Abstract
Objectives
Inflammatory joint disease (IJD) is associated with an increased risk of developing cardiovascular disease (CVD). Arterial stiffness is both a risk factor and a surrogate marker for CVD. This study aims to compare arterial stiffness across patients with rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis, and, by extension, to explore the relationship between arterial stiffness and the estimated CVD risk by the Systematic COronary Risk Evaluation (SCORE) algorithm.
Method
During the study period, from April 2017 to June 2018, 196 patients with IJD visited the Preventive Cardio-Rheuma Clinic in Oslo, Norway. A CVD risk stratification was performed, including the assessment of traditional risk factors and the measurement of arterial stiffness.
Results
Thirty-six patients (18.4%) had elevated aortic pulse wave velocity (aPWV) (≥ 10 m/s). After adjustment for age and heart rate, arterial stiffness was comparable across the IJD entities (p = 0.69). Associated factors, revealed by regression analysis, were age, blood pressure, heart rate, presence of carotid plaques, establis hed CVD, non-steroidal anti-inflammatory drugs, and statin use. Furthermore, aPWV was positively correlated with estimated CVD risk (r = 0.7, p < 0.001) and patients with a very high predicted CVD risk (SCORE ≥ 10%) had significantly higher aPWV than patients at lower CVD risk (9.2 vs 7.5 m/s, p < 0.001).
Conclusion
The degree of arterial stiffness was comparable across the IJD entities and was highly associated with the estimated CVD risk. Our findings support the need for an increased focus on prevention of CVD in all patients with IJD.
According to the World Health Organization (WHO), 17.7 million deaths are attributable to cardiovascular diseases (CVDs) annually, representing 31% of all deaths globally (Citation1). This vast number underlines the need to identify susceptible populations requiring preventive measures.
Established risk factors for CVD include age, male gender, hyperlipidaemia, hypertension, tobacco use, physical inactivity, obesity, unhealthy diets, and diabetes (Citation2). Large observational studies have revealed an increased CVD risk among patients with inflammatory joint diseases (IJDs), which is most likely caused by chronic inflammation in the vascular wall (Citation3–7). Patients with rheumatoid arthritis (RA) have a 1.5–2-fold increased risk of CVD, which is comparable to the risk of CVD in patients with type 2 diabetes mellitus (Citation6, Citation8). There is also increasing evidence of elevated CVD risk among patients with other IJD entities, such as psoriatic arthritis (PsA) (Citation8–10) and ankylosing spondylitis (AS) (Citation9, Citation11, Citation12).
In clinical practice, CVD risk is often estimated by the use of risk calculators, such as the Systematic COronary Risk Evaluation (SCORE) algorithm, endorsed by the European Society of Cardiology (ESC) (Citation13, Citation14). However, owing to observations of inaccurate predictions by the traditional CVD risk calculators in patients with RA, substantial efforts have been made to investigate novel biomarkers of CVD risk which may improve the precision of the CVD risk calculators in this patient population. Increased arterial stiffness is an early stage of the atherosclerotic process that can easily be assessed by non-invasive measurements. The two most common measures of arterial stiffness, the augmentation index (AIx) and aortic pulse wave velocity (aPWV), are validated surrogate CVD endpoints and have been shown to predict CVD events in both RA and non-RA populations (Citation15–19). Indeed, both AIx and aPWV are increased in patients with RA (Citation13, Citation20).
During the past few decades, treatment opportunities for patients with IJD have evolved with the introduction of effective disease-modifying anti-rheumatic drugs (DMARDs). Current observational studies suggest that both conventional/synthetic disease-modifying anti-rheumatic drugs (sDMARDs) and biological disease-modifying anti-rheumatic drugs (bDMARDs) not only improve disease-specific outcomes but also may be associated with a reduced risk of CVD events in patients with RA and PsA; however, data are contradictory and prone to confounding by indication bias (Citation21–24).
Differences in arterial stiffness or CVD risk between the IJD entities may lead to more or less aggressive CVD risk management strategies, and hence the aim of the present study was, first, to compare arterial stiffness measured by aPWV and AIx across patients with RA, PsA, and AS. Secondly, we aimed to assess the relationship between arterial stiffness and estimated CVD risk by the SCORE algorithm in IJD patients. Thirdly, we aimed to explore possible associations between arterial stiffness and CVD risk factors, haemodynamic indices, and frequently used anti-rheumatic medication.
