Abstract
Objective
To estimate the prevalence of psoriatic arthritis (PsA), axial spondyloarthritis (axSpA) and rheumatoid arthritis (RA) and the use of biologic agents in these diseases in Norway.
Methods
From the Norwegian Patient Registry (NPR), we identified as PsA, axSpA and RA patients ≥18 years those with ≥2 recorded episodes with diagnostic coding for index disease (L40.5, M07.0-M07.3 for PsA; M45, M46.0, M46.1, M46.8 and M46.9 for axSpA; M05-M06 for RA). We calculated the point prevalence of PsA, axSpA and RA as per the 1st of January 2017 in the Norwegian adult population (age ≥18). Dispensed disease-modifying antirheumatic drug (DMARD) prescriptions were obtained from the Norwegian Prescription Database and biologic DMARDs given in hospitals from the NPR.
Results
The point prevalence of PsA, axSpA, RA, and any of these diseases in total was 0.46%, 0.41%, 0.78%, and 1.56%, respectively. Among women, the prevalence of PsA, axSpA, and RA was 0.50%, 0.37%, and 1.10%, and among men 0.43%, 0.45%, and 0.46%, respectively. In 2017, 27.3% of RA patients, 25.7% of PsA patients and 35.1% of axSpA patients used biologic DMARDs. Treatment with biologics was more frequent in younger age groups in all three diseases, and became more infrequent especially after age ≥55 years.
Conclusion
In Norway, the combined prevalence of PsA, axSpA, and RA was over 1.5%. Reflecting the good overall access to highly effective but costly biologic treatments, more than a fourth of these patients used biologic agents, which corresponds to over 0.4% of Norwegian adult population.
Psoriatic arthritis (PsA), axial spondyloarthritis (axSpA), and rheumatoid arthritis (RA) are among the most common inflammatory joint diseases (IJDs), and their diagnostics and treatment comprise a significant part of rheumatologists’ work. In Norway, the prevalence of RA was estimated to be 0.43% in the County of Oslo in 1994, and 0.47% in the catchment area of University Hospital of Tromsø in 1994 (Citation1, Citation2). Three previous studies have evaluated the prevalence of PsA in Norway, although yielding contrasting results (0.13% in Tromsø, Northern Norway, in 1996; 0.20% in Hordaland, Western Norway, in 2003; and as high as 0.67% in Nord-Trøndelag, Central Norway, in 2008) (Citation3–5). Regarding axSpA, the prevalence of ankylosing spondylitis (AS) was estimated to be 0.21% in 1990 in Northern Norway (Citation6), although another study in the same region in 1979–1980 yielded a prevalence estimate as high as 1.1–1.4% (Citation7). Along with true changes in disease frequency, improvements in early diagnostics of IJDs may have affected the prevalence of IJDs during the past few decades. In addition, ageing of the Norwegian population is likely to have resulted in an increase in the prevalence of chronic IJDs. Thus, updated and nationwide estimates of the prevalence of IJDs in Norway are needed.
Since the 2000s, the introduction of biological disease-modifying anti-rheumatic drugs (bDMARDs) has revolutionized the treatment of PsA, axSpA, and RA. Patients with inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs) and/or non-steroidal anti-inflammatory drugs (NSAIDs) have an indication to be treated with bDMARDs. In general, the direct costs of bDMARDs are substantially higher than those of csDMARDs. Owing to often restricted healthcare budgets, differences in IJD patients’ accessibility to bDMARDs exist between countries. In Norway, IJD patients’ access to bDMARDs is generally good, with almost full reimbursement and no strict requirements of disease activity measures (Citation8–10). Based on data from the NOR-DMARD registry (covering 29% of the Norwegian IJD population), the estimated proportions of patients with RA, PsA, and AS prescribed with a bDMARD during 2000–2011 were 19.8%, 17.3%, and 19.2%, respectively, with a marked increase in first-time bDMARD prescriptions apparent during the study period, especially in PsA and AS (Citation11).
Using data from Norwegian nationwide registries, our aim was to provide updated estimates of the prevalence of PsA, axSpA, and RA, and the use of bDMARDs for these diseases in Norway.
Method
Register data and linkage
We used data from the Norwegian Cardio-Rheuma Register, which is a register linkage study combining data from multiple nationwide registries on the total adult Norwegian population (age ≥ 18 years) between 2008 and 2017. Organized under the Norwegian Directorate of Health, the Norwegian Patient Registry (NPR) provided individual-level data on episodes in both public and private specialized healthcare, covered by the public reimbursement policy. The NPR does not cover episodes in primary healthcare. For all inpatient, day, and outpatient episodes in specialized care, the NPR includes up to 20 diagnostic ICD-10 codes as well as medical procedure codes according to the Norwegian Classification of Medical Procedures (NCMP), including bDMARDs given in hospitals. Completeness of diagnostic codes in the NPR is good, with the main diagnosis recorded in 100% of episodes in 2017 public somatic hospital records (Citation12). The Norwegian Prescription Database (NorPD) at the Norwegian Institute of Public Health provided data on dispensed prescriptions according to Anatomical Therapeutic Chemical (ATC) classification codes.
