Abstract
Objective
The aim of this study was to investigate pentraxin-3 (PTX3) as a potential biomarker of inflammatory activity in patients with anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) at baseline and 6 month follow-up in a longitudinal cohort.
Method
Plasma PTX3 levels were measured in 79 newly diagnosed or relapsing AAV patients at baseline and 6 month follow-up, and in 23 healthy controls. Urinary PTX3 levels were measured in 34 of the patients. C-reactive protein (CRP), creatinine, and albuminuria were measured and the cumulative glucocorticoid dose at inclusion was calculated. The Birmingham Vasculitis Activity Score (BVAS) was assessed at baseline and follow-up.
Results
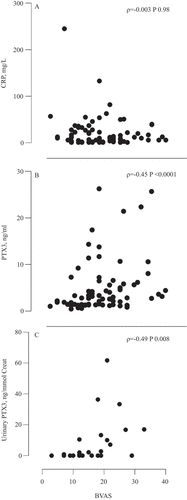
Plasma PTX3 levels were significantly higher at baseline than at 6 months (2.85 vs 1.23 ng/mL, p < 0.001). Plasma and urinary PTX3 levels correlated with BVAS at baseline (ρ = 0.45, p < 0.001, and ρ = 0.49, p = 0.008, respectively). A significant correlation between both plasma and urinary PTX3 levels and estimated glomerular filtration rate and albuminuria was found. However, there was no correlation between plasma and urinary PTX3 levels. At baseline, plasma and urinary PTX3 levels were significantly higher in patients with kidney involvement. PTX3 levels did not correlate with CRP, nor was there a correlation between CRP levels and BVAS at baseline.
Conclusion
Plasma and urinary PTX3 seem to reflect disease activity in AAV better than the commonly used CRP. PTX3 may have a potential role as a biomarker in monitoring disease activity in AAV patients, particularly in patients with kidney involvement.
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a group of necrotizing vasculitides that predominantly affect small to medium-sized vessels (Citation1). Clinical manifestations vary widely, from a limited form restricted to the upper respiratory tract to a severe generalized disease with vital organ failure (Citation2). Kidney involvement occurs in approximately 80–90% of patients and can lead to end-stage kidney disease (Citation3). Untreated disease carries a 1 year mortality of around 80% (Citation4). Although treatment options have improved, there are still no fully reliable methods available for monitoring disease activity, predicting relapse, and guiding individual treatment. It is thus essential to identify biomarkers that could enable tailored individual treatment, thwart relapses, and potentially reduce adverse treatment effects.
The pathogenesis of AAV is complex and not fully understood. Primed neutrophils are activated by ANCA and activated neutrophils undergo NETosis, a form of cell death with degranulation, respiratory burst, and release of neutrophil extracellular traps (NETs) (Citation5–9). The formation of NETs and release of reactive oxygen species from activated neutrophils leads to endothelial damage (Citation7). NETs induced by ANCA have been shown to activate the alternative complement pathway (Citation10), which, in turn, leads to the generation of C5a, which is believed to play a role in the priming of neutrophils. This causes an inflammatory amplification loop that contributes to the pathogenesis of AAV (Citation11).
Pentraxin-3 (PTX3) is a protein structurally similar to the short pentraxin C-reactive protein (CRP). In contrast to CRP, which is mainly produced in the liver, PTX3 is widely produced by a variety of cells, including dendritic cells, macrophages, vascular endothelial cells, and kidney epithelial cells, in response to various inflammatory stimuli. It is believed to reflect local tissue inflammatory activity more closely than CRP (Citation12, Citation13).
PTX3 is a soluble pattern recognition receptor (Citation14) that has the capacity to recognize a number of different ligands, including extracellular matrix proteins, apoptotic cells, pathogens, and complement components (Citation12, Citation14). It has been shown to contribute to complement activation as well as regulation (Citation15–19). Moreover, neutrophils store vast amounts of PTX3 in granules, which is released upon inflammatory stimulation and can be found in NETs (Citation20), and PTX3 also appears to have a regulatory role on NETs (Citation21). In addition, PTX3 affects the glycosylation-dependent regulation of inflammation and dampens neutrophil recruitment (Citation22). As the alternative complement pathway and NET formation are believed to play an important part in the pathogenesis of AAV (Citation5), PTX3 may be an interesting candidate biomarker in AAV patients.
Previous cross-sectional studies have assessed the PTX3 levels in AAV patients and found that the levels were higher in patients with active disease compared with inactive patients and healthy controls (Citation23–25).
To the best of our knowledge, this is the first longitudinal study to investigate PTX3 levels in patients with AAV in relation to the disease activity and immunosuppressive treatment. Our aim was to further delineate the evolution and potential role of PTX3 as a biomarker in a longitudinal cohort of AAV patients.
