Abstract
Objectives
The use of rituximab (MabThera®), an anti-CD20 monoclonal antibody, is the most significant development in the management of anti-neutrophil cytoplasm antibody (ANCA)-associated vasculitis (AAV) since the introduction of cytotoxic therapy in 1950. Truxima® is the first anti-CD20 biosimilar approved for the same indications, and has been available in the UK since 2017. Significant cost savings have been reported when switching to biosimilars, which could lead to greater patient access to such treatment. Therefore, it is important to know whether patients’ clinical and laboratory parameters respond equally well to biosimilars as to reference medicines, tested in clinical trials.
Method
We retrospectively reviewed the clinical outcomes and laboratory parameters in 257 consecutive patients treated with anti-CD20 depletion therapy using MabThera or Truxima, for induction and maintenance of remission, in two tertiary renal centres between 2010 and 2019.
Results
We demonstrated no difference between patients treated with MabThera or Truxima in rates of remission, relapse, and hospitalization with infection when used for either induction or maintenance of remission of AAV. In one hospital subgroup analysis, we showed comparable levels of hypogammaglobulinaemia, B-cell depletion, and frequency of infusion reactions, with no significant differences.
Conclusion
The efficacy and safety of the rituximab biosimilar Truxima are not inferior to the originator MabThera in patients with AAV. Truxima represents a cheaper and safe therapeutic alternative that could increase patient access to rituximab.
Anti-neutrophil cytoplasm antibody (ANCA)-associated vasculitis (AAV) is a rare systemic autoimmune disease characterized by severe necrotizing inflammation of predominantly small vessels. The group of ANCA-associated vasculitides includes granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), and eosinophilic granulomatosis with polyangiitis (EGPA). These are associated with the presence of circulating ANCAs directed against myeloperoxidase (MPO) or proteinase-3 (PR3), although 10% of patients are ANCA negative and have similar clinical features, and therapeutic responses, to those who are positive (Citation1).
The use of rituximab, a murine chimeric monoclonal antibody against the B-cell surface marker CD20, for the induction and remission–maintenance of AAV is the most significant development in the management of AAV since the introduction of cyclophosphamide in the mid-twentieth century. B cells are central to the pathogenesis of the disease. They produce pathogenic ANCAs, their numbers correlate with disease activity (Citation2), and B-cell repopulation after rituximab may predict relapse (Citation3). Clinical trials are increasingly supporting the use of rituximab in both induction [PEXIVAS (Citation4)] and remission–maintenance therapy [MAINRITSAN (Citation5), RITAZAREM (Citation6)] of AAV.
Currently, the two types of rituximab that are widely used are the originator MabThera® (Roche Pharmaceuticals) and the biosimilar Truxima® (Napp Pharmaceutical Group, Cambridge, UK). The European Medicines Agency (EMA) defines a biosimilar as a product that is similar to a biological medicine that has already been authorized. There is a significant financial incentive in switching to the biosimilar, as the price of Truxima could be up to 60–70% lower than that of MabThera, representing a substantial societal health gain with more patient access to life-saving treatment (Citation7).
In 2017, UK institutions switched to using Truxima for all rituximab indications following EMA guidelines (Citation8). Here, we investigate the efficacy and safety of Truxima in the treatment of AAV and compare it to contemporaneous cohorts treated with MabThera.
Method
We retrospectively reviewed the outcomes of consecutive patients treated with either MabThera or Truxima, for the induction or maintenance of remission of AAV in two tertiary renal centres in the UK between 2010 and 2019.
Patients with a diagnosis of systemic vasculitis consistent with the Chapel Hill Consensus Conference criteria and positive ANCA serology were included. ANCA-negative vasculitis was defined as disease that was consistent with clinical and histological features of AAV on the renal biopsy in the absence of positive ANCA serology. Remission was defined as a Birmingham Vasculitis Activity Score (BVAS) of 0 and prednisolone dose of < 10 mg/day by 3 months. Relapse was defined as an increase in BVAS requiring an increase in immunosuppression. Rituximab was dosed at either 375 mg/m2 per week for up to 4 weeks or 1 g for 2 weeks. Considerations for rituximab therapy for both renal centres included active disease or disease that progressed despite the course of cyclophosphamide lasting for 3–6 months, contraindication to cyclophosphamide, concern that treatment with cyclophosphamide may materially affect fertility, further cyclophosphamide treatment exceeding the maximum cumulative dose, or the presence of urothelial malignancy. None of the patients analysed in this cohort received rituximab for both induction and maintenance of disease. The maintenance regimen for patients induced with either cyclophosphamide or rituximab was the same: azathioprine 2 weeks after induction therapy, along with reducing the steroid regimen, aiming for 10 mg/day by 3 months. If azathioprine was not tolerated, patients received mycophenolate mofetil.