The whole Norwegian adult population (≥ 18 years) on 1 January 2017 was identified from the Norwegian National Population Register, administered by the Norwegian Tax Administration. From the same register, we obtained data on date of birth, death, immigration/emigration, gender, and county of residence/health region (Supplementary Table S1). Statistics Norway provided annual data on the highest educational level.
The data from these registries were linked and pseudonymized by the register keeper (NorPD) before data transfer to a secure research server at Diakonhjemmet Hospital.
Approval for the study was obtained from the Norwegian Data Protection Authority (16/00482-11/CDG), the South East Norway Regional Ethical Committee (2016/588), and the Data Protection Officer at Oslo University Hospital (2016/924) and at Diakonhjemmet Hospital (7/12-2019). No written consent from study subjects was necessary, since the data set comprises only routinely recorded administrative data.
Prevalence of IJDs
Point prevalence of IJDs was calculated as per 1 January 2017. Out of all Norwegian residents aged ≥ 18 years, alive in January 2017 and recorded in the Norwegian population register, we identified PsA, axSpA, and RA patients with linkage to NPR data. Identification of patients relied on International Classification of Diseases, 10th revision (ICD-10) codes: M07.0–M07.3 and L40.5 for PsA; M45, M46.0, M46.1, M46.8, and M46.9 for axSpA; and M05–M06 for RA. To be identified as a PsA, axSpA, or RA case, a person had to have NPR records of: (i) a first inpatient or outpatient episode in the NPR with a diagnostic ICD-10 code for PsA/axSpA/RA as the main or contributory diagnosis between January 2008 and December 2016; and (ii) a second episode with an ICD-10 code for PsA/axSpA/RA in December 2017 at the latest. If a person had only one episode listing an ICD-10 code for PsA, axSpA, or RA, they would not fulfil the base-case definition. Patients were included in all those IJD cohorts for which they fulfilled the case identification criteria. When calculating the combined prevalence of PsA, axSpA, and RA, however, patients who fulfilled the case definition for more than one IJD were calculated only once.
As a sensitivity analysis, we evaluated how much the prevalence estimates and cohort characteristics changed if only at least one episode with an ICD-10 code for PsA/axSpA/RA was required, or if at least one episode with a diagnostic code was required to be recorded in either the internal medicine or rheumatology unit.
We approximated seropositivity in RA by ICD-10 codes (M05 for seropositive and M06 for seronegative RA). When both M05 and M06 were present, RA cases were defined as seropositive if the number of episodes with a recorded M05 code was equal to or exceeded the number with M06. To allow easier comparison with the previous literature, we also approximated the prevalence of ankylosing spondylitis (AS) and non-radiographic axSpA (nr-axSpA), defined by ICD-10 codes (M45 for AS; and M46.0, M46.1, M46.8, and M46.9 for nr-axSpA).
Use of DMARDs, NSAIDs, and glucocorticoids
To estimate the current use of DMARDs during 2017, we identified patients with any dispensed DMARD prescriptions from NorPD and/or bDMARDs given in hospitals from the NPR during the calendar year 2017 (ATC and NCMP codes in Supplementary Table S2). To be classified as a user of a certain DMARD, glucocorticoids, or NSAIDs, a patient had to have at least one dispensed prescription for the medicinal product of interest (or, in the case of bDMARDs, a dispensed prescription or a record of at least one bDMARD infusion in the NPR), during the 12 month period between 1 January and 31 December 2017. There was no lower limit for the number or size of packages purchased. For NSAIDs and glucocorticoids, only prescriptions for oral forms were included.
Statistical analysis
The point prevalence of PsA, axSpA, and RA, or any of these diseases, was estimated in the whole Norwegian adult population, by gender, age, educational level, and health region. The standardized prevalence by education level and health region was calculated using the direct standardization method (standardization by gender and age in 5 year age strata (18–24, 25–29, … ≥ 90 years). The Norwegian population aged ≥ 18 years on 1 January 2017 was the standard population. The 95% confidence intervals were based on the Poisson distribution. Statistical analyses were carried out using R version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
There were 4 094 425 people (alive and aged ≥ 18 years) in the Norwegian population register on 1 January 2017 (the population’s age structure is shown in Supplementary Figure S1). Of them, 18 896 (0.46%) had PsA, 16 948 (0.41%) had axSpA, 31 885 (0.78%) had RA, and 63 748 (1.56%) had either PsA, axSpA, or RA, based on two or more NPR episodes with a recorded diagnostic code for the disease of interest (base-case definition). Their age, gender, and use of DMARDs in 2017 are shown in . There was some overlap between PsA, axSpA, and RA diagnoses: 2013 patients had both at least two RA and PsA diagnoses (6.3% of RA patients and 10.7% of PsA patients), 1026 had both RA and axSpA diagnoses (3.2% of RA patients and 6.1% of axSpA patients), and 1104 had both PsA and axSpA diagnoses (5.8% of PsA patients and 6.5% of axSpA patients). Furthermore, 162 fulfilled case definition criteria for all three IJDs (0.9% of PsA patients, 1.0% of axSpA patients, and 0.5% of RA patients).