Method
Patients with IJD, including RA, AS, and PsA, were referred either from the rheumatology outpatient clinic at Diakonhjemmet Hospital, Oslo, Norway, or by primary care physicians, for a CVD risk evaluation (April 2017 to June 2018). Referral criteria to the Preventive Cardio-Rheuma Clinic were: (i) a patient with IJD has asked for a CVD risk evaluation; (ii) the physician or the patient has knowledge of one or more CVD risk factor(s); (iii) the presence of symptoms or signs of a CVD risk factor, e.g. headache due to hypertension; and (iv) premature familial CVD.
This is a quality assurance report, and ethical approval was therefore not required. Data collection and publication were approved by the Data Protection Officer at Oslo University Hospital, Oslo, Norway (2011/7318), who decided that informed patient consent was not required.
CVD risk factors
Traditional CVD risk factors were collected at baseline (). Blood pressure was measured after the patient had been in a supine position for at least 10 min in a semi-dark room, using a Mobile-O-Graph apparatus (PWA Monitor; IEM, Stolberg, Germany). Hypertension was defined as blood pressure levels ≥ 140/90 mmHg and/or use of anti-hypertensive medication. Lipid levels were measured at the hospital laboratory (European Standard Accredited 2009) by routine procedures in a cobas 8000 (Roche Diagnostics, Oslo, Norway) (Citation25). Low-density lipoprotein cholesterol (LDL-c) was calculated according to Friedewald’s formula (Citation26). Dyslipidaemia was defined as total cholesterol (TC) > 5.2 mmol/L (200 mg/dL), LDL-c > 3.4 mmol/L (130 mg/dL), high-density lipoprotein cholesterol (HDL-c) < 1.0 mmol/L (39 mg/dL) in males, HDL-c < 1.3 mmol/L (50 mg/dL) in females, triglycerides > 1.7 mmol/L (150 mg/dL), the use of lipid-lowering medication (statins), or a combination thereof (Citation27). Family history of premature CVD was defined as coronary heart disease in first degree relatives prior to the age of 55 years in males and 65 years in females. Diabetes mellitus was defined as the presence of a physician-verified diagnosis and/or the use of oral hypoglycaemic medications/insulin.
Table 1. Patient characteristics
Systematic COronary Risk Evaluation
The estimated 10 year risk for a fatal atherosclerotic CVD event was calculated according to the SCORE low-risk algorithm (Citation14), as Norway is regarded as a low CVD risk country. The traditional risk assessment model is only applicable to patients aged 40–64 years. For the risk estimation of patients aged 65–79 years, the SCORE OP was used (Citation28). Consequently, it was not possible to assess an estimated 10 year risk for fatal CVD event in patients older than 79 years, but they were considered as patients at ‘very high risk’. Patients younger than 40 years were truncated to have an estimated CVD risk of zero. The CVD risk factors included in the SCORE algorithm are age, gender, smoking status, TC/HDL-c, and systolic blood pressure. As recommended by the ESC at the time of the project, a calculated risk by SCORE of ≥ 5% indicates a high risk of CVD, and lipid-lowering therapy is recommended to reduce LDL-c to < 2.6 mmol/L. A calculated risk by SCORE of ≥ 10% or established CVD is defined as ‘very high risk’ for future or recurrent fatal CVD, with a lower LDL-c goal for lipid-lowering treatment at < 1.8 mmol/L. Even lower LDL-c goals are recommended in the current ESC guidelines (Citation29).
Arterial stiffness
Arterial stiffness was measured after the patient had been in the supine position for at least 10 min in a semi-dark room, using the Mobil-O-Graph, and was characterized by AIx corrected to a pulse of 75 beats/min (AIx@75) and aPWV. This device first records oscillometric brachial blood pressure, then the cuff reinflates in the diastolic phase for 10 s to record brachial pulse waves with the use of a high-fidelity pressure sensor (Citation30). Determination of the brachial pulse waveforms is based on the oscillometric brachial systolic and diastolic blood pressures. The aortic pulse waveform is generated via the inbuilt ARCSolver algorithm (AIT Austrian Institute of Technology, Vienna, Austria), based on a three-element Windkessel model (Citation30, Citation31). In addition, the Mobil-O-Graph hypertension management software (HMS) analyses wave separation by decomposing the aortic pulse waveform into forward (incident) and backward (reflected) travelling pulse waves with the use of an uncalibrated aortic flow waveform (Citation32). aPWV is estimated from the time difference between the derived incident and reflected waves. Analysis of the aPWV also provides the AIx@75, which represents the percentage of the central pulse pressure (Citation33). It is derived from the ratio of augmentation pressure to aortic pulse pressure. AIx@75 is dependent on the heart rate and thus AIx@75 was reported normalized to a heart rate of 75 beats/min (AIx@75), to facilitate comparisons (Citation34). The Mobil-O-Graph has been extensively validated, with significant reproducibility in both invasive and non-invasive comparison studies (Citation30, Citation35–37).