We examined the efficacy of MabThera and Truxima on the induction and maintenance of remission of AAV, rates of relapse, renal outcomes, and B-cell depletion (in one centre), while the adverse effects were assessed by rates of hospitalization with infection, infusion reactions, and rates of hypogammaglobulinaemia. Data on cyclophosphamide use and steroid increase within the year before receiving rituximab were collected. B-cell depletion was defined as an absolute CD19+ cell count below 0.005 × 109 cells/L.
Continuous data were found to be non-parametric using the Shapiro–Wilk test of normality. For comparison of continuous variables, a Mann–Whitney test was performed to detect statistically significant difference between treatments. The Wilcoxon signed-rank test was used to compare non-parametric paired data.
For comparison of categorical variables between groups, the chi-squared test was used. Multiple logistic regression analysis was used to ascertain odds ratios of factors associated with dependent variables (induction of remission, maintenance of remission, relapse, and infection). Covariates included in the regression analysis were age, gender, follow-up duration, diagnosis of GPA, cyclophosphamide use, or steroid increase within the year before receiving rituximab. Data are presented as odds ratios (ORs), with 95% confidence interval (CI) and p value. Graphs were constructed and statistical analysis was performed using Prism 8.0 (GraphPad Software, La Jolla, CA, USA).
Results
We identified a total of 257 patients in the two tertiary renal centres who received Truxima or MabThera between 2010 and 2019 for the induction or maintenance of remission of AAV. Of these, 56.8% (n = 146) were female and the median [interquartile range (IQR)] age of the population was 64 (52–73) years. Fifty one per cent of the patients had GPA, 29.6%, MPA, 10% ANCA negative vasculitis, 6.3% unclassified ANCA-positive vasculitis, and 3.1% EGPA. All patients had renal involvement. In total, 137 patients (53.3%) received rituximab for the induction of remission and 120 (46.7%) for maintenance. In the entire cohort of patients, 127 (49.4%) received MabThera and 130 (50.6%) Truxima.
show the unadjusted comparison between MabThera and Truxima and multivariable analysis in patients who received treatment for the induction or maintenance of remission of AAV.
Table 1. Comparison of characteristics of a two-centre patient cohort that received rituximab (RTX) therapy with either MabThera or Truxima for the induction and maintenance of remission of anti-neutrophil cytoplasm antibody-associated vasculitis
Table 2. Factors associated with remission at 3 months, relapse, and hospitalization with infection in patients treated with MabThera or Truxima for the induction and maintenance of remission of anti-neutrophil cytoplasm antibody-associated vasculitis
In the MabThera induction remission group, there were more patients with GPA (62.9% MabThera vs 32.8% Truxima, p < 0001), the group had longer follow-up [median (IQR) 41 (29–58) MabThera vs 8 (4–12) months Truxima, p < 0.001], and patients were more likely to have received cyclophosphamide and/or an increase in steroids within the year before receiving rituximab compared to those in the Truxima group (37.1% MabThera vs 10.4% Truxima, p < 0.001). Importantly, there was no difference between the two preparations in the rates of induction of remission, relapse, or hospitalizations with infection. In the multivariable analysis, the preparation of rituximab used had no influence on the induction of remission, relapse, or hospitalization with infection.
In the maintenance of remission cohort, the group of patients who received MabThera were younger [56 (40.8–68) years MabThera vs 65 (52–74) years Truxima, p = 0.03], had longer follow-up [46 (31.5–75) months MabThera vs 19 (16–21) months Truxima, p < 0.001], and were more likely to have received cyclophosphamide and/or an increase in steroids within the year before receiving rituximab compared to the Truxima group (33.3% MabThera vs 4.8% Truxima, p < 0.001). The MabThera patients relapsed more frequently (24.6% vs 3.1%, p < 0.001), whereas the Truxima group had more hospitalizations with infection (14% vs 33.3%, p = 0.02). However, in the multivariate analysis, the only significant difference between the two groups associated with relapse was follow-up duration (OR 1.03, 95% CI 1.00–1.06, p = 0.02), whereas the use of either MabThera or Truxima did not influence relapse or hospitalization with infection.