Table 1. Baseline characteristics, extent of overlap between inflammatory joint diseases (IJDs), and use of disease-modifying anti-rheumatic drugs (DMARDs) and glucocorticoids during 2017 (12 month period), based on both dispensed prescriptions and records of biological disease-modifying anti-rheumatic drugs (bDMARDs) given in hospitals.
Compared to the base-case definition, the prevalence estimates were 32%, 37%, and 27% higher for PsA, axSpA, and RA, respectively, when only one or more episode with a recorded diagnostic code for the IJD of interest was required (). The prevalence estimates changed by +10% for PsA, +12% for axSpA, and −0.5% for RA when we identified all patients with at least one episode with a recorded diagnostic code for the disease of interest in either the internal medicine of rheumatology unit, and by −9% for PsA, −8% for axSpA, and −11% for RA when we identified all patients with two or more episodes with a diagnostic code recorded anywhere, of which at least one had to be registered in either the internal medicine or the rheumatology unit. The characteristics for the cohorts based on these alternative definitions are shown in Supplementary Table S3. The patients who were excluded from the base-case definition because they had only one episode with a recorded diagnostic code for the IJD of interest were, on average, less often treated with DMARDs and bDMARDs, and more often had records of diagnostic codes for other IJDs.
Table 2. Prevalence of psoriatic arthritis (PsA), axial spondyloarthritis (axSpA), and rheumatoid arthritis (RA), by different case definitions.
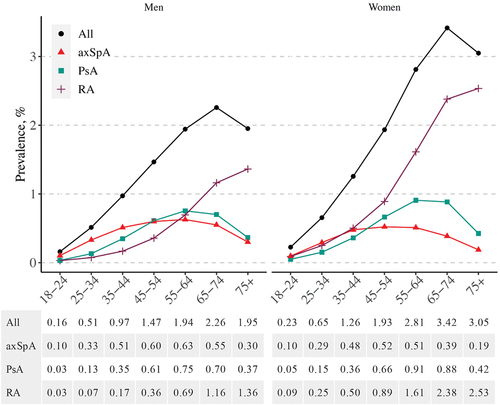
The prevalence of PsA was slightly higher among women compared to men, at 0.50% and 0.43%, respectively. The prevalence of axSpA was 0.37% among women and 0.45% among men. The prevalence of RA was higher, 1.10%, among women compared to 0.46% among men.
The prevalence of seropositive RA (M05) was 0.52% (21 238 cases) and the prevalence of seronegative RA (M06) was 0.26% (10 647 cases). Based on ICD-10 codes, the prevalence of AS (M45) was 0.28% (11 598 cases) and the prevalence of non-radiographic axSpA (M46.0, M46.1, M46.8, or M46.9) was 0.13% (5350 cases).
In both men and women, RA was clearly more common than PsA and axSpA among adults aged ≥ 65 years (). For women aged < 45 years, the prevalences of PsA, axSpA, and RA were quite similar, whereas among young men, axSpA was the most common diagnosis.
Figure 1. Point prevalence (%) of axial spondyloarthritis (axSpA), psoriatic arthritis (PsA), rheumatoid arthritis (RA), and any of these diseases on 1 January 2017, by age and gender.
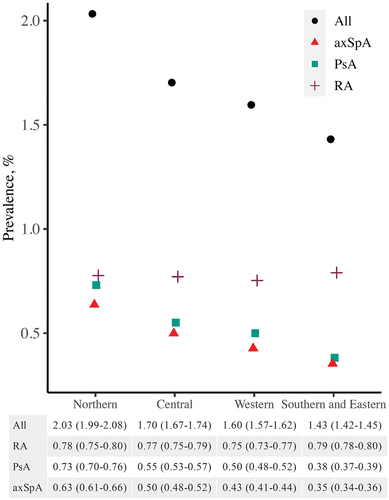
The estimated prevalence of PsA and RA was lower for people with higher education compared to upper secondary or below upper secondary education, whereas the prevalence of axSpA was more similar across educational levels (age- and gender-standardized prevalences in ; crude prevalences in Supplementary Table S4). AxSpA and PsA prevalence varied between the four health regions, with higher prevalence estimates in the north and lower estimates in the south, but RA prevalence was comparable across the health regions (age- and gender-standardized prevalences in ; crude prevalences in Supplementary Table S4).
Figure 2. Age- and gender-standardized point prevalence (%) of axial spondyloarthritis (axSpA), psoriatic arthritis (PsA), rheumatoid arthritis (RA), and any of these diseases (with 95% confidence intervals) on 1 January 2017, by level of education.
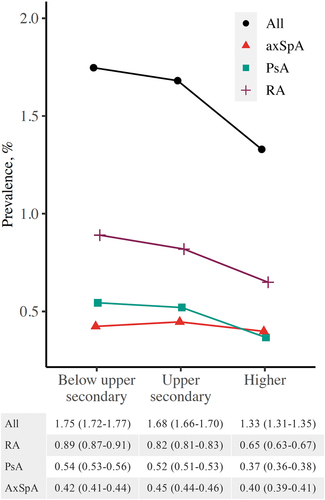
Figure 3. Age- and gender-standardized point prevalence (%) of axial spondyloarthritis (axSpA), psoriatic arthritis (PsA), rheumatoid arthritis (RA), and any of these diseases (with 95% confidence intervals) on 1 January 2017, by health region.
