Abstract
Objective
To evaluate trends of acute myocardial infarction (AMI) and ischaemic heart disease (IHD) in rheumatoid arthritis (RA) patients compared with the general population over time.
Method
We performed a retrospective cohort study of 1821 RA patients diagnosed from 1972 to 2013. Aggregated counts of the total population of the same county (Hordaland, Norway) and period were used for comparison. Information on AMI and IHD events was obtained from hospital patient administrative systems or cardiovascular registries. We estimated incidence rates and excess of events [standardized event ratio (SER) with 95% confidence interval (CI)] compared with the general population by Poisson regression.
Results
There was an average annual decline of 1.6% in age- and gender-adjusted AMI incidence rates from 1972 to 2017 (p < 0.035). The difference in events (excess events) in RA patients compared with the general population declined on average by 1.3% per year for AMI and by 2.3% for IHD from 1972 to 2014. There were no significant excess AMI (SER 1.05, 95% CI 0.82–1.35) or IHD events (SER 1.02, 95% CI 0.89–1.16) for RA patients diagnosed after 1998 compared with the general population.
Conclusion
Incidence rates and excess events of AMI and IHD in RA patients declined from 1972 to 2017. There were no excess AMI or IHD events in RA patients diagnosed after 1998 compared with the general population.
Rheumatoid arthritis (RA) patients have 1.5–2.0 times increased risk of acute myocardial infarction (AMI) and ischaemic heart disease (IHD) compared with the general population (Citation1, Citation2). Some studies indicate that cardiovascular disease (CVD) risk could be increased even before the diagnosis of RA (Citation3, Citation4), and a study on blood donors found an atherogenic lipid profile up to 10 years before the onset of RA symptoms (Citation5). However, two large population-based case–control studies from Sweden found no increased occurrence of IHD before the onset of RA symptoms (Citation6). Mortality rates for RA patients are about 50% higher than in the general population, and CVD is the primary cause of death in about 40% of patients (Citation7).
RA involves systemic inflammation that favours the development of atherosclerosis. The AMI risk and development of atherosclerosis in RA increase with higher levels of inflammation and higher disease activity (Citation8–12). Treatment of RA with disease-modifying anti-rheumatic drugs (DMARDs) reduces inflammation and disease activity, and is associated with a lower AMI risk (Citation13–17).
RA treatment has improved vastly over the past two decades, in particular owing to the focus on early and aggressive treatment, tight control strategies, and the use of biological DMARDs for patients with insufficient response to synthetic DMARDs. Several studies have found higher rates of remission and better long-term outcomes in patients with early introduction of DMARDs (Citation18). This window of opportunity may be a critical phase for intervention against the development of atherosclerosis in RA. Some studies suggest a decline in the overall mortality gap between RA patients and the general population in the twenty-first century, which parallels the improvement in RA treatment (Citation19–24). Other studies still found excess mortality in RA patients during the twenty-first century (Citation25, Citation26). However, there is little information about the AMI and IHD risk in RA patients diagnosed after the introduction of modern RA treatment.
Our aim was therefore to assess whether the introduction of improved RA treatment was associated with a lower risk of AMI and IHD. We evaluated trends in AMI and IHD incidence in RA patients diagnosed from 1972 to 2013. RA patients were compared with the entire population of the same county and period to estimate possible excess AMI and IHD events and to adjust for secular changes in the diagnosis of IHD. The estimates of excess events were stratified by time of RA diagnosis to address possible RA treatment-related changes.
Method
Study design and setting
We performed a retrospective cohort study of 1821 RA patients compared with aggregated counts of the total population from the same county (Hordaland, Norway) during 1972–2014 to investigate the possible excess of AMI and IHD events in RA patients. We also evaluated time trends of AMI and IHD events for RA patients from 1972 to 2017.
RA patient cohort
We evaluated all outpatient contacts or hospital admissions with an RA diagnosis at the Department of Rheumatology, Haukeland University Hospital, in the period 1972–2013. Almost all RA patients in Hordaland county are treated at this hospital. The selection process is shown in and explained in detail in the Supplementary material, along with a comparison between the included and excluded RA patients. We obtained consent from 2649 patients through the Norwegian Arthritis Registry (n = 1456) and letters of consent from patients not included in the Norwegian Arthritis Registry (n = 214). Exemption from consent was allowed for patients who were dead (n = 979), by the Regional Committee for Medical and Health Research Ethics. The included RA patients were diagnosed during 1972–2013 by the treating rheumatologist. Most RA patients are in secondary care for several years. We therefore excluded patients with fewer than five hospital contacts, assuming them to have been initially misdiagnosed, miscoded, or treated at a different institution.