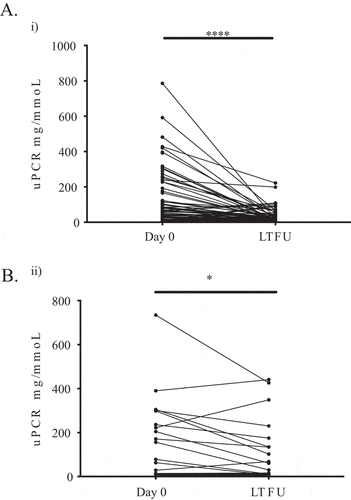
We analysed a single-centre subgroup of 140 patients with AAV who had received MabThera (79.3%) or Truxima (20.7%) and in whom we had more granular laboratory data (). The median cumulative dose was 2 g in both groups (IQR: 1.65–3 g MabThera vs 1.5–2 g Truxima). There was a significant decrease in proteinuria from presentation to last follow-up in both groups treated with MabThera [median (IQR) from a urine protein:creatinine ratio of 63 (16.5–201) to 17 (11–37) mg/mmoL, p < 0.001] and Truxima [median (IQR) from 29 (9.5–229) to 16 (7–135) mg/mmoL, p = 0.04] (). In the MabThera group, the estimated glomerular filtratin rate [median (IQR)] changed from 60 (29–90) to 66.5 (45.8–85.5) mL/min/1.73 m2 (p < 0.001) and in the Truxima group from 57.5 (16.8–75.7) to 58 (21–83) mL/min/1.73 m2 (p = 0.37) at last follow-up. There were no significant differences between the two groups with regard to CD19 depletion (p = 0.52) or in the development of hypogammaglobulinaemia, with immunoglobulin A (IgA) < 0.7 g/L (p = 0.68), IgG < 7 g/L (p = 0.2), and IgM < 0.4 g/L (p = 0.63) at the last clinic follow-up. The rate of hospitalization due to infection was 13.8% in the Truxima group and 9% in the MabThera group (p = 0.48). The relapse rate was higher in the MabThera group (15.3%) compared to Truxima (0%) (p = 0.04), but the median (IQR) follow-up duration was significantly shorter in the Truxima group [0.4 (0.25–0.98) years] than in the MabThera group [3.4 (2.5–51) years] (p < 0.001).
Table 3. Subpopulation comparison of a single-centre patient cohort with anti-neutrophil cytoplasm antibody (ANCA)-associated vasculitis that received rituximab (RTX) therapy with MabThera or Truxima
Discussion
Rituximab is an effective treatment option in the care of patients with autoimmune disease, including rheumatoid arthritis and AAV. As the financial burden to healthcare systems of treating such disorders escalates globally, the availability of new biosimilar products may help to overcome cost-related barriers to treatment and improve patient access. Therefore, it is important to know that clinical and laboratory parameters respond equally to biosimilars and reference medicines.
We demonstrated no differences between MabThera and Truxima in terms of remission, relapse, and hospitalization with infection. We also showed comparable levels of proteinuria, hypogammaglobulinaemia, B-cell depletion, and infusion reactions.
Differences between the two groups were noted in the unadjusted analysis, with patients receiving MabThera having longer follow-up and being more likely to have received cyclophosphamide or an increase in their steroid dose within the year before receiving rituximab than the Truxima group. These differences can be partly explained by Truxima only being introduced in 2017, whereas patients had been receiving MabThera since 2010, coupled with the increasing effort over the past decade for corticosteroid minimization in the treatment of AAV (Citation9). In addition, given the longer follow-up, the group of patients who received MabThera for the maintenance of remission is likely to be more heterogeneous in terms of previous cumulative immunosuppression doses, comorbidity, and presence of relapsing disease.
Our findings confirm and extend previous observations in patients with vasculitis, where the preparation of rituximab used did not influence the induction of remission, relapse, or all-cause mortality (Citation10, Citation11), by adding the comparison to laboratory parameters. Comparative studies of MabThera and Truxima have also been conducted in other disciplines. Phase III clinical trials in rheumatoid arthritis and lymphoma revealed similar pharmacodynamics, immunogenicity, and safety profiles between MabThera and Truxima (Citation12). Similarly, in patients with immune thrombocytopenic purpura (Citation13) and multiple sclerosis (Citation14), there were no differences between the biosimilar and the originator in clinical outcomes, infective complications, infusion reactions, and B-cell depletion. In addition, several second generation anti-CD20 drugs are in development. For example, ofatumumab, a fully humanized anti-CD20 monoclonal antibody, tested in a small case series of eight patients with AAV, showed therapeutic benefit (Citation15).
Significant budget savings have already been reported in European healthcare systems when switching to biological biosimilars (Citation16). A budget analysis published in 2017 demonstrated that the introduction of Truxima in the European Union would save €90.04 million in the first year, allowing a 6.4% increase in patients with access to rituximab (Citation7). In our centre, based on an average patient weight and a four-dose course (based on 375 mg/m2 dosing), the mean saving by switching from a MabThera to Truxima treatment course was approximately £4000 per patient (Citation13).