The proportion of IJD patients using different DMARDs and/or glucocorticoids in 2017 is shown in , and the use of DMARDs ever during the study period 2008–2017 is shown in Supplementary Table S5. Supplementary Figure S2 illustrates that the proportion of patients with use of any DMARD either during 2017 or ever during the study period 2008–2017 decreased with increasing age, especially after ≥ 65 years, in all three IJDs, whereas the proportion of patients with glucocorticoid use in 2017 increased with age.
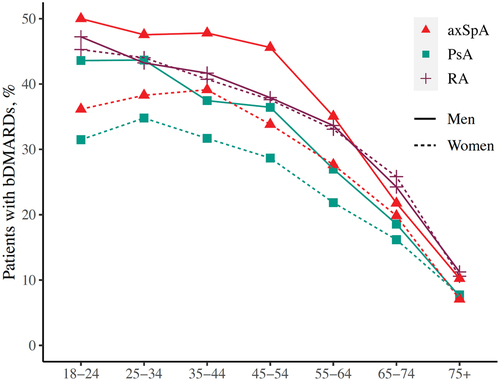
Up to 26% of PsA patients, 35% of axSpA patients, and 27% of RA patients used bDMARDs in 2017, based on both dispensed prescriptions from pharmacies and hospital procedure codes for bDMARDs. The proportions of bDMARD users ever in 2008–2017 were even higher, at 32%, 44%, and 36% for PsA, axSpA, and RA, respectively (Supplementary Table S5). Across the three IJDs and in both genders, the proportion of bDMARD users decreased steeply with age, especially after 55 years (). A similar proportion of men and women of the same age with RA used bDMARDs, whereas out of men and women of the same age with PsA or axSpA, a larger proportion of men used bDMARDs ().
Discussion
In this Norwegian nationwide register study, we showed that the prevalences of clinically diagnosed PsA, axSpA, and RA were 0.46%, 0.41%, and 0.78%, respectively, totalling 1.56% of the Norwegian adult population. Direct comparisons to studies with other methodological approaches are challenging, but compared to studies dating back to the 1990s and early 2000s from smaller Norwegian regions, our prevalence estimates were higher for all three diseases (Citation1–3, Citation5, Citation6). In contrast, compared to a more recent estimate of PsA prevalence in the Nord-Trøndelag Health Study 3 (HUNT 3) during 2006–2008, our PsA prevalence estimate was more comparable (0.55% in Central Norway in our data vs 0.67% in HUNT) (Citation4). Our results may indicate an increase in the prevalence of IJDs, which may be related to factors including ageing of the Norwegian population, improvements in early diagnostics, development of more sensitive classification criteria (Citation13–16), and, in the case of AS, recognition of nr-axSpA as part of the disease spectrum.
To our knowledge, our study is the first published nationwide prevalence study on not only AS but also nr-axSpA. Our estimate of AS prevalence, 0.28%, was quite well aligned with previous evidence: according to a systematic review of 36 studies, average AS prevalence in Europe was 0.24%, which was lower than in North America (0.32%) but higher than in Asia (0.17%), Latin America (0.10%), and Africa (0.07%) (Citation17). The prevalence of AS/axSpA is affected by the frequency of human leucocyte antigen (HLA)-B27 in the background population, which is especially frequent among the native populations of the arctic and subarctic regions of Eurasia and North America (Citation18).
As with axSpA prevalence, RA and PsA prevalences are known to be quite high in the Nordic countries compared to many other parts of the world (Citation19–21). The global prevalence of PsA was estimated to be 0.13% in a meta-analysis of 27 studies, which is substantially lower than our estimate of 0.46% (Citation19). For RA, our prevalence estimate of 0.78% was substantially higher than a pooled global RA prevalence estimate of 0.46%, reported by a meta-analysis published in 2021 (Citation22), and higher than the estimated RA prevalence of 0.25% by the Global Burden of Disease study (Citation21). Regardless, comparisons of prevalence estimates between studies should be interpreted with caution because of methodological differences, for example differences in data collection and case definitions, as well as differences in the age structure of the background population.
Our prevalence estimates for IJDs were also slightly higher than in recent Nordic studies. In Denmark, a nationwide register study showed that while PsA incidence increased rapidly between 1997 and 2011, PsA prevalence was 0.28% of Danes aged ≥ 18 years in 2012 (Citation23). Regarding RA, a Swedish nationwide study showed a slightly lower prevalence of RA in 2008 compared to ours in 2017: RA prevalence was 0.77% if all patients with at least one visit with an RA diagnosis were included (in our data, 0.99%), and only 0.59% if at least two visits with an RA diagnosis were required (in our data, 0.78%) (Citation24). The prevalence of AS in the Swedish population aged 16–64 years in 2009 was 0.18% (Citation25). For comparison, in our study, if only AS patients with ICD-10 code M45 on at least two occasions were included, the prevalence of AS in the population aged 16–64 years was 0.27%.