Figure 1. Selection of patients with rheumatoid arthritis (RA), Hordaland, Western Norway. NorArthritis, the Norwegian Arthritis Registry.
Data on AMI and IHD events in RA patients were obtained from the Western Norway Cardiovascular Registry (WENOCARD) (1972–2006) and from the hospital’s patient administrative system (2007–2017) (Citation27, Citation28). We only included events where Hordaland was the current county of residence. Cause of death was obtained from the Norwegian Cause of Death Registry (Citation29). The Norwegian personal identification number, unique to each Norwegian resident, was used to link the data sources.
Comparison cohort
The entire population of Hordaland county, including RA patients, was used as a comparison cohort. Aggregated population counts and data on AMI and IHD events were obtained from WENOCARD (1972–2006) and the Cardiovascular Diseases in Norway project (CVDNOR) (2007–2014) (27, 28). We did not have data beyond 2014 for the comparison cohort. All data for the comparison cohort were aggregated by year, gender, and 5 year age groups. A description of WENOCARD and CVDNOR can be found in the Supplementary material.
Definition of outcomes
The outcome of our study was AMI and IHD events. Incident events were used to estimate time trends in AMI and IHD, while multiple events were used to evaluate time trends in excess events compared with the general population, as well as to estimate standardized event ratios (SERs).
An incident event of AMI was defined as a hospitalization with AMI [International Classification of Diseases (ICD)-8/9: 410; and ICD-10: I21, I22] as primary or secondary discharge diagnosis or death from AMI (ICD-8/9: 410–414; and ICD-10: I20–I25) without any AMI hospitalizations prior to RA diagnosis (Citation30). An incident event of IHD (ICD-8/9: 410–414; and ICD-10; I20–I25) was defined as hospitalization with IHD as primary or secondary diagnosis or death with IHD as the underlying cause of death without any IHD hospitalizations prior to RA diagnosis. Consequently, the IHD outcome included both AMI and chronic coronary heart disease (e.g. angina).
When counting multiple AMI events and IHD events per person, a new hospitalization was counted as a new event if it occurred > 28 days after the previous hospitalization. If the new hospitalization occurred ≤ 28 days after the previous one, it was defined as a part of the previous event (27).
Data on AMI and IHD events for the RA and comparison cohort were collected from different sources, but are all based on the same discharge diagnosis codes from hospitals in Western Norway. A comparison between WENOCARD and CVDNOR showed little deviation of AMI counts in the overlapping period (1994–2006) of 0–1.06% (Citation31).
Statistics
We estimated a required sample size of 7769 person-years’ follow-up for RA and non-RA patients to detect a standardized incidence ratio of 1.5 with 95% confidence and 80% power under the assumption of an incidence rate of 50 per 10 000 in non-RA patients.
The RA patients were divided into three cohorts by period of RA diagnosis according to the main RA treatment trends: 1972–1985 (pre-methotrexate era), 1986–1998 (methotrexate era), and 1999 onwards (biologics era). Continuous variables are presented as means with standard deviations and categorical variables as numbers and proportions. For baseline characteristics of RA patients (), trends were estimated by linear regression for continuous variables, logistic regression for binary variables, and ordinal logistic regression for variables with multiple categories. Missing values were excluded from the analyses.
Table 1. Baseline characteristics of patients with rheumatoid arthritis (RA) according to time of diagnosis, Hordaland, Western Norway.
Time trends in AMI and IHD incidence were explored during 1972–2017 () for the total RA cohort. Incidence rate ratios (IRRs) and 95% confidence intervals (CIs) per 1 year change in calendar time were estimated using Poisson regression with year included as a continuous independent variable and adjustment for age, body mass index (BMI), serological status, and smoking status (and gender for total estimates). Annual percentage changes in rates were calculated by subtracting IRR from 1. Person-years were calculated from 1 January of the year in which RA was diagnosed to the first event, death, or end of the study period (31 December 2017). We also performed a subanalysis of time trends from 2002 to 2017, because of the introduction of troponins in the diagnosis of AMI at the beginning of the twenty-first century. Missing values for BMI, serological status, and smoking status were noted in less than 5% of cases and replaced by multiple imputation using the mi function in STATA, with weight and gender as additional predictors.
Table 2. Annual change in rates of acute myocardial infarction (AMI) and ischaemic heart disease (IHD) for rheumatoid arthritis patients, 1972–2017.