Statistical analysis
The data were presented as mean with standard deviation, median with interquartile range (IQR), or number with percentage, as appropriate. Normality of the data distribution for continuous variables was assessed by the Shapiro–Wilk test. Non-normally distributed variables were natural logarithm (ln) transformed after adding a constant value to the data to correct for negative values before comparison. Differences across IJD entities and across degree of arterial stiffness were analysed using Welch’s t-test, analysis of variance (ANOVA), Mann–Whitney U-test, Kruskal–Wallis test, or chi-squared test, based on the distribution and type of data. Tests were two sided, with the significance level set at p < 0.05. Patients were dichotomized into groups with increased aPWV (≥ 10 m/s) and normal aPWV (< 10 m/s). This cut-off was chosen as a conservative estimate of significant alterations in aortic function based on both cross-sectional and longitudinal data (Citation38). Pearson and Spearman correlation analyses were used, as appropriate, to explore correlations. To study the factors associated with increased arterial stiffness, a univariate logistic regression analysis was conducted. In the next step, variables of interest (p < 0.10) were analysed using multiple logistic regression, to obtain adjusted odds ratios (ORs) and confidence intervals (CIs). Blood pressure, presence/number of carotid plaques, established CVD, and medication were excluded from the final model, as their cause–effect relationships with aPWV have not yet been fully clarified (Citation39). In addition, blood pressure, presence/number of carotid plaques, and established CVD are correlated with age. Previous studies have revealed that heart rate is a modulator of aPWV, and therefore it was included in the model (Citation40). Model fit was assessed using the Hosmer–Lemeshow test, the area under the receiver operating characteristics curve (AuROC), and Nagelkerke’s R2. Subsequently, analysis of covariance (ANCOVA) was used to compare arterial stiffness across the IJD entities and to evaluate whether various medications had an effect on arterial stiffness, adjusting for relevant variables associated with arterial stiffness (i.e. age and heart rate). The non-parametric Kruskal–Wallis test was used to compare the estimated risk of fatal CVD by SCORE across the IJD entities. All statistical analyses were performed using IBM SPSS Statistics for Mac, version 25.0 (IBM Corp., Armonk, NY, USA). All graphs were designed with GraphPad Prism (GraphPad Software, La Jolla, CA, USA).
Results
Patient characteristics
Patient characteristics are shown in . In total, 196 patients with IJD, including RA (n = 109), AS (n = 49), PsA (n = 32), and spondyloarthropathy (n = 6) (aged 27–84 years), were referred for CVD risk evaluation. The six patients diagnosed with spondyloarthropathy were counted in the AS group.
There were more women in the RA group than in the AS and PsA groups (73%, 45%, and 50%, respectively), and the AS patients were youngest. Two-thirds of the IJD patients were current or former smokers, and hypertension was present in 43%. All patients with PsA had dyslipidaemia, compared to 89% of the RA patients and 73% of the AS patients (p = 0.001). Approximately 8% of all patients had established CVD and more than half of all referred patients had asymptomatic carotid atherosclerotic plaque(s).
Arterial stiffness across IJD entities
Thirty-six patients (18.4%) had an elevated arterial stiffness, as measured by an aPWV ≥ 10 m/s, and the majority were patients with RA (24.8% vs 7.3% of AS and 15.6% of PsA patients, p = 0.022). Likewise, the mean aPWV was higher in the RA group than in AS and PsA (8.7, 7.7, and 8.5 m/s, respectively, p < 0.001) (). After adjusting for age and heart rate, no significant differences across the three disease entities were identified (p = 0.688).
Figure 1. Differences in arterial stiffness, assessed by mean aortic pulse wave velocity (aPWV) and median augmentation index (AIx@75), across the inflammatory joint disease (IJD) entities: rheumatoid arthritis (RA), ankylosing spondylitis (AS), and psoriatic arthritis (PsA). • Unadjusted data; ○ adjusted data. Error bars represent the standard error. *Significant difference (p < 0.05) from RA. aPWV adjusted for age and heart rate; AIx@75 adjusted only for age.