The age and gender distribution of RA and PsA prevalence followed previously described patterns (Citation23, Citation26). RA was over twice as common among women compared to men, and PsA was also slightly more common among women. Of our AS patients, men comprised 62.1%, and of all axSpA patients, 54.9%. For AS, a traditionally reported male:female ratio is around 3:1, but over time, this ratio has been decreasing, with up to 46% of AS patients being women in more recent studies (Citation20, Citation27).
RA and PsA were less prevalent among people with higher education compared to those with upper secondary or lower education. This has been previously shown by a few studies regarding RA (Citation24, Citation28), but not PsA. Educational inequalities in health may stem from physical, behavioural, and psychosocial risk factors (Citation29). Smoking is an established risk factor for RA, whereas alcohol consumption has an inverse association with RA incidence (Citation30). Heavy alcohol consumption and obesity are associated with PsA (Citation31, Citation32). These risk factors are also linked to lower educational level, and may partly explain our results.
We found differences in axSpA and PsA prevalence between Norwegian health regions, with higher prevalence estimates in the north compared to the south. Such a gradient was not apparent for RA. AxSpA and PsA are both spondyloarthritides and linked to HLA-B27: up to 90% of patients with axSpA, 60–70% of patients with axial PsA, and 25% of patients with peripheral PsA are HLA-B27 positive (Citation20). HLA-B27 is especially common in Northern Norway, and this may explain our results (Citation33). Studies from Northern Norway dating back to the 1980s have reported very high AS prevalence estimates: among a population of Samis in two municipalities, in whom the prevalence of HLA-B27 is 24%, the prevalence of AS was estimated to be as high as 1.8% (Citation34).
In general, IJD patients’ access to bDMARDs is good in Norway compared to many other European countries (Citation8, Citation9). Based on our results, up to 27% of Norwegian RA patients, 26% of PsA patients, and 35% of axSpA patients used bDMARDs in 2017. The proportion of IJD patients using bDMARDs decreased with age in both genders, especially after the age of 55 years. This finding of reduced likelihood of bDMARD initiation or longer delay to first bDMARD prescription with older age has been made previously in many other countries as well (Citation24, Citation35–38). This phenomenon may partly be related to concerns about adverse events, especially infection risk, among older adults (Citation39). Previous research suggests that multimorbidity, which is a common finding among older adults, may be associated with reduced initiation of bDMARD therapy (Citation40). However, since bDMARD penetration already started to decrease among relatively young patients, aged 55–64 years, we assume that this finding is not fully explained by severe comorbidities that would hinder the use of bDMARDs. While unravelling the potential causes for this gradient in bDMARD use by age is outside the scope of this study, our results may suggest a disadvantage in access to bDMARDs for older IJD patients, and display an unmet need in treatment.
In RA, we found no gender differences in bDMARD use, but for PsA and axSpA, men used bDMARDs more often than women. Similarly, in a Swedish nationwide study on AS patients, men were more often treated with tumour necrosis factor (TNF) inhibitors compared to women (Citation25). Men with axSpA may more often have HLA-B27 and present with more imaging abnormalities despite similar symptom duration compared to women with axSpA (Citation41). The progression of spinal ankylosis is faster among men compared to women with AS (Citation27). These differences in the natural course of axSpA may explain the observed difference in received treatment.
The proportions of RA, PsA, and axSpA patients using any DMARD during 2017 were 66.5%, 56.4%, and 41.3%, respectively. Among RA patients aged < 65 years, this proportion was approximately 75%. This proportion may seem low, but is well aligned with previous Scandinavian estimates among prevalent RA patients (Citation24). In addition to the possibility of drug-free remission, these rates may be affected by poor drug adherence, side effects, contraindications, or significant comorbidities that prevent the use of DMARDs. Simultaneously with a drop in any DMARD and bDMARD use in older age groups, the proportion of patients with dispensed oral glucocorticoids increased with age. This may demonstrate another gap in the care of older IJD patients, since glucocorticoids should be used sparingly also among the elderly because of their notorious side effects (Citation42).
The main strengths of our study are its nationwide setting, including both public and private specialized care, and access to data on both dispensed prescriptions and hospital-administered bDMARDs. Our prevalence estimates reflect the diagnostic prevalence of IJDs, i.e. the proportion of people who have a known diagnosis of the disease, but fail to include patients with undiagnosed disease. This may be especially relevant for axSpA and PsA, in which diagnostic delays are known to be significant and underdiagnosis common (Citation43, Citation44). In addition, our study fails to identify IJD patients with no contacts with specialized care in 2008–2017, perhaps leading to more likely exclusion of patients with longer disease duration and/or milder disease course. This may also be reflected in the decreasing prevalence of PsA and axSpA in the older age groups of ≥ 65 years, which has also been seen in previous research (Citation23). Moreover, the patients over 65, and especially over 75 years of age in 2017 have lived their young adulthood in an era with less developed diagnostics for PsA and axSpA. Thus, more cases with milder forms of SpA may have gone undiagnosed at that time.