SERs were estimated by Poisson regression () from counts of the total and RA population in Hordaland, Norway, at the middle of each calendar year. SERs are similar to standardized mortality ratios (SMRs) but with events as the outcome of interest instead of deaths. An SER of 2 indicates that twice as many events were observed in the RA cohort as expected based on age-, gender-, and calendar year-specific rates from the total population. Specific reference rates for each calendar year, gender, and 5 year age interval from the total population were used to estimate expected counts of AMI and IHD in the RA cohort. The expected counts were included in the Poisson model as an offset to obtain indirect standardization. The model was not adjusted for additional variables such as smoking, since they were unavailable for the total population. We calculated separate estimates for the total follow-up period (total estimate) and for events occurring during three consecutive periods (1972–1985, 1986–1998, and 1999–2014) for the total RA cohort and for the three cohorts defined by time of RA diagnosis. Separate estimates were also calculated for men, women, and three age groups. There were no missing values in these analyses. Sensitivity analyses were performed, restricting the analysis to RA patients fulfilling the American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) criteria.
Time trends in excess events of AMI and IHD in RA patients compared with the general population were explored by Poisson regression during 1972–2014. Counts of events in the RA population were specified as the outcome, calendar year was included as a continuous variable, and expected counts was included as an offset. The exponentiated regression coefficient for calendar year can be interpreted as a ratio of event ratios (ERR) for annual relative change in SER, assuming a log-linear trend over calendar years, reported with 95% CIs. There were no missing values in these analyses.
We used robust standard errors to calculate 95% CIs when using Poisson regression to adjust for overdispersion. A p-value below 0.05 was considered significant.
Analyses were conducted using Stata Statistical Software (Release 16; StataCorp, College Station, TX, USA) and R version 3.6.3.
Results
Patient characteristics
Age, body mass index (BMI), prevalence of diabetes, and use of anti-hypertensives and statins at the time of RA diagnosis increased across the diagnostic cohorts ().
The total RA cohort had a follow-up time of 23 808 person-years from RA diagnosis to death or 31 December 2014, while the diagnostic cohorts (1972–1985, 1986–1998, and 1999–2013) had, respectively, 5600, 9536, and 8672 person-years of follow-up.
AMI and IHD incidence rates
In total, 240 incident AMI and 327 incident IHD events occurred in the 1821 RA patients from 1972 to 2017 during, respectively, 25 890 and 24 345 person-years of observation.
There was an average annual decline in the AMI incidence rates of 1.6% (IRR 0.986, 95% CI 0.970–0.999) adjusted for age, gender, BMI, and serological and smoking status (). Similarly, adjusted IHD incidence rates declined on average by 3.2% per year (IRR 0.968, 95% CI 0.958–0.978). On average, there was an increase in the AMI and IHD incidence in RA patients older than 85 years (), mostly in men. Furthermore, there was no decline in AMI and IHD incidence in women aged 16–64 years. Trends in AMI and IHD incidence in the 2002–2017 subgroup (Supplementary Table 1) were similar to those in the total period (1972–2017).
Excess AMI and IHD events
In total, 269 AMI and 1325 IHD events occurred from 1972 to 2014 in the RA cohort, while 35 614 AMI and 126 974 IHD events occurred in the general population. The overall SER in RA patients compared with the general population was 1.49 (95% CI 1.30–1.69) for AMI and 1.63 (95% CI 1.52–1.74) for IHD from 1972 to 2014. There were no excess events in patients older than 85 years. When stratifying by diagnostic cohort and year of event (), significant excess events of AMI and IHD were seen in the total estimates for the two first diagnostic cohorts, but not in the 1999–2013 cohort (AMI: SER 1.05, 95% CI 0.82–1.35; IHD: SER 1.02, 95% CI 0.89–1.16). A subanalysis which included only patients diagnosed with RA during 1999–2009 revealed similar results to the 1999–2013 RA cohort (numbers not shown). There were significant excess IHD events, but no excess AMI events for patients in the early phase of RA diagnosed during 1985–1998. Exclusion of autoantibody-negative patients or patients not fulfilling the ACR/EULAR criteria did not change the estimates significantly. Men and women had similar excess events in total and in the three diagnostic cohorts.