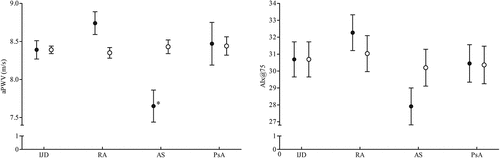
The median AIx@75 was numerically lower in the AS group (Citation23) than in RA and PsA patients (29 for both), although this difference did not reach statistical significance.
Associations between arterial stiffness and traditional CVD risk factors, haemodynamic factors, and medication use
IJD patients with aPWV ≥ 10 m/s were older (p < 0.001), had more hypertension (p < 0.001), higher heart rates (p < 0.001), and higher levels of HDL-c (p = 0.028), and were more likely to have established CVD (p < 0.001) than those with an aPWV < 10 m/s (). The number of carotid plaques in the aPWV ≥ 10 m/s group was higher than in those with aPWV < 10 m/s (p = 0.001). The use of prednisolone, non-steroidal anti-inflammatory drugs (NSAIDs), and statins was significantly higher among patients with aPWV ≥ 10 m/s (p = 0.008, p = 0.034, and p < 0.001, respectively).
Table 2. Comparison of traditional cardiovascular disease (CVD) risk factors, haemodynamic factors, and medication use between patients with high and low aortic pulse wave velocity (aPWV)
Age, gender, blood pressure, heart rate, HDL-c, carotid plaques, established CVD, NSAID use, and statin use were associated with arterial stiffness in univariate analysis (p < 0.1) (). When all relevant variables were entered simultaneously in multivariate analysis, only age and heart rate remained significantly associated with arterial stiffness (p < 0.001 and p = 0.008, respectively). The results did not change using a forward or backward procedure. The fit of the model was satisfactory on the basis of the Hosmer–Lemeshow test (p = 0.285). After adjusting for age and heart rate, this combination of variables explained 70% of the variation in the outcome (Nagelkerke’s R2 = 0.698). The model possessed sufficient discriminatory ability to distinguish between patients with increased aPWV and normal aPWV (adjusted AuROC = 0.962).
Table 3. Associations between arterial stiffness and cardiovascular disease (CVD) risk factors, haemodynamic factors, and medication use
Relationship between estimated risk of CVD and arterial stiffness
The median (IQR) estimated risk for future CVD by SCORE was low at 1.5 (1.0–3.0) and was significantly different between the groups [RA: 2.0 (1.0–4.0), AS: 1.0 (0.0–2.0), PsA: 1.0 (1.0–3.0), p = 0.036]. However, 106 out of 196 patients (54%) had an estimated very high risk [≥ 10%, presence of carotid plaque(s) and/or established CVD].
Both in the overall population and within the individual IJD disease entities, aPWV was positively correlated with the estimated CVD risk (r = 0.7, p < 0.001) (), and patients at very high CVD risk had significantly higher aPWV than those in lower risk categories (9.2 vs 7.5 m/s, p < 0.001).
Figure 2. Relationship between cardiovascular disease (CVD) risk estimation by the Systematic COronary Risk Evaluation (SCORE) algorithm and arterial stiffness, expressed as aortic pulse wave velocity (aPWV) and augmentation index (AIx@75). y(PWV) = 7.52 + 0.32x; r2 (PWV) = 0.49. y(AIx@75) = 24.57 + 0.26x; r2 (AIx@75) = 0.0038. Solid black line: regression line of aPWV; dashed black line: confidence interval of regression line of aPWV; solid grey line: regression line of AIx@75; dashed grey line: confidence interval of regression line of AIx@75.
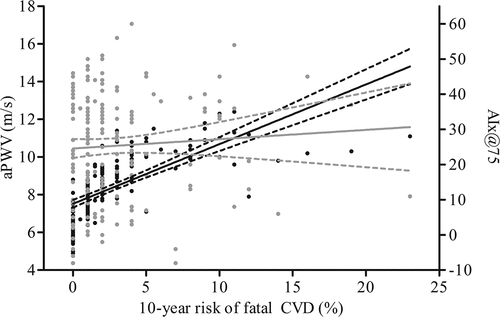
AIx@75 and estimated CVD risk by SCORE were not significantly correlated. A significantly higher AIx@75 was observed only in RA patients, and in those categorized in the very high CVD risk group [≥ 10%, presence of carotid plaque(s) and/or established CVD], compared to patients in lower risk categories (33 vs 23, respectively, p = 0.03).