Our case identification relied on ICD-10 codes recorded by physicians, and we lack data on the fulfilment of classification criteria for PsA, axSpA, and RA, as well as data on clinical investigations or data collected straight from the study subjects. We have not validated our case identification method in the NPR, which is an important limitation. The positive predictive values (PPVs) for IJD diagnostic codes in similar patient registries from other Scandinavian countries have varied, with PPVs as high as 82–90% for RA, 79–89% for axSpA, and 63–92% for PsA (Citation45–48). From the NPR, we included only patients with at least two recorded diagnostic codes for the index disease, because it has previously been shown that in similar administrative patient registers, requiring diagnostic codes to appear multiple times improves specificity (Citation45, Citation47, Citation49). We believe that by requiring two or more visits with a diagnostic code for the index disease we have reduced the probability of overlapping IJD diagnoses due to unintentional coding errors. Some of the observed overlap between IJDs may not represent true overlap but changes in diagnosis over time, especially regarding the overlap of RA and PsA. On the other hand, PsA and axSpA belong under the same umbrella term of SpA, and their overlap is expected, to some extent. Since axial PsA and AS with psoriasis can be hard to distinguish even in a clinical setting (Citation50), we decided to include in both disease groups the approximately 6% of PsA and axSpA patients who had diagnostic codes for both diseases, although we recognize that in real life, some of these patients are likely to have had a diagnosis of PsA with axial involvement.
Conclusion
The prevalences of clinically diagnosed PsA, axSpA, and RA in Norway were 0.46%, 0.41%, and 0.78%, respectively. Especially for RA and axSpA, these estimates are higher than in previous Norwegian studies from earlier decades (Citation1, Citation2, Citation6), and highlight that IJDs demand considerable resources from the Norwegian healthcare system. Of all PsA, axSpA, and RA patients, 28% used bDMARDs in 2017, corresponding to over 0.4% of Norwegian adults, which reflects good overall access to bDMARDs in Norway. However, a clear gradient of decreasing bDMARD use with older age was observed in all three IJDs, potentially indicating an age inequality in treatment.
Supplemental Material
Download MS Word (326.4 KB)Acknowledgements
We sincerely thank Trude Solbakken from the Norwegian Directorate of Health, Fredrick Blikeng from Statistics Norway, and Maria Amberger from NorPD for their kind collaboration in extracting the register data, as well as health project advisor Merete Tholin from Norsk Revmatikerforbund, interest policy advisor Ann Kristin Bakke from Norsk Revmatikerforbund, and Thalita Blanck from Diakonhjemmet Hospital’s REVMA Pasientråd for their kind help with the dissemination and communication of our results to patients and their spouses.
Disclosure statement
AMK has received speaker fees from Boehringer-Ingelheim, has attended advisory boards of Pfizer, Gilead and Boehringer-Ingelheim, and received congress sponsorship from Pfizer, Celgene, UCB Pharma, Mylan and Roche. SR: None. AK: None. GW: None. JS: None. NM: None. MK: None. TK has received fees for speaking from Amgen, Celltrion, Egis, Evapharma, Ewopharma, Hikma, Oktal, Sandoz, Sanofi; fees for consulting from AbbVie, Amgen, Biogen, Celltrion, Eli Lilly, Gilead, Mylan, Novartis, Pfizer, Sandoz, Sanofi and research funding to Diakonhjemmet Hospital from AbbVie, Amgen, BMS, MSD, Novartis, Pfizer and UCB. EH has received consulting fees from Abbvie, Novartis, Pfizer, Gilead, Eli Lilly, Galapagos, Janssen and speaking fees from Pfizer and Abbvie. AGS has received speaker honoraria and/or consulting fees from: AbbVie, Bayer, Lilly, Novartis, and Sanofi, and a collaborative agreement for independent research from Eli Lilly who had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/03009742.2021.1997436.
Additional information
Funding
References
- Kvien TK, Glennås A, Knudsrød OG, Smedstad LM, Mowinckel P, Førre Ø. The prevalence and severity of rheumatoid arthritis in Oslo: results from a county register and a population survey. Scand J Rheumatol 1997;26:412–8.
- Riise T, Jacobsen BK, Gran JT. Incidence and prevalence of rheumatoid arthritis in the county of Troms, northern Norway. J Rheumatol 2000;27:1386–9.
- Madland TM, Apalset EM, Johannessen AE, Rossebö B, Brun JG. Prevalence, disease manifestations, and treatment of psoriatic arthritis in Western Norway. J Rheumatol 2005;32:1918–22.
- Hoff M, Gulati AM, Romundstad PR, Kavanaugh A, Haugeberg G. Prevalence and incidence rates of psoriatic arthritis in central Norway: data from the Nord-Trøndelag Health Study (HUNT). Ann Rheum Dis 2015;74:60–4.
- Nossent JC, Gran JT. Epidemiological and clinical characteristics of psoriatic arthritis in northern Norway. Scand J Rheumatol 2009;38:251–5.