Figure 2. Excess acute myocardial infarction (AMI) and ischaemic heart disease(IHD) events in rheumatoid arthritis (RA) patients compared with the general population. RA patients are divided into three groups by time of RA diagnosis (A: 1972–1985; B: 1986–1998; and C: 1999–2013). Excess events are estimated for three separate periods (1972–1985, 1986–1998, and 1999–2014) and the entire follow-up period (total estimate). Point estimates are standardized event ratios comparing the number of events in the RA cohort to the expected number of events calculated from reference rates in the general population and standardized for age, gender, and year of the event. All estimates are given with robust 95% confidence intervals. Both the RA patients and the general population were obtained from Hordaland, Western Norway.
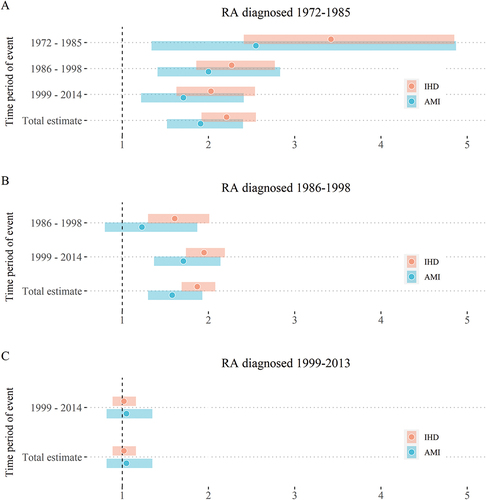
There was an average annual decline in the excess events of 1.3% for AMI (IRR 0.987, 95% CI 0.972–1.002) and 2.3% for IHD (ERR 0.977, 95% CI 0.69–0.985).
Discussion
In this large and long-term RA cohort, we found no excess of AMI and IHD events in patients diagnosed from 1999 onwards, while patients diagnosed before 1999 had a significant excess of events compared with the general population. Furthermore, from 1972 to 2014 there was a decline in the excess of AMI and IHD events in RA patients compared with the general population. The estimates of excess events were similar for both genders and autoantibody-positive RA patients compared with the total RA cohort. In addition, we found a significant overall decline in the incidence rates of AMI and IHD among RA patients from 1972 to 2017. However, patients older than 85 years experienced an increase in AMI incidence rates.
Most previous studies have included patients with long-standing RA, diagnosed before the introduction of modern RA treatment, which could explain excess AMI risk found after the year 2000 (1, 2, 16, Citation32–36). Two recent studies found an increased risk of AMI for the total RA cohort but not for subgroups of incident RA (Citation37, Citation38). Myasoedova et al found no excess in CVD in RA patients diagnosed in the 2000s compared with non-RA patients (Citation39). We similarly found an excess of AMI and IHD events compared with the general population for the total RA cohort, but not for RA patients diagnosed after 1998. Both Holmqvist et al and Lindhardsen et al found an elevated risk of AMI in incident RA cohorts diagnosed from 1995 and 1997 to 2006 compared with the general population (Citation40, Citation41). In the study by Lindhardsen et al, the risk estimate in RA patients was comparable to that of patients with diabetes mellitus. Yazdani et al found an elevated AMI risk in RA patients diagnosed from 1997 to 2004 compared with the general population during a 10 year follow-up period (25). As the treat-to-target strategy was not implemented at the time of RA diagnosis in these cohorts, the rates of patients in remission were probably lower than for patients with a later diagnosis, treated according to new treatment strategies (Citation42, Citation43). This could be a possible explanation for the elevated AMI risks in these studies, as RA patients in remission appear to have a CVD risk similar to the general population (10).
Treatment with methotrexate and biologics is associated with a lower risk of cardiovascular events (13–17), while the use of non-steroidal anti-inflammatory drugs (NSAIDs) and corticosteroids is associated with higher CVD risk (13). Both increased use of methotrexate and biologics, and a decreased use of NSAIDs and corticosteroids over time are evident in our cohort, as shown in a previous study (Citation44). This could be one of several possible explanations for the lower AMI and IHD occurrence in RA patients diagnosed after 1998 compared with the general population.
A study by Crowson et al established that smoking, hypertension, and dyslipidaemia contributed more to the CVD risk in RA patients than RA characteristics (Citation45). Changes in the prevalence of traditional CVD risk factors over time could explain some of our findings. Unfortunately, we did not have data on hypertension and dyslipidaemia in the RA cohort or general population, and had smoking data only for RA patients. The general population in Norway has shown a significant decline in the proportion of active smokers, from 41% in 1972 to 15% in 2013 (Citation46). Since smoking is both a known CVD risk factor and associated with less response to DMARDs and more severe RA (8–11), smoking cessation could be more effective in lowering the CVD risk in RA patients compared with the general population. Myasoedova et al recently published findings similar to ours, where adjustment for smoking, hypertension, and dyslipidaemia attenuated associations for excess cardiovascular events in RA compared with non-RA patients in 1980 and 1990, but not 2000 (39). Changes in the prevalence of smoking over time could therefore partly explain our findings, but are unlikely to be the sole reason for the decrease in excess AMI and IHD events across diagnostic cohorts.