Effect of medications on arterial stiffness
After adjustment for age and heart rate, no significant differences in aPWV across the three IJD entities were observed between users and non-users of NSAIDs, bDMARDs, sDMARDs, and anti-hypertensive medication. In the overall group, statin users and prednisolone users had significantly higher aPWV compared to non-users (8.7 vs 8.3 m/s, p = 0.004, and 8.6 vs 8.3, p = 0.033, respectively). A significant difference in aPWV between users and non-users of statins was also observed in the RA group (p = 0.012) and between users and non-users of prednisolone in the AS group (p = 0.014).
AIx@75 was significantly different in the overall group of statin users compared to the non-users (35.7 vs 29.8, respectively, p = 0.024).
Discussion
In the past decade, arterial stiffness has become an acknowledged surrogate marker of CVD. Numerous studies have demonstrated that patients with IJD have increased arterial stiffness and, thus, an increased risk of developing CVD (Citation6, Citation12, Citation41). Standardized mortality ratios compared to the general population are between 1.3 and 2.3 for RA, between 1.6 and 1.9 for AS, and between 0.8 and 1.6 for PsA (Citation9). To our knowledge, this is the first study to reveal comparable arterial stiffness levels across the three major IJD entities: RA, AS, and PsA.
PsA patients had a more unfavourable risk profile than patients with RA and AS. All of the PsA patients had dyslipidaemia and their median body mass index (BMI) was at least 2 kg/m2 higher than in the other IJD groups. This is in line with a cross-sectional study from Labitigan et al, who found a relatively high prevalence of abdominal obesity and diabetes in PsA patients compared to RA patients (Citation42). Differences in impaired glucose tolerance and hypertension were not identified in our study population, in contrast to the findings of Wibetoe et al (Citation43). An overrepresented cardiovascular risk in patients with PsA compared with matched subjects from the general population was reported by Landgren et al (Citation44).
After adjustment for age and heart rate, we did not identify differences in arterial stiffness across the IJD entities. Differences in arterial stiffness before the adjustment of associated factors were as a result of the higher average age of the RA patients. Age and blood pressure are known to be the major determinants of aPWV (Citation45). Whether hypertension is indeed the cause of arterial stiffness or, rather, the consequence remains an ongoing academic discussion (Citation39). In line with this, patients with increased arterial stiffness were older, and had a higher heart rate and more risk factors for CVD events such as hypertension, carotid plaques, or already established CVD, compared to those with normal arterial stiffness. Against expectations, HDL-c was higher in the group of patients with increased arterial stiffness. This can be explained by the higher number of statin users in this group.
aPWV and AIx are two common measures of arterial stiffness, and have previously been shown to be elevated in patients with RA (Citation13, Citation20) and predictive of future cardiovascular events in the general population (Citation17–19). However, in patients with RA only aPWV, and not AIx, has been found to be a predictor of future CVD events (Citation16). This falls in line with the results in our study, where AIx was not associated with the calculated CVD risk using the SCORE algorithm. In a study of 3000 subjects from the general population, AIx was reported to predict CVD events in men, but not in women (Citation46). Considering that there is a strong female preponderance in RA, it may be that AIx is not suitable as a surrogate marker of CVD in these patients. However, larger studies are needed to confirm this hypothesis.
Prednisolone users had a higher aPWV than non-users. An association between glucocorticoid use and a dose-dependent increased risk of cardiovascular mortality has been shown in RA patients, probably due to toxic effects (Citation47). The higher aPWV among prednisolone users in our patients may be due to glucocorticoid-induced hypertension, which may be caused by an activation of the renin–angiotensin system, among other factors (Citation48). These findings support the recommendation that glucocorticoids should be prescribed at the lowest clinically effective dose possible.
Increased arterial stiffness was not associated with longer disease duration or elevated IJD disease activity markers (C-reactive protein and erythrocyte sedimentation rate). This is in agreement with previous studies but in contrast to others, as has been pointed out in a meta-analysis by Weng et al (Citation49). It appears that increased arterial stiffness results primarily from the thickening and reduced elasticity of large conduit arteries over time, rather than the cumulative burden of inflammation. This concurs well with a study demonstrating accelerated vascular stiffening in children with progeria syndrome, who are ageing prematurely (Citation50). Low inflammation parameters in patients with high aPWV that do not differ from patients with low aPWV may also result from more aggressive anti-inflammatory therapy.