- Bakland G, Nossent HC, Gran JT. Incidence and prevalence of ankylosing spondylitis in northern Norway. Arthritis Rheum 2005;53:850–5.
- Gran JT, Husby G, Hordvik M. Prevalence of ankylosing spondylitis in males and females in a young middle-aged population of Tromso, northern Norway. Ann Rheum Dis 1985;44:359–67.
- Putrik P, Ramiro S, Kvien TK, Sokka T, Pavlova M, Uhlig T, et al. Inequities in access to biologic and synthetic DMARDs across 46 European countries. Annals Rheum Dis 2014;73:198–206.
- Kaló Z, Vokó Z, Östör A, Clifton-Brown E, Vasilescu R, Battersby A, et al. Patient access to reimbursed biological disease-modifying antirheumatic drugs in the European region. J Mark Access Health Policy 2017;5:1345680.
- Glintborg B, Lindström U, Aaltonen K, Kristianslund EK, Gudbjornsson B, Chatzidionysiou K, et al. Biological treatment in ankylosing spondylitis in the Nordic countries during 2010–2016: a collaboration between five biological registries. Scand J Rheumatol 2018;47:465–74.
- Lie E, Fagerli KM, Mikkelsen K, Rødevand E, Lexberg Å, Kalstad S, et al. First-time prescriptions of biological disease-modifying antirheumatic drugs in rheumatoid arthritis, psoriatic arthritis and axial spondyloarthritis 2002-2011: data from the NOR-DMARD register. Ann Rheum Dis 2014;73:1905–6.
- Mangerud W, Kjelvik M, Krokan T. Aktivitetsdata for somatisk spesialisthelsetjeneste 2017: norsk pasientregister [in Norwegian]. Helsedirektoratet. IS-2711. 2018. Cited 2021 Jun 1. Available from: https://www.helsedirektoratet.no/rapporter/aktivitetsdata-for-somatisk-spesialisthelsetjeneste/Aktivitetsdata%20for%20somatisk%20spesialisthelsetjeneste%202017.pdf/_/attachment/inline/ec8ed607-d173-4e5f-9489-8264a93231ad:949f3925cf6df6c4a1dca4d5e147282b1a47dc9a/Aktivitetsdata%20for%20somatisk%20spesialisthelsetjeneste%202017.pdf
- Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83.
- Rudwaleit M, Landewé R, van der Heijde D, Listing J, Brandt J, Braun J, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis 2009;68:770–6.
- Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–73.
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81.
- Dean LE, Jones GT, Macdonald AG, Downham C, Sturrock RD, Macfarlane GJ. Global prevalence of ankylosing spondylitis. Rheumatology (Oxford) 2014;53:650–7.
- Gabriel SE, Michaud K. Epidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseases. Arthritis Res Ther 2009;11:229.
- Scotti L, Franchi M, Marchesoni A, Corrao G. Prevalence and incidence of psoriatic arthritis: a systematic review and meta-analysis. Semin Arthritis Rheum 2018;48:28–34.
- Stolwijk C, Boonen A, van Tubergen A, Reveille JD. Epidemiology of spondyloarthritis. Rheum Dis Clin North Am 2012;38:441–76.
- Safiri S, Kolahi AA, Hoy D, Smith E, Bettampadi D, Mansournia MA, et al. Global, regional and national burden of rheumatoid arthritis 1990-2017: a systematic analysis of the Global Burden of Disease study 2017. Annals Rheum Dis 2019;78:1463–71.
- Almutairi K, Nossent J, Preen D, Keen H, Inderjeeth C. The global prevalence of rheumatoid arthritis: a meta-analysis based on a systematic review. Rheumatol Int 2021;41:863–77.
- Egeberg A, Kristensen LE, Thyssen JP, Gislason GH, Gottlieb AB, Coates LC, et al. Incidence and prevalence of psoriatic arthritis in Denmark: a nationwide register linkage study. Ann Rheum Dis 2017;76:1591–7.
- Neovius M, Simard JF, Askling J. Nationwide prevalence of rheumatoid arthritis and penetration of disease-modifying drugs in Sweden. Ann Rheum Dis 2011;70:624–9.
- Exarchou S, Lindström U, Askling J, Eriksson JK, Forsblad-d’Elia H, Neovius M, et al. The prevalence of clinically diagnosed ankylosing spondylitis and its clinical manifestations: a nationwide register study. Arthritis Res Ther 2015;17:118.
- Hunter TM, Boytsov NN, Zhang X, Schroeder K, Michaud K, Araujo AB. Prevalence of rheumatoid arthritis in the United States adult population in healthcare claims databases, 2004–2014. Rheumatol Int 2017;37:1551–7.
- Feldtkeller E, Bruckel J, Khan MA. Scientific contributions of ankylosing spondylitis patient advocacy groups. Curr Opin Rheumatol 2000;12:239–47.
- Bae SC, Lee YH. Causal relationship between years of education and the occurrence of rheumatoid arthritis. Postgrad Med J 2019;95:378–81.
- Kurtze N, Eikemo TA, Kamphuis CBM. Educational inequalities in general and mental health: differential contribution of physical activity, smoking, alcohol consumption and diet. Eur J Public Health 2013;23:223–9.