Our findings are in line with several studies that did not find excess cardiovascular mortality after 2004 (19, 21, 23, 24, Citation47). The decline in mortality was attributed to improved RA treatment, but could also be due to improved treatment and primary prevention of CVD, which may have influenced RA patients to a greater extent than non-RA individuals. Mortality data tend to underestimate IHD, and hospitalization data are preferable due to a higher positive predictive value (PPV) for AMI (Citation48, Citation49). Our study corroborates the significant decline in CVD in RA patients in the twenty-first century using data from hospital records. Overall, there was a decline in AMI and IHD incidence rates in RA patients during the study period. This matches findings in the general population of Norway and other countries in Europe (Citation50–52). However, we found an increase in elderly RA patients, which is likely to be due to non-RA-related factors since there were no excess events in this age group. Possible explanations could be a lower threshold for hospitalization of older patients and increased detection of type 2 AMI after the introduction of troponins. Similar findings have been reported in the general population in Denmark and the UK (Citation53, Citation54). The lack of a declining trend seen in younger women with RA is in accordance with several previous studies of the general population showing an increasing trend in younger women from around 1988 until 2009 (27, Citation55, Citation56).
The main strengths of our study are the long period of study of an unselected RA population stratified by time of RA diagnosis and the use of the general population as a comparison cohort. Data from region-wide registries and hospital records have ensured completeness of the outcome variables. The comparison cohort made it possible to adjust estimates of excess events for changes in the definition and diagnosis of IHD over time.
Our study has some limitations. Substantially more patients in our cohort were diagnosed with RA in the most recent period (1999–2013). This could be due to the lack of effective RA treatment in the earlier periods and hence fewer referrals to secondary care, especially for milder forms of RA. It is therefore possible that RA patients diagnosed during the first period had a more serious illness, which could correspond to a higher risk of IHD. We used two different sources for outcomes from 2007 onwards for the RA and the comparison cohort, but both were based on hospital discharge diagnosis codes. Furthermore, we did not have data on relevant confounders for the comparison cohort and could therefore not adjust the estimates for excess AMI and IHD events for factors such as smoking status, dyslipidaemia, and hypertension. Another limitation is the use of recurrent AMI and IHD in comparison to the total population, which are likely to be less precise outcomes than incident AMI. A study using the Danish National Patient Registry, which is comparable to the registries in our study, found a higher PPV for incident AMI (97%) compared to recurrent myocardial infarction (88%) and IHD (88–93% for stable and unstable angina) from 2010 to 2012.
Conclusion
RA patients have historically had an excess risk of IHD compared with the general population. Our study found a decline in IHD and excess IHD events from 1972 to 2014 and there was no excess of such events in RA patients diagnosed after 1998. Our findings may reflect improved management of RA, CVD prevention, or changes in the case-mix of RA patients over time.
Supplemental Material
Download MS Word (19.1 KB)Acknowledgements
The authors thank Tomislav Dimoski at the Norwegian Knowledge Centre for the Health Services, Norway, for his contribution in developing the software necessary for obtaining data from Norwegian hospitals, conducting the data collection, and quality assurance of data in the CVDNOR project. The interpretation and reporting of the data are the sole responsibility of the authors, and no endorsement by the Norwegian Cause of Death Registry is intended or should be inferred.
Disclosure statement
CL Alsing reports grants from Western Norway Regional Health Authority during the conduct of the study. BT Fevang reports attendance of an advisory board meeting by Eli Lilly Norge A.S Customer Meeting Services where travel expenses were paid by the company.
Supplemental data
Supplemental data for this article can be accessed https://doi.org/10.1080/03009742.2022.2040116.
Additional information
Funding
References
- Schieir O, Tosevski C, Glazier RH, Hogg-Johnson S, Badley EM. Incident myocardial infarction associated with major types of arthritis in the general population: a systematic review and meta-analysis. Ann Rheum Dis 2017;76:1396–404.
- Han C, Robinson DW, Hackett MV, Paramore LC, Fraeman KH, Bala MV. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol 2006;33:2167–72.
