMM) for radiological progression (change from baseline) at 6 month, 1 year, 3 year, and 6 year follow-up
Table 4. Multivariable negative binomial generalized linear mixed model (GLMM) for radiological progression (change from baseline) at 6 month, 1 year, 3 year, and 6 year follow-up
Variables associated with RRP
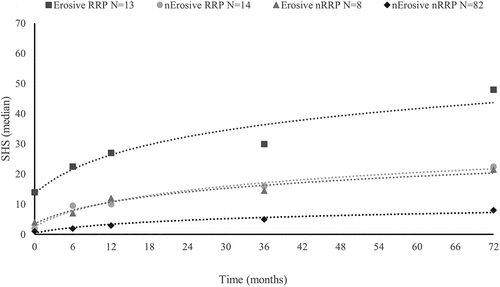
Except for the SHS scores at baseline, none of the independent variables violated the assumption of linearity. Therefore, we excluded total SHS, erosion SHS, and JSN SHS at baseline from the logistic regression analyses. Higher age, higher swollen joint count, higher CRP, and erosive disease (p < 0.001) at baseline, and not reaching DAS28 remission after 3 months (p = 0.08) were significantly or marginally univariately associated with developing RRP as defined as an increase of at least 4 units on the SHS (). These variables were simultaneously entered into multivariable logistic analysis. The resulting multivariable model showed an adequate fit to the data [Hosmer and Lemeshow test: χ2(8) = 3.38, p = 0.91] (). The model as a whole explained 28.7% of the variance in developing RRP and correctly classified 85.0% of cases. As shown in , only higher baseline CRP and baseline erosive disease remained independently associated. The variable most strongly associated with developing early clinically relevant progression was baseline erosive disease, with an OR of 8.77. This indicated that the odds for patients with at least three erosions in different places at baseline of developing RRP was over eight times greater than the odds for patients with fewer than three or no erosions at baseline. In the multivariable model using an increase of at least 6 units on the SHS as definition of RRP, higher age and not reaching DAS28 remission after 3 months were no longer significantly associated with RRP, and CRP was only marginally associated in univariate analysis. In the resulting multivariable model, only baseline erosive disease remained an independently associated variable, and together the predictors explained 17% of the variance in developing RRP. In , radiological progression between 6 months and 6 years is plotted separately for erosive and non-erosive patients with and without RRP. This figure, representing only patients with available data at all three time-points (baseline, 6 months, and 6 years), illustrates three groups of patients with different rates of progression. Patients who were erosive at baseline and experienced RRP developed the most radiological damage between 6 months and 6 years. Patients with no erosions at baseline and no RRP had the most favourable course with respect to 6 year radiological progression.
Table 5. Univariate logistic regression for rapid radiological progression (RRP)
Table 6. Multivariable logistic regression for rapid radiological progression (RRP)
Figure 2. Course of median Sharp/van der Heijde score (SHS) over time plotted separately for each group. Lines are logarithmic fits to the data. The number of patients represents only the patients with available radiological data at all three time-points (baseline, 6 months, and 6 years). Number of total patients = 117 (the difference from 129 available SHS at 6 years in is explained by the exclusion of patients with available SHS at 6 years, but missing SHS at 6 months). Number of patients with rapid radiological progression (RRP) = 27 (the difference from 36 in is explained by exclusion of patients with available SHS at 6 months, but missing SHS at 6 years). RRP is defined as increase in SHS ≥ 4 after 6 months; nErosive, non-erosive at baseline; nRRP, non-rapid radiological progression.
Discussion
Our study shows the course of radiological damage in a real-world RA population which was treated to the target of remission. Despite quickly achieving low disease activity on average, a substantial proportion of patients developed RRP and erosive disease. Elevated CRP and baseline erosive disease at baseline were independently associated with RRP. Anti-CCP positivity, baseline erosive disease, and RRP were significant independent predictors of radiological progression over the course of 6 years.