Conclusion
In the era of biosimilars therapy, we have presented the largest comparative analysis of patients with vasculitis between the reference rituximab and the biosimilar, and have demonstrated no differences between the two preparations in either clinical outcomes or laboratory parameters. The rising treatment costs of autoimmune diseases pose a challenge to constrained healthcare budgets worldwide. Biosimilars may provide an effective solution to this conundrum, reducing treatment costs and thereby increasing patient access to rituximab therapy.
Funding
This research is funded by the Medical Research council grant No. MR/P001777/1. MA is a Medical Research Council-funded Clinical Research Fellow.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Arthritis Rheum 2013;65:1–11.
- Jones RB, Cohen Tervaert JW, Hauser T, Luqmani R, Morgan MD, Peh CA, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 2010;363:211–20.
- Alberici F, Smith RM, Jones RB, Roberts DM, Willcocks LC, Chaudhry A, et al. Long-term follow-up of patients who received repeat-dose rituximab as maintenance therapy for ANCA-associated vasculitis. Rheumatology (Oxford) 2015;54:1153–60.
- Walsh M, Merkel PA, Peh CA, Szpirt W, Guillevin L, Pusey CD, et al. Plasma exchange and glucocorticoid dosing in the treatment of anti-neutrophil cytoplasm antibody associated vasculitis (PEXIVAS): protocol for a randomized controlled trial. Trials 2013;14:73.
- Charles P, Perrodeau É, Samson M, Bonnotte B, Néel A, Agard C, et al. Long-term rituximab use to maintain remission of antineutrophil cytoplasmic antibody–associated vasculitis. Ann Intern Med 2020;173:179–87.
- Smith RM, Jones RB, Specks U, Bond S, Nodale M, Aljayyousi R, et al. Rituximab as therapy to induce remission after relapse in ANCA-associated vasculitis. Ann Rheum Dis 2020;79:1243–9.
- Gulácsi L, Brodszky V, Baji P, Rencz F, Péntek M. The rituximab biosimilar CT-P10 in rheumatology and cancer: a budget impact analysis in 28 European countries. Adv Ther 2017;34:1128–44.
- EMA. Assessment report: Truxima. International non‐proprietary name: rituximab (2016). Procedure No. EMEA/H/C/004112/0000. EMA/CHMP/75695/2017. London, UK: European Medicines Agency, 2016. https://www.ema.europa.eu/documents/assessment-report/truxima-eparpublic-assessment-report_en.pdf.
- Hendra H, Salama AD. Steroids as treatment for glomerulonephritis: time for a rethink. Nephrol Dial Transplant 2020;gfaa267. doi:https://doi.org/10.1093/ndt/gfaa267. Online ahead of print.
- Kwon HC, Kim MK, Song JJ, Park YB, Lee SW. Rituximab biosimilar prevents poor outcomes of microscopic polyangiitis and granulomatosis with polyangiitis as effectively as rituximab originator. Yonsei Med J 2020;61:712–9.
- Mittal S, Naidu GSRSNK, Jha S, Rathi M, Nada R, Minz RW, et al. Experience with similar biologic rituximab in 77 patients of granulomatosis with polyangiitis-a real-life experience. Clin Rheumatol 2021;40:645–51.
- Coiffier B. Pharmacokinetics, efficacy and safety of the rituximab biosimilar CT-P10. Expert Rev Clin Pharmacol 2017;10:923–33.
- Stubbs MJ, Low R, McGuckin S, Newton R, Thomas M, Westwood JP, et al. Comparison of rituximab originator (MabThera) to biosimilar (Truxima) in patients with immune-mediated thrombotic thrombocytopenic purpura. Br J Haematol 2019;185:912–7.
- Perez T, Rico A, Boutière C, Maarouf A, Roudot M, Honoré S, et al. Comparison of rituximab originator (MabThera®) to biosimilar (Truxima®) in patients with multiple sclerosis. Mult Scler 2020;27:1352458520912170.
- McAdoo SP, Bedi R, Tarzi R, Griffith M, Pusey CD, Cairns TD. Ofatumumab for B cell depletion therapy in ANCA-associated vasculitis: a single-centre case series. Rheumatology (Oxford) 2016;55:1437–42.
- Tabernero J, Vyas M, Giuliani R, Arnold D, Cardoso F, Casali PG, et al. Biosimilars: a position paper of the European Society for Medical Oncology, with particular reference to oncology prescribers. ESMO Open 2016;1:e000142.