- Nikiphorou E, de Lusignan S, Mallen CD, Khavandi K, Bedarida G, Buckley CD, et al. Cardiovascular risk factors and outcomes in early rheumatoid arthritis: a population-based study. Heart 2020;106:1566–72.
- Maradit-Kremers H, Crowson CS, Nicola PJ, Ballman KV, Roger VL, Jacobsen SJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum 2005;52:402–11.
- van Halm VP, Nielen MMJ, Nurmohamed MT, van Schaardenburg D, Reesink HW, Voskuyl AE, et al. Lipids and inflammation: serial measurements of the lipid profile of blood donors who later developed rheumatoid arthritis. Ann Rheum Dis 2007;66:184–8.
- Holmqvist ME, Wedrén S, Jacobsson LTH, Klareskog L, Nyberg F, Rantapää-Dahlqvist S, et al. No increased occurrence of ischemic heart disease prior to the onset of rheumatoid arthritis: results from two Swedish population-based rheumatoid arthritis cohorts. Arthritis Rheum 2009;60:2861–9.
- Sokka T, Abelson B, Pincus T, Holmqvist ME, Wedrén S. Mortality in rheumatoid arthritis: 2008 update. Clin Exp Rheumatol 2008;26(5 Suppl 51):S35–61.
- de Thurah A, Andersen IT, Tinggaard AB, Riis AH, Therkildsen J, Bøtker HE, et al. Risk of major adverse cardiovascular events among patients with rheumatoid arthritis after initial CT-based diagnosis and treatment. RMD Open 2020;6(1):e001113.
- Innala L, Möller B, Ljung L, Magnusson S, Smedby T, Södergren A, et al. Cardiovascular events in early RA are a result of inflammatory burden and traditional risk factors: a five year prospective study. Arthritis Res Ther 2011;13:R131.
- Myasoedova E, Chandran A, Ilhan B, Major BT, Michet CJ, Matteson EL, et al. The role of rheumatoid arthritis (RA) flare and cumulative burden of RA severity in the risk of cardiovascular disease. Ann Rheum Dis 2016;75:560–5.
- Rao VU, Pavlov A, Klearman M, Musselman D, Giles JT, Bathon JM, et al. An evaluation of risk factors for major adverse cardiovascular events during tocilizumab therapy. Arthritis Rheumatol 2015;67:372–80.
- Björsenius I, Rantapää-Dahlqvist S, Berglin E, Södergren A. Extent of atherosclerosis after 11-year prospective follow-up in patients with early rheumatoid arthritis was affected by disease severity at diagnosis. Scand J Rheumatol 2020;49:443–51.
- Roubille C, Richer V, Starnino T, McCourt C, McFarlane A, Fleming P, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis 2015;74:480–9.
- Lee JL, Sinnathurai P, Buchbinder R, Hill C, Lassere M, March L. Biologics and cardiovascular events in inflammatory arthritis: a prospective national cohort study. Arthritis Res Ther 2018;20:171.
- Low ASL, Symmons DPM, Lunt M, Mercer LK, Gale CP, Watson KD, et al. Relationship between exposure to tumour necrosis factor inhibitor therapy and incidence and severity of myocardial infarction in patients with rheumatoid arthritis. Ann Rheum Dis 2017;76:654–60.
- Ljung L, Askling J, Rantapää-Dahlqvist S, Jacobsson L, ARTIS Study Group. The risk of acute coronary syndrome in rheumatoid arthritis in relation to tumour necrosis factor inhibitors and the risk in the general population: a national cohort study. Arthritis Res Ther 2014;16:R127.
- Widdifield J, Abrahamowicz M, Paterson JM, Huang A, Thorne JC, Pope JE, et al. Associations between methotrexate use and the risk of cardiovascular events in patients with elderly-onset rheumatoid arthritis. J Rheumatol 2019;46:467–74.
- Monti S, Montecucco C, Bugatti S, Caporali R. Rheumatoid arthritis treatment: the earlier the better to prevent joint damage. RMD Open 2015;1:e000057.
- Provan SA, Lillegraven S, Sexton J, Angel K, Austad C, Haavardsholm EA, et al. Trends in all-cause and cardiovascular mortality in patients with incident rheumatoid arthritis: a 20-year follow-up matched case-cohort study. Rheumatology 2020;59:505–12.
- Abhishek A, Nakafero G, Kuo C-F, Mallen C, Zhang W, Grainge MJ, et al. Rheumatoid arthritis and excess mortality: down but not out. A primary care cohort study using data from clinical practice research datalink. Rheumatology 2018;57:977–81.