Our analyses revealed that more than half of the patients had an estimated CVD risk ≥ 10%, carotid plaque(s), or already established CVD, confirming the previously mentioned increased risk of CVD in IJD patients. In this regard, and in view of the fact that development of CVD is an insidious process and usually causes no symptoms, initiation of CVD risk management may be considered at the time of IJD diagnosis. Although this has been known for decades, implementing measures to reduce this risk in clinical practice remains challenging (Citation51). There is often a lack of awareness and patients are often undertreated with CVD-preventive medication.
The risk calculation by SCORE was highly positive correlated with aPWV, but not with AIx@75. The correlation indicates that aPWV and the estimated CVD risk by SCORE carry similar information regarding the prediction of future CVD events. Although aPWV has been shown to predict CVD events, further prospective studies are warranted to confirm the agreement of aPWV with the calculated estimated risk of future CVD. The additive value of using aPWV beyond traditional risk factors in the general population has been examined in two meta-analyses, and was found to be of most use in better predicting cardiovascular outcomes in the intermediate-risk stratum (Citation17, Citation19).
Unlike other research carried out in this area, we did not find a significant difference in arterial stiffness between users and non-users of bDMARDs. Large clinical trials have demonstrated that treatment with tumour necrosis factor inhibitors is associated with significantly decreased arterial stiffness (Citation52). After adjusting for age and heart rate, only statin use was associated with arterial stiffness. Statin users in the overall group had a significantly higher aPWV and AIx@75. The effect of medications on arterial stiffness is debatable since we did not conduct a randomized controlled trial, and thus the results may be affected by indication bias.
A limitation of our study is the cross-sectional design; hence, we could not investigate causal relationships between arterial stiffness and risk factors. Furthermore, owing to the lack of longitudinal data, we were not able to generalize the finding that users of bDMARDs did not have lower arterial stiffness.
A pitfall of the SCORE algorithm is the intended applicable age range of 40–65 years. It is thought that the cardiovascular risk in patients aged under 40 years and without major CVD risk factors can be neglected. However, this may not be the case for patients with IJD or others with elevated individual CVD risk factors, and clinicians should be aware that truncating their estimated CVD risk to zero may be an underestimation of their actual risk of CVD.
In interpreting our results, one should also be aware of a possible selection bias due to inclusion of patients following referral to a preventive cardio-rheumatology clinic for CVD risk evaluation. The referred patients may have higher CVD risk than patients attending a rheumatology outpatient clinic, but it is not likely that the selection of patients differed between the IJD diagnoses. The main objective of this project was to compare arterial stiffness across patients with RA, AS, and PsA, and therefore it is less likely that a selection bias would influence the results. A control group would have been beneficial to evaluate whether there is increased arterial stiffness among patients with IJD compared to the general population; however, this has previously been demonstrated for patients with RA and PsA (Citation49, Citation53).
Conclusion
This study has shown that patients with IJD have comparable levels of arterial stiffness. The degree of arterial stiffness was associated with the estimated CVD risk by SCORE, indicating a high impact of traditional CVD risk factors on arterial stiffness. Our findings support the need for an increased focus on preventing CVD in all patients with IJD, not only those with RA.
Acknowledgements
This work was supported by the Erasmus+ grant to KF and research grants from the South Eastern Regional Health Authorities of Norway to SR [grant number 2016063] and AGS [grant number 2013064].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- World Health Organization. World health statistics 2018: monitoring health for the SDGs, sustainable development goals.Geneva: World Health Organization, 2018.
- Greenland P, Knoll MD, Stamler J, Neaton JD, Dyer AR, Garside DB, et al. Major risk factors as antecedents of fatal and nonfatal coronary heart disease events. JAMA 2003;290:891–7.
- Agca R, Heslinga SC, Rollefstad S, Heslinga M, McInnes IB, Peters MJ, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis 2017;76:17–28.
- Sattar N, McCarey DW, Capell H, McInnes IB. Explaining how “high-grade” systemic inflammation accelerates vascular risk in rheumatoid arthritis. Circulation 2003;108:2957–63.
- Avina-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum 2008;59:1690–7.
- Avina-Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis 2012;71:1524–9.
- Han C, Robinson DW Jr., Hackett MV, Paramore LC, Fraeman KH, Bala MV. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol 2006;33:2167–72.