In comparison with historical data from before the use of modern T2T treatment strategies, the overall radiological progression at group level was limited. Our data extend previous findings of reduction in radiological damage in RA over recent decades (Citation28, Citation29). Carpenter et al evaluated data from 10 longitudinal observational studies, which recruited patients between 1965 and 2000 and followed them up for a duration of 5–10 years. They found that baseline radiological scores did not differ significantly between cohorts recruiting patients pre- and post-1990; however, the annual rate of progression was significantly reduced in the post-1990 cohort (Citation29). Our data show a further improvement in the treatment of RA patients, probably caused by the introduction of bDMARDs and the implementation of T2T. Another study, comparing radiological outcomes between two inception cohorts in the UK, found that patients with early RA with onset between 2002 and 2013 had significantly lower baseline and radiological progression compared to those with onset between 1986 and 2001. This reduction was also clinically meaningful; whereas 74% of patients in the earlier cohort progressed on average more than the minimal clinically important difference (MCID) of ≥ 5 SHS units per year over the 5 year period of follow-up considered, just 27% of patients in the later cohort did (Citation30).
Our study confirms anti-CCP positivity as a significant and independent predictor of long-term radiological damage. This in line with data from a Swedish real-world RA cohort (2006–2009), which showed that anti-CCP positivity was associated with more radiological damage during 3 years of follow-up (Citation16). Our study shows that about one in five patients developed RRP (18.8%) in the first 6 months despite quickly achieving low disease activity at the group level. These patients also developed more radiological damage in subsequent years than the patients without this early joint damage. This finding is in line with previously found associations between early radiological progression and long-term damage (Citation10, Citation31). Remarkably, this concerns both data from the time before the implementation of T2T treatment strategies and data from a recent clinical trial with remission-steered treatment. Other prospective cohort studies have also demonstrated that even in clinical remission, some patients still experience radiological progression (Citation9).
Over the past decade, correlating and predictive factors for baseline erosions and RRP have been identified in data from observational studies and randomized clinical trials. Rydell et al found that a history of smoking, RF and/or anti-CCP positivity, early erosions, and high initial disease activity increased the risk of RRP (defined as an increase of 5 or more SHS points per year) (Citation17). Patients with baseline erosions had higher levels of interleukin-6 and were more often immunoglobulin A-RF positive (Citation32). In addition, the prevalence of baseline erosive disease appeared to be particularly increased in patients with positive autoantibodies combined with smoking habits (Citation33). Composite prediction models, arranged in matrices, were constructed to identify patients at risk for RRP (Citation13, Citation14, Citation34). Predictive factors used in these matrices are swollen joint count, autoantibody status, inflammatory parameters ESR and CRP, baseline erosions, gender, and smoking. The Leiden Early Arthritis Clinic (a population-based prospective observational cohort) also identified older age, male gender, longer symptom duration at first visit, and the involvement of lower extremities as risk factors (Citation12). More recently, some studies found a predictive value of a multi-biomarker disease activity score on early radiological progression (Citation35, Citation36). Except for baseline erosive disease and CRP, we did not find independent associated factors for RRP. We also showed this in our previous study, which compared different treatment strategies in the real world (Citation19). In this study, only the treatment strategy appeared to be an important predictor for the occurrence of radiological progression after controlling for other potential associations. Also, as previously mentioned by Heimans et al, traditional risk factors disappear in modern well-treated early RA cohorts (Citation18). Heimans et al showed that prediction models for joint damage based on data from previous trials and patient populations, in eras with less strict treatment strategies, may no longer be relevant for current and future patients.
Strengths of the current study are the real-world setting and consecutive inclusion of non-selected patients, which is most appropriate for identifying risk factors for the disease course, making the results generalizable. Strict T2T and tight control treatment was consistently applied and adherence to the protocol was high (Citation37). The proportion of patients lost to follow-up is comparable to that in clinical trials. Finally, the calculated SDC in our cohort data corresponds closely to the most widely used MCID of ≤ 5 SHS points (Citation38).
However, our research is also subject to some limitations. Although a strict T2T strategy was applied, the medication and dosages used in the current study are not quite up to date. For example, the targeted synthetic DMARDs are not yet included in the treatment protocol. Furthermore, our database does not contain all possible predictors of radiological damage, such as smoking status, bone marrow oedema, or biomarkers. In addition, radiological follow-up is not available for all participants. Because of the limited number of patients with RRP, the statistical power of the multivariable logistic regression models for RRP may be a potential limitation for these exploratory models. Also, the method of variable selection (only factors with a univariate association with p < 0.10) in the multivariable analyses can lead to biased estimates and model overfitting. Therefore, the findings from these analyses need to be replicated in larger samples. Nevertheless, our data confirm one already known predictor for RRP, i.e. baseline erosive disease. Finally, in real-world treatment settings, patients and their physician make shared decisions that sometimes deviate from the protocol, and it is well known that non-adherence in the real world remains an issue in a considerable number of patients (Citation39).