Method
Patients and methods
Seventy-nine patients with active AAV were included in the study and followed prospectively. Samples from 23 healthy controls were obtained for comparison. Disease activity was assessed using the Birmingham Vasculitis Activity Score 2003 (BVAS) at baseline and at the 6 month follow-up (Citation26). Disease remission was defined as a BVAS of 0. Kidney involvement was defined as kidney biopsy findings consistent with pauci-immune vasculitis or as a clinical presentation with affected kidney function and/or significant haematuria (defined as ≥ 2 on dipstick urinalysis or ≥ 10 erythrocytes per high-power field on urinary sediment).
The cumulative glucocorticoid (GC) dose that patients had received at the baseline sampling was calculated in prednisolone-equivalent milligrams (mg). For the patients with a relapse, the GC dose was calculated from the relapse diagnosis, when a higher dose was restarted. Low doses (up to 10 mg) given before the diagnosis of a relapse were not included in the analysis. The GC dose at the time of the 6 month follow-up was also registered.
The study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the local ethics committee, the Regional Ethical Review Board in Stockholm, and the Swedish Ethical Review Authority. Informed written consent was obtained from all participants.
Laboratory analysis
Peripheral venous blood (heparin plasma) samples and urine samples were obtained from patients at baseline and at the 6 month follow-up. Samples were frozen within 4 h and stored at −70°C for future analysis. Serum CRP and plasma creatinine were measured, and urine analysis was performed using routine methods at the Department of Clinical Chemistry at the Karolinska University Hospital. Albuminuria was assessed with morning urine albumin to creatinine ratio (mg/mmol), and estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (Citation27). ANCA serology was analysed with an enzyme-linked immunosorbent assay (ELISA) (direct EliA; EURO Diagnostic, Malmö, Sweden) or multiplex (BioPlex TM 2200; Bio-Rad, Hercules, California, USA) according to clinical routine at the Department of Clinical Immunology at Karolinska University Hospital. Plasma and urinary levels of PTX3 were analysed using a commercially available ELISA kit from R&D Systems Europe (Abingdon, UK). The urinary pentraxin-3 to creatinine ratio (uPTX3/Cr) was calculated (ng/mmol).
Statistics
Normally distributed variables are presented as mean and standard deviation (sd), non-normally distributed variables as median and 25th to 75th percentiles, and categorical values as frequency and percentage. The Shapiro–Wilk test was used to examine distributions. The non-parametric Wilcoxon rank-sum test was used to assess the difference between two groups. The Wilcoxon signed-rank test was used for comparison over time and Kruskal–Wallis analysis of variance (ANOVA) for differences between time points and controls. Fisher’s exact test was used for comparison of categorical data. Correlations were calculated using the non-parametric Spearman rank test. To assess the prognostic value of baseline PTX3 in predicting kidney function at 6 months, receiver operator characteristics (ROC) curve analysis was performed. Statistical significance was set at the level of p < 0.05. Statistical analyses were performed with JMP software (version 14; SAS, Cary, NC, USA) and GraphPad Prism (version 9; GraphPad Software, La Jolla, CA, USA).
Results
Patients and controls
Seventy-nine patients with active AAV were included in the study and followed prospectively from baseline to a 6 months follow-up (median 195 days from inclusion). Forty-three were men and 36 women, with a median age of 58 years (age range 19–86 years). Seventy patients had a newly diagnosed disease and nine had a relapse of a previously diagnosed AAV. Fifty-four patients were diagnosed with granulomatosis with polyangiitis (GPA), 21 with microscopic polyangiitis (MPA), and four with eosinophilic granulomatosis with polyangiitis (EGPA). All patients were ANCA positive, 48 patients were positive for proteinase-3 (PR3) ANCA and 31 for myeloperoxidase (MPO) ANCA.
Twenty-three randomly selected healthy controls from the Swedish Population Registry, described in a previous study (Citation28), were included for comparison. The baseline characteristics of the patients and controls are given in .
Table 1. Characteristics of patients and controls at baseline.
Induction treatment
All but eight patients were included and sampled after the onset of treatment (median 5 days, range 0–37). All patients received treatment with GCs. Thirty-seven (46.8%) of the patients had received induction remission treatment with cyclophosphamide (CYC), rituximab (RTX), mycophenolate mofetil (MMF), or methotrexate (MTX) at the time of inclusion. The majority of these patients had recently started treatment with CYC (n = 25), but only one patient had received two doses of intravenous CYC before inclusion. Two patients received high-dose GCs only owing to localized ear, nose, and throat (ENT) involvement. The remainder of the patients received induction treatment after inclusion, as shown in .
Maintenance treatment
At the 6 month follow-up, 58 of the patients had started maintenance treatment with azathioprine (AZA), MMF, RTX, or MTX in combination with a low dose of GCs.