- Schieir O, Tosevski C, Glazier RH, Hogg-Johnson S, Badley EM. Incident myocardial infarction associated with major types of arthritis in the general population: a systematic review and meta-analysis. Ann Rheum Dis 2017;76:1396–404.
- Agca R, Heslinga SC, van Halm VP, Nurmohamed MT. Atherosclerotic cardiovascular disease in patients with chronic inflammatory joint disorders. Heart 2016;102:790–5.
- Polachek A, Li S, Chandran V, Gladman DD. Clinical enthesitis in a prospective longitudinal psoriatic arthritis cohort: incidence, prevalence, characteristics, and outcome. Arthritis Care Res (Hoboken) 2017;69:1685–91.
- Mathieu S, Gossec L, Dougados M, Soubrier M. Cardiovascular profile in ankylosing spondylitis: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2011;63:557–63.
- Mathieu S, Pereira B, Soubrier M. Cardiovascular events in ankylosing spondylitis: an updated meta-analysis. Semin Arthritis Rheum 2015;44:551–5.
- Ambrosino P, Tasso M, Lupoli R, Di Minno A, Baldassarre D, Tremoli E, et al. Non-invasive assessment of arterial stiffness in patients with rheumatoid arthritis: a systematic review and meta-analysis of literature studies. Ann Med 2015;47:457–67.
- Perk J, de Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J 2012;33:1635–701.
- Vlachopoulos C, Xaplanteris P, Aboyans V, Brodmann M, Cifkova R, Cosentino F, et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis 2015;241:507–32.
- Ikdahl E, Rollefstad S, Wibetoe G, Olsen IC, Berg IJ, Hisdal J, et al. Predictive value of arterial stiffness and subclinical carotid atherosclerosis for cardiovascular disease in patients with rheumatoid arthritis. J Rheumatol 2016;43:1622–30.
- Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 2010;55:1318–27.
- Vlachopoulos C, Aznaouridis K, O’Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J 2010;31:1865–71.
- Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 2014;63:636–46.
- Provan SA, Angel K, Semb AG, Mowinckel P, Agewall S, Atar D, et al. Early prediction of increased arterial stiffness in patients with chronic inflammation: a 15-year followup study of 108 patients with rheumatoid arthritis. J Rheumatol 2011;38:606–12.
- Holmqvist M, Ljung L, Askling J. Mortality following new-onset rheumatoid arthritis: has modern rheumatology had an impact? Ann Rheum Dis 2018;77:85–91.
- Li L, Hagberg KW, Peng M, Shah K, Paris M, Jick S. Rates of cardiovascular disease and major adverse cardiovascular events in patients with psoriatic arthritis compared to patients without psoriatic arthritis. J Clin Rheumatol 2015;21:405–10.
- Roubille C, Richer V, Starnino T, McCourt C, McFarlane A, Fleming P, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis 2015;74:480–9.
- Westlake SL, Colebatch AN, Baird J, Kiely P, Quinn M, Choy E, et al. The effect of methotrexate on cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology (Oxford) 2010;49:295–307.
- van Gammeren AJ, van Gool N, de Groot MJ, Cobbaert CM. Analytical performance evaluation of the Cobas 6000 analyzer – special emphasis on trueness verification. Clin Chem Lab Med 2008;46:863–71.
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502.
- Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol 2014;63:2889–934.
- Cooney MT, Selmer R, Lindman A, Tverdal A, Menotti A, Thomsen T, et al. Cardiovascular risk estimation in older persons: score o.P. Eur J Prev Cardiol 2016;23:1093–103.
- Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 esc/eas guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–88.
- Wassertheurer S, Kropf J, Weber T, van der Giet M, Baulmann J, Ammer M, et al. A new oscillometric method for pulse wave analysis: comparison with a common tonometric method. J Hum Hypertens 2010;24:498–504.
- Papaioannou TG, Protogerou AD, Stamatelopoulos KS, Vavuranakis M, Stefanadis C. Non-invasive methods and techniques for central blood pressure estimation: procedures, validation, reproducibility and limitations. Curr Pharm Des 2009;15:245–53.
- Hametner B, Wassertheurer S, Kropf J, Mayer C, Holzinger A, Eber B, et al. Wave reflection quantification based on pressure waveforms alone–methods, comparison, and clinical covariates. Comput Methods Programs Biomed 2013;109:250–9.
- Hametner B, Weber T, Mayer C, Kropf J, Wassertheurer S. Calculation of arterial characteristic impedance: a comparison using different blood flow models. Math Comput Model Dyn Syst 2013;19:319–30.