The introduction of early diagnosis and treatment, T2T, and tight control treatment strategies, including the introduction of bDMARDs, have been shown to inhibit progression of radiological damage for most patients. This makes it increasingly difficult to identify patients with RRP and unfortunate radiological outcome. However, our study shows that patients with baseline erosive disease are still at risk for developing RRP and that baseline erosive disease, anti-CCP positivity, and RRP predict radiological damage in the long term. Routine monitoring of radiological progression, especially for patients with anti-CCP positivity and erosive disease at baseline, in the first year of treatment, may be useful to identify patients at risk for unfortunate radiological outcome and eligible for intensification of DMARD therapy. The additional value of frequent radiological monitoring, and treatment intensification on indication, in patients at risk for unfortunate radiological damage remains to be demonstrated.
Conclusion
Compared to historical data, the overall radiological damage is limited in real-world RA patients treated to the target of remission. However, RRP still occurs in about one out of five patients and is most strongly predicted by baseline erosive disease. Together with anti-CCP positivity, baseline erosive disease and RRP remain significantly associated with long-term radiological outcome.
Acknowledgements
The authors thank all patients, rheumatologists, and nurses at the Departments of Rheumatology at the participating hospitals. No funding has been received from public, commercial, or non-profit organizations to carry out the work described in this article.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data sets generated and/or analysed during the current study are not publicly available for legal and ethical reasons but are available from the corresponding author on reasonable request.
References
- Govoni M, Caporali R. Predicting and managing the progression of structural damage in rheumatoid arthritis: where do we stand? Clin Exp Rheumatol 2012;30:459–63.
- Welsing PMJ, Van Gestel AM, Swinkels HL, Kiemeney LALM, Van Riel PLCM. The relationship between disease activity, joint destruction, and functional capacity over the course of rheumatoid arthritis. Arthritis Rheum 2001;44:2009–17.
- Korpela M, Laasonen L, Hannonen P, Kautiainen H, Leirisalo-Repo M, Hakala M, et al. Retardation of joint damage in patients with early rheumatoid arthritis by initial aggressive treatment with disease-modifying antirheumatic drugs: five-year experience from the FIN-RACo study. Arthritis Rheum 2004;50:2072–81.
- Grigor C, Capell H, Stirling A, McMahon AD, Lock P, Vallance R, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet 2004;364:263–9.
- Klarenbeek NB, Güler-Yüksel M, Van Der Kooij SM, Han KH, Ronday HK, Kerstens PJSM, et al. The impact of four dynamic, goal-steered treatment strategies on the 5-year outcomes of rheumatoid arthritis patients in the BeSt study. Ann Rheum Dis 2011;70:1039–46.
- Vermeer M, Kuper HH, Moens HJB, Drossaers-Bakker KW, Van Der Bijl AE, Van Riel PLCM, et al. Sustained beneficial effects of a protocolized treat-to-target strategy in very early rheumatoid arthritis: three-year results of the Dutch Rheumatoid Arthritis Monitoring remission induction cohort. Arthritis Care Res (Hoboken) 2013;65:1219–26.
- Schipper LG, Vermeer M, Kuper HH, Hoekstra MO, Haagsma CJ, Den Broeder AA, et al. A tight control treatment strategy aiming for remission in early rheumatoid arthritis is more effective than usual care treatment in daily clinical practice: a study of two cohorts in the Dutch Rheumatoid Arthritis Monitoring registry. Ann Rheum Dis 2012;71:845–50.
- Cohen G, Gossec L, Dougados M, Cantagrel A, Goupille P, Daures JP, et al. Radiological damage in patients with rheumatoid arthritis on sustained remission. Ann Rheum Dis 2007;66:358–63.
- Molenaar ETH, Voskuyl AE, Dinant HJ, Bezemer PD, Boers M, Dijkmans BAC. Progression of radiologic damage in patients with rheumatoid arthritis in clinical remission. Arthritis Rheum 2004;50:36–42.