- Hedenstierna L, Bellocco R, Ye W, Adami HO, Åkerstedt T, Trolle Lagerros Y, et al. Effects of alcohol consumption and smoking on risk for RA: results from a Swedish prospective cohort study. RMD Open 2021;7:e001379.
- Solmaz D, Eder L, Aydin SZ. Update on the epidemiology, risk factors, and disease outcomes of psoriatic arthritis. Best Pract Res Clin Rheumatol 2018;32:295–311.
- Ogdie A, Weiss P. The Epidemiology of Psoriatic Arthritis. Rheum Dis Clin North Am 2015;41:545–68.
- Johnsen SS, Bakland G, Nossent JC. The distribution of HLA-B27 subtype in patients with ankylosing spondylitis in Northern Norway. Scand J Rheumatol 2014;43:296–300.
- Johnsen K, Gran JT, Dale K, Husby G. The prevalence of ankylosing spondylitis among Norwegian Samis (Lapps). J Rheumatol 1992;19:1591–4.
- Putrik P, Ramiro S, Lie E, Keszei AP, Kvien TK, van der Heijde D, et al. Less educated and older patients have reduced access to biologic DMARDs even in a country with highly developed social welfare (Norway): results from Norwegian cohort study NOR-DMARD. Rheumatology (Oxford) 2016;55:1217–24.
- Tatangelo M, Tomlinson G, Paterson JM, Ahluwalia V, Kopp A, Gomes T, et al. Association of patient, prescriber, and region with the initiation of first prescription of biologic disease-modifying antirheumatic drug among older patients with rheumatoid arthritis and identical health insurance coverage. JAMA Netw Open 2019;2:e1917053.
- Kim G, Barner JC, Rascati K, Richards K. Factors associated with the initiation of biologic disease-modifying antirheumatic drugs in Texas Medicaid patients with rheumatoid arthritis. J Manag Care Spec Pharm 2015;21:401–7.
- Radovits BJ, Fransen J, Eijsbouts A, van Riel PL, Laan RF. Missed opportunities in the treatment of elderly patients with rheumatoid arthritis. Rheumatology (Oxford) 2009;48:906–10.
- Borren NZ, Ananthakrishnan AN. Safety of biologic therapy in older patients with immune-mediated diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2019;17:1736–1743.e4.
- Armagan B, Sari A, Erden A, Kilic L, Erdat EC, Kilickap S, et al. Starting of biological disease modifying antirheumatic drugs may be postponed in rheumatoid arthritis patients with multimorbidity: single center real life results. Medicine (Baltimore) 2018;97:e9930.
- Ortolan A, van Lunteren M, Ramiro S, Ramonda R, Landewé RBM, Dagfinrud H, et al. Are gender-specific approaches needed in diagnosing early axial spondyloarthritis? Data from the SPondyloArthritis Caught Early cohort. Arthritis Res Ther 2018;20:218.
- Lahaye C, Tatar Z, Dubost JJ, Tournadre A, Soubrier M. Management of inflammatory rheumatic conditions in the elderly. Rheumatology (Oxford) 2019;58:748–64.
- Danve A, Deodhar A. Axial spondyloarthritis in the USA: diagnostic challenges and missed opportunities. Clin Rheumatol 2019;38:625–34.
- Villani AP, Rouzaud M, Sevrain M, Barnetche T, Paul C, Richard MA, et al. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: systematic review and meta-analysis. J Am Acad Dermatol 2015;73:242–8.
- Löfvendahl S, Theander E, Svensson Å, Carlsson KS, Englund M, Petersson IF. Validity of diagnostic codes and prevalence of physician-diagnosed psoriasis and psoriatic arthritis in southern Sweden – a population-based register study. PLoS One 2014;9:e98024.
- Lindström U, Exarchou S, Sigurdardottir V, Sundström B, Askling J, Eriksson JK, et al. Validity of ankylosing spondylitis and undifferentiated spondyloarthritis diagnoses in the Swedish National Patient Register. Scand J Rheumatol 2015;44:369–76.
- Paltta J, Heikkilä H, Pirilä L, Eklund K, Huhtakangas J, Isomäki P, et al. The validity of rheumatoid arthritis diagnoses in Finnish biobanks. Scand J Rheumatol 2021 ;1–9.
- Eriksson JK, Neovius M, Ernestam S, Lindblad S, Simard JF, Askling J. Incidence of rheumatoid arthritis in Sweden: a nationwide population-based assessment of incidence, its determinants, and treatment penetration. Arthritis Care Res (Hoboken) 2013;65:870–8.
- Curtis JR, Harrold LR, Asgari MM, Deodhar A, Salman C, Gelfand JM, et al. Diagnostic prevalence of ankylosing spondylitis using computerized health care data, 1996 to 2009: underrecognition in a US Health Care Setting. Perm J 2016;20:15–151.
- Feld J, Chandran V, Haroon N, Inman R, Gladman D. Axial disease in psoriatic arthritis and ankylosing spondylitis: a critical comparison. Nat Rev Rheumatol 2018;14:363–71.