- Stoner L, Faulkner J, Lowe A, Lambrick DM, Young JM, Love R, et al. Should the augmentation index be normalized to heart rate? J Atheroscler Thromb 2014;21:11–16.
- Luzardo L, Lujambio I, Sottolano M, da Rosa A, Thijs L, Noboa O, et al. 24-h ambulatory recording of aortic pulse wave velocity and central systolic augmentation: a feasibility study. Hypertens Res 2012;35:980–7.
- Hametner B, Wassertheurer S, Kropf J, Mayer C, Eber B, Weber T. Oscillometric estimation of aortic pulse wave velocity: comparison with intra-aortic catheter measurements. Blood Press Monit 2013;18:173–6.
- Feistritzer HJ, Reinstadler SJ, Klug G, Kremser C, Seidner B, Esterhammer R, et al. Comparison of an oscillometric method with cardiac magnetic resonance for the analysis of aortic pulse wave velocity. PLoS One 2015;10:e0116862.
- Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens 2012;30:445–8.
- Safar ME, Asmar R, Benetos A, Blacher J, Boutouyrie P, Lacolley P, et al. Interaction between hypertension and arterial stiffness. Hypertension 2018;72:796–805.
- Lantelme P, Mestre C, Lievre M, Gressard A, Milon H. Heart rate an important confounder of pulse wave velocity assessment. Hypertension 2002;39:1083–7.
- Horreau C, Pouplard C, Brenaut E, Barnetche T, Misery L, Cribier B, et al. Cardiovascular morbidity and mortality in psoriasis and psoriatic arthritis: a systematic literature review. J Eur Acad Dermatol Venereol 2013;27:12–29.
- Labitigan M, Bahce-Altuntas A, Kremer JM, Reed G, Greenberg JD, Jordan N, et al. Higher rates and clustering of abnormal lipids, obesity, and diabetes mellitus in psoriatic arthritis compared with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2014;66:600–7.
- Wibetoe G, Ikdahl E, Rollefstad S, Olsen IC, Bergsmark K, Kvien TK, et al. Cardiovascular disease risk profiles in inflammatory joint disease entities. Arthritis Res Ther 2017;19:153.
- Landgren AJ, Bilberg A, Eliasson B, Larsson I, Dehlin M, Jacobsson L, et al. Cardiovascular risk factors are highly overrepresented in Swedish patients with psoriatic arthritis compared with the general population. Scand J Rheumatol 2020;49:195–9.
- Reference Values for Arterial Stiffness Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J 2010;31:2338–50.
- Janner JH, Godtfredsen NS, Ladelund S, Vestbo J, Prescott E. High aortic augmentation index predicts mortality and cardiovascular events in men from a general population, but not in women. Eur J Prev Cardiol 2013;20:1005–12.
- del Rincón I, Battafarano DF, Restrepo JF, Erikson JM, Escalante A. Glucocorticoid dose thresholds associated with all-cause and cardiovascular mortality in rheumatoid arthritis. Arthritis Rheumatol 2014;66:264–72.
- Saruta T. Mechanism of glucocorticoid-induced hypertension. Hypertens Res 1996;19:1–8.
- Wang P, Huang L, Xu Q, Xu L, Deng FY, Lei SF. Assessment of aortic stiffness in patients with rheumatoid arthritis using pulse wave velocity: an update meta-analysis. Arch Med Res 2019;50:401–12.
- Gerhard-Herman M, Smoot LB, Wake N, Kieran MW, Kleinman ME, Miller DT, et al. Mechanisms of premature vascular aging in children with Hutchinson-Gilford progeria syndrome. Hypertension 2012;59:92–7.
- Semb AG, Ikdahl E, Wibetoe G, Crowson C, Rollefstad S. Atherosclerotic cardiovascular disease prevention in rheumatoid arthritis. Nat Rev Rheumatol 2020;16:361–79.
- Vlachopoulos C, Gravos A, Georgiopoulos G, Terentes-Printzios D, Ioakeimidis N, Vassilopoulos D, et al. The effect of TNF-a antagonists on aortic stiffness and wave reflections: a meta-analysis. Clin Rheumatol 2018;37:515–26.
- Costa L, Caso F, D’Elia L, Atteno M, Peluso R, del Puente A, et al. Psoriatic arthritis is associated with increased arterial stiffness in the absence of known cardiovascular risk factors: a case control study. Clin Rheumatol 2012;31:711–5.