- Van Den Broek M, Dirven L, De Vries-bouwstra JK, Dehpoor AJ, Goekoop-Ruiterman YPM, Gerards AH, et al. Rapid radiological progression in the first year of early rheumatoid arthritis is predictive of disability and joint damage progression during 8 years of follow-up. Ann Rheum Dis 2012;71:1530–3.
- Akdemir G, Verheul MK, Heimans L, Wevers-De Boer KVC, Goekoop-Ruiterman YPM, Van Oosterhout M, et al. Predictive factors of radiological progression after 2 years of remission-steered treatment in early arthritis patients: a post hoc analysis of the IMPROVED study. RMD Open 2016;2:1–9.
- De Rooy DPC, Van Der Linden MPM, Knevel R, Huizinga TWJ, Van Der Helm-van AHM. Predicting arthritis outcomes – what can be learned from the Leiden Early Arthritis Clinic? Rheumatology 2011;50:93–100.
- Visser K, Goekoop-Ruiterman YPM, De Vries-Bouwstra JK, Ronday HK, Seys PEH, Kerstens PJSM, et al. A matrix risk model for the prediction of rapid radiographic progression in patients with rheumatoid arthritis receiving different dynamic treatment strategies: post hoc analyses from the BeSt study. Ann Rheum Dis 2010;69:1333–7.
- Fautrel B, Granger B, Combe B, Saraux A, Guillemin F, Le Loet X. Matrix to predict rapid radiographic progression of early rheumatoid arthritis patients from the community treated with methotrexate or leflunomide: results from the ESPOIR cohort. Arthritis Res Ther 2012;14:R249.
- Hetland ML, Østergaard M, Stengaard-Pedersen K, Junker P, Ejbjerg B, Jacobsen S, et al. Anti-cyclic citrullinated peptide antibodies, 28-joint Disease Activity Score, and magnetic resonance imaging bone oedema at baseline predict 11 years’ functional and radiographic outcome in early rheumatoid arthritis. Scand J Rheumatol 2019;48:1–8.
- Ziegelasch M, Boman A, Martinsson K, Thyberg I, Jacobs C, Nyhäll-Wåhlin BM, et al. Anti-cyclic citrullinated peptide antibodies are associated with radiographic damage but not disease activity in early rheumatoid arthritis diagnosed in 2006–2011. Scand J Rheumatol 2020;49:434–42.
- Rydell E, Forslind K, Nilsson J-Å, Jacobsson LTH, Turesson C. Smoking, body mass index, disease activity, and the risk of rapid radiographic progression in patients with early rheumatoid arthritis. Arthritis Res Ther 2018;20:20.
- Heimans L, Boer KVCW, Ronday HK, Collée G, De Sonnaville PBJ, Grillet BAM, et al. Can we prevent rapid radiological progression in patients with early rheumatoid arthritis? Clin Rheumatol 2015;34:163–6.
- Steunebrink LMM, Versteeg LGA, Vonkeman HE, Klooster PM, Hoekstra M, Van De Laar MAFJ. Radiographic progression in early rheumatoid arthritis patients following initial combination versus step-up treat-to- target therapy in daily clinical practice: results from the DREAM registry. BMC Rheumatol 2018;2:1.
- Vermeer M, Kuper HH, Hoekstra M, Haagsma CJ, Posthumus MD, Brus HLM, et al. Implementation of a treat-to-target strategy in very early rheumatoid arthritis: results of the Dutch Rheumatoid Arthritis Monitoring remission induction cohort study. Arthritis Rheum 2011;63:2865–72.
- Prevoo MLL, Van’T Hof MA, Kuper HH, Van Leeuwen MA, Van De Putte LBA, Van Riel PLCM. Modified disease activity scores that include twenty-eight-joint counts development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8.
- Van Der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol 2000;27:261–3.
- Bruynesteyn K, Boers M, Kostense P, Van Der Linden S, Van Der Heijde D. Deciding on progression of joint damage in paired films of individual patients: smallest detectable difference or change. Ann Rheum Dis 2005;64:179–82.
- Van Der Heijde D, Van Der Helm-van Mil AHM, Aletaha D, Bingham CO, Burmester GR, Dougados M, et al. EULAR definition of erosive disease in light of the 2010 ACR/EULAR rheumatoid arthritis classification criteria. Ann Rheum Dis 2013;72:479–81.