- Lacaille D, Avina-Zubieta JA, Sayre EC, Abrahamowicz M. Improvement in 5-year mortality in incident rheumatoid arthritis compared with the general population-closing the mortality gap. Ann Rheum Dis 2017;76:1057–63.
- van den Hoek J, Boshuizen HC, Roorda LD, Tijhuis GJ, Nurmohamed MT, van den Bos GAM, et al. Mortality in patients with rheumatoid arthritis: a 15-year prospective cohort study. Rheumatol Int 2017;37:487–93.
- Puolakka K, Kautiainen H, Pohjolainen T, Virta L. No increased mortality in incident cases of rheumatoid arthritis during the new millennium. Ann Rheum Dis 2010;69:2057–8.
- van Nies JAB, de Jong Z, van der Helm-van Mil AHM, Knevel R, Le Cessie S, Huizinga TWJ. Improved treatment strategies reduce the increased mortality risk in early RA patients. Rheumatology 2010;49:2210–6.
- Yazdani K, Xie H, Avina-Zubieta JA, Zheng Y, Abrahamowicz M, Lacaille D. Has the excess risk of acute myocardial infarction in rheumatoid arthritis relative to the general population declined? A population study of trends over time. Semin Arthritis Rheum 2021;51:442–9.
- Holmqvist M, Ljung L, Askling J. Mortality following new-onset rheumatoid arthritis: has modern rheumatology had an impact? Ann Rheum Dis 2018;77:85–91.
- Sulo G, Vollset SE, Nygård O, Igland J, Egeland GM, Ebbing M, et al. Trends in acute myocardial infarction event rates and risk of recurrences after an incident event in Norway 1994 to 2009 (from a Cardiovascular Disease in Norway Project).Am J Cardiol 2014;113:1777–81.
- Øyen N, Nygård O, Igland J, Tell GS, Nordrehaug JE, Irgens LM, et al. Hospital admission rates for cardiovascular diseases in Western Norway, 1992-2001 [in Norwegian]. Tidsskr Nor Laegeforen 2008;128:17–23.
- Pedersen AG, Ellingsen CL. Data quality in the causes of death registry. Tidssk Nor Laegeforen 2015;135:768–70.
- Sulo G, Igland J, Vollset SE, Nygård O, Egeland GM, Ebbing M, et al. Effect of the lookback period’s length used to identify incident acute myocardial infarction on the observed trends on incidence rates and survival: Cardiovascular Disease in Norway project. Circ Cardiovasc Qual Outcomes 2015;8:376–82.
- Igland J, Tell GS, Ebbing M, Nygård O, Vollset SE, Dimoski T. The CVDNOR project: Cardiovascular Disease in Norway 1994–2009. Description of data and data quality. 2013. (https://cvdnor.w.uib.no/files/2013/08/CVDNOR-Data-and-Quality-Report1.pdf). Accessed 2 May 2021.
- Curtis JR, Yang S, Singh JA, Xie F, Chen L, Yun H, et al. Is rheumatoid arthritis a cardiovascular risk-equivalent to diabetes mellitus? Arthritis Care Res 2018;70:1694–9.
- Ljung L, Rantapää-Dahlqvist S, Jacobsson LTH, Askling J. Response to biological treatment and subsequent risk of coronary events in rheumatoid arthritis. Ann Rheum Dis 2016;75:2087–94.
- Fernández-Gutiérrez B, Perrotti PP, Gisbert JP, Domènech E, Fernández-Nebro A, Cañete JD, et al. Cardiovascular disease in immune-mediated inflammatory diseases: a cross-sectional analysis of 6 cohorts. Medicine 2017;96:e7308.
- Solomon DH, Goodson NJ, Katz JN, Weinblatt ME, Avorn J, Setoguchi S, et al. Patterns of cardiovascular risk in rheumatoid arthritis. Ann Rheum Dis 2006;65:1608–12.
- Wolfe F, Michaud K. The risk of myocardial infarction and pharmacologic and nonpharmacologic myocardial infarction predictors in rheumatoid arthritis: a cohort and nested case-control analysis. Arthritis Rheum 2008;58:2612–21.
- Pujades-Rodriguez M, Duyx B, Thomas SL, Stogiannis D, Rahman A, Smeeth L, et al. Rheumatoid arthritis and incidence of twelve initial presentations of cardiovascular disease: a population record-linkage cohort study in England. PLoS One 2016;11:e0151245.
- Kronzer VL, Crowson CS, Sparks JA, Myasoedova E, Davis JM 3rd. Comorbidities as risk factors for rheumatoid arthritis and their accrual after diagnosis. Mayo Clin Proc 2019;94:2488–98.
- Myasoedova E, Davis JM, Roger VL, Achenbach SJ, Crowson CS. Improved incidence of cardiovascular disease in patients with incident rheumatoid arthritis in the 2000s: a population-based cohort study. J Rheumatol 2021;48:1379–87.
- Lindhardsen J, Ahlehoff O, Gislason GH, Madsen OR, Olesen JB, Torp-Pedersen C, et al. The risk of myocardial infarction in rheumatoid arthritis and diabetes mellitus: a Danish nationwide cohort study. Ann Rheum Dis 2011;70:929–34.
- Holmqvist ME, Wedrén S, Jacobsson LTH, Klareskog L, Nyberg F, Rantapää-Dahlqvist S, et al. Rapid increase in myocardial infarction risk following diagnosis of rheumatoid arthritis amongst patients diagnosed between 1995 and 2006. J Intern Med 2010;268:578–85.
- Grigor C, Capell H, Stirling A, McMahon AD, Lock P, Vallance R, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet 2004;364:263–9.
- Fransen J. Effectiveness of systematic monitoring of rheumatoid arthritis disease activity in daily practice: a multicentre, cluster randomised controlled trial. Ann Rheum Dis 2005;64:1294–8.
- Nystad TW, Fenstad AM, Furnes O, Fevang BT. Predictors for orthopaedic surgery in patients with rheumatoid arthritis: results from a retrospective cohort study of 1010 patients diagnosed from 1972 to 2009 and followed up until 2015. Scand J Rheumatol 2018;47:282–90.
- Crowson CS, Rollefstad S, Ikdahl E, Kitas GD, van Riel PLCM, Gabriel SE, et al. Impact of risk factors associated with cardiovascular outcomes in patients with rheumatoid arthritis. Ann Rheum Dis 2018;77:48–54.
- Statistics Norway. Tobacco, alcohol and other drugs. 05307: percentage daily smokers and occasional smokers, by sex and age (per cent) 1973–2020 (https://www.ssb.no/en/statbank/table/05307/). Accessed 11 November 2021.
- Myasoedova E, Gabriel SE, Matteson EL, Davis JM 3rd, Therneau TM,Crowson CS. Decreased cardiovascular mortality in patients with incident rheumatoid arthritis (RA) in recent years: dawn of a new era in cardiovascular disease in RA? J Rheumatol 2017;44:732–9.
- Alfsen GC, Mæhlen J. The value of autopsies for determining the cause of death. Tidsskr Nor Laegeforen 2012;132:147–51.
- McCormick N, Lacaille D, Bhole V, Avina-Zubieta JA. Validity of myocardial infarction diagnoses in administrative databases: a systematic review. PLoS One 2014;9:e92286.
- Sulo G, Igland J, Vollset SE, Ebbing M, Egeland GM, Ariansen I, et al. Trends in incident acute myocardial infarction in Norway: an updated analysis to 2014 using national data from the CVDNOR project. Eur J Prev Cardiol 2018;25:1031–9.
- Dégano IR, Salomaa V, Veronesi G, Ferriéres J, Kirchberger I, Laks T, et al. Twenty-five-year trends in myocardial infarction attack and mortality rates, and case-fatality, in six European populations. Heart 2015;101:1413–21.
- Yang D, Dzayee DAM, Beiki O, de Faire U, Alfredsson L, Moradi T. Incidence and case fatality after day 28 of first time myocardial infarction in Sweden 1987-2008. Eur J Prev Cardiol 2012;19:1304–15.
- Alzuhairi KS, Søgaard P, Ravkilde J, Gislason G, Køber L, Torp-Pedersen C. Incidence and outcome of first myocardial infarction according to gender and age in Denmark over a 35-year period (1978–2012). Eur Heart J Qual Care Clin Outcomes 2015;1:72–8.
- Bhatnagar P, Wickramasinghe K, Wilkins E, Townsend N. Trends in the epidemiology of cardiovascular disease in the UK. Heart 2016;102:1945–52.
- Towfighi A. Sex-specific trends in midlife coronary heart disease risk and prevalence. Arch Intern Med 2009;169:1762–6.
- Arora S, Stouffer GA, Kucharska-Newton AM, Qamar A, Vaduganathan M, Pandey A, et al. Twenty year trends and sex differences in young adults hospitalized with acute myocardial infarction. Circulation 2019;139:1047–56.