- Landewé R, Van Der Heijde D. Radiographic progression depicted by probability plots: presenting data with optimal use of individual values. Arthritis Rheum 2004;50:699–706.
- Park GS, Wong WK, Khanna D, Gold RH, Paulus HE. Examining radiographic outcomes over time. Rheumatol Int 2014;34:271–9.
- Versteeg GA, Steunebrink LMM, Vonkeman HE, Ten Klooster PM, Van Der Bijl AE, Van De Laar MAFJ. Long-term disease and patient-reported outcomes of a continuous treat-to-target approach in patients with early rheumatoid arthritis in daily clinical practice. Clin Rheumatol 2018;37:1189–97.
- Sokka T, Kautiainen H, Häkkinen A, Hannonen P. Radiographic progression is getting milder in patients with early rheumatoid arthritis. Results of 3 cohorts over 5 years. J Rheumatol 2004;31:1073–82.
- Carpenter L, Nikiphorou E, Sharpe R, Norton S, Rennie K, Bunn F, et al. Have radiographic progression rates in early rheumatoid arthritis changed? A systematic review and meta-analysis of long-term cohorts. Rheumatology (Oxford) 2016;55:1053–65.
- Carpenter L, Norton S, Nikiphorou E, Jayakumar K, McWilliams DF, Rennie KL, et al. Reductions in radiographic progression in early rheumatoid arthritis over twenty-five years: changing contribution from rheumatoid factor in two multicenter UK inception cohorts. Arthritis Care Res 2017;69:1809–17.
- Tiippana-Kinnunen T, Laasonen L, Kautiainen H, Paimela L, Leirisalo-Repo M. Impact of early radiographic remission on the 15-year radiographic outcome in patients with rheumatoid arthritis. Scand J Rheumatol 2011;40:263–8.
- Fedele AL, Petricca L, Tolusso B, Alivernini S, Canestri S, Di Mario C, et al. Interleukin-6 and IgA-rheumatoid factor are crucial for baseline erosiveness, and anti-citrullinated peptide antibodies for radiographic progression in early rheumatoid arthritis treated according to a treat-to-target strategy. Scand J Rheumatol 2018;47:351–9.
- Krol A, Garred P, Heegaard NHH, Christensen AF, Hetland ML, Stengaard-Pedersen K, et al. Interactions between smoking, increased serum levels of anti-CCP antibodies, rheumatoid factors, and erosive joint disease in patients with early, untreated rheumatoid arthritis. Scand J Rheumatol 2015;44:8–12.
- Vastesaeger N, Xu S, Aletaha D, Clair EWS, Smolen JS. A pilot risk model for the prediction of rapid radiographic progression in rheumatoid arthritis. Rheumatology 2009;48:1114–21.
- Brahe CH, Østergaard M, Johansen JS, Defranoux N, Wang X, Bolce R, et al. Predictive value of a multi-biomarker disease activity score for clinical remission and radiographic progression in patients with early rheumatoid arthritis: a post-hoc study of the OPERA trial. Scand J Rheumatol 2019;48:9–16.
- Hambardzumyan K, Bolce R, Saevarsdottir S, Cruickshank SE, Sasso EH, Chernoff D, et al. Pretreatment multi-biomarker disease activity score and radiographic progression in early RA: results from the SWEFOT trial. Ann Rheum Dis 2015;74:1102–9.
- Vermeer M, Kuper HH, Bernelot Moens HJ, Hoekstra M, Posthumus MD, Van Riel PLCM, et al. Adherence to a treat-to-target strategy in early rheumatoid arthritis: results of the DREAM remission induction cohort. Arthritis Res Ther 2012;14:R254.
- Bruynesteyn K, Van Der Heijde D, Boers M, Saudan A, Peloso P, Paulus H, et al. Determination of the minimal clinically important difference in rheumatoid arthritis joint damage of the Sharp/van der Heijde and Larsen/Scott scoring methods by clinical experts and comparison with the smallest detectable difference. Arthritis Rheum 2002;46:913–20.
- Van Den Bemt BJF, Zwikker HE, Van Den Ende CHM. Medication adherence in patients with rheumatoid arthritis: a critical appraisal of the existing literature. Expert Rev Clin Immunol 2012;8:337–51.