Disease activity and phenotype
Disease activity measured as BVAS decreased from a median of 15 at baseline to a median of 0 at the 6 month follow-up (p < 0.001) (). Forty-seven patients had kidney involvement at baseline, 43 confirmed with kidney biopsy and four patients diagnosed clinically. A failure to achieve complete remission of disease activity (BVAS > 0) was seen in five out of 79 patients at follow-up. All of these patients had ENT involvement, two had lung involvement, but none had active kidney disease.
Table 2. Birmingham Vasculitis Activity Score (BVAS) and laboratory analysis at baseline and at 6 month follow-up.
Laboratory results
Plasma PTX3 levels
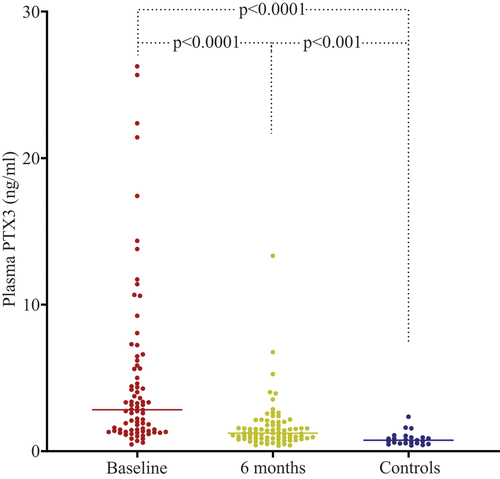
The plasma PTX3 levels in patients were significantly higher at baseline than at follow-up (median 2.85 vs 1.23 ng/mL, p < 0.001, Wilcoxon signed-rank test). Levels were also significantly higher at baseline and at 6 months in AAV patients compared to controls (p < 0.001, non-parametric ANOVA Kruskal–Wallis H-test) ( and ). Plasma PTX3 levels correlated with BVAS at baseline (ρ = 0.45, p < 0.001). All except five patients were in remission at the follow-up, and no significant difference in PTX3 plasma levels was found between patients in remission and those with active disease (1.25 ng/mL vs 0.75 ng/mL, p = 0.13). The results are shown in and . PTX3 levels at baseline were higher in patients with newly diagnosed disease than in patients with relapsing disease (p = 0.046, Wilcoxon rank-sum test).
Table 3. Spearman rank correlation coefficients between plasma pentraxin-3 (PTX3) and clinical and laboratory variables at baseline.
Urinary PTX3 levels
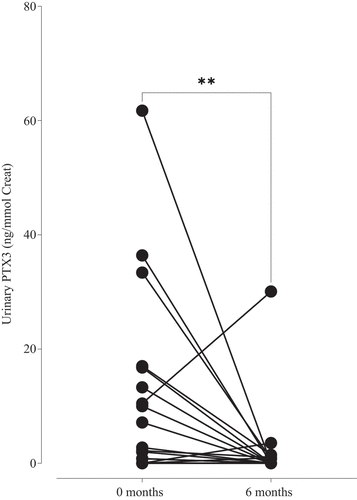
Urine samples for PTX3 analysis were available in a subset of the patients, 34 patients at baseline and 33 at follow-up. uPTX3/Cr could be calculated in 28 patients at baseline and 31 at follow-up, 26 of whom were available at both time points for comparison over time. Levels of uPTX3/Cr were significantly higher at baseline than at follow-up (p = 0.01, Wilcoxon signed-rank test) (). A significant correlation was found between uPTX3/Cr and BVAS at baseline (ρ = 0.49, p = 0.008) (). At follow-up, there was no significant difference in urinary PTX3 levels between patients in remission and those with active disease. No significant correlation was found between plasma PTX3 levels and the uPTX3/Cr at baseline ().
PTX3 levels and kidney involvement
Plasma and urinary PTX3 levels were significantly higher in patients with kidney involvement at baseline compared to those without (p = 0.001 and p = 0.005, respectively, Wilcoxon rank-sum test). Moreover, a significant correlation was found between plasma PTX3 and creatinine, eGFR, and albuminuria at baseline, but not at 6 months (). Similarly, significant correlations were found between uPTX3/Cr and eGFR (ρ = −0.42, p = 0.025) and between uPTX3/Cr and albuminuria at baseline (ρ = 0.54, p = 0.002), but no significant correlation was found at 6 months ().
CRP levels
CRP decreased from a median of 9.0 mg/L at baseline sampling to 2.0 mg/L at the 6 month follow-up (p < 0.001, Wilcoxon signed-rank test) (). However, PTX3 levels did not significantly correlate with CRP, and there was no significant correlation between CRP levels and BVAS at baseline ( and ). There was also no significant difference in CRP levels between patients with and those without kidney involvement or between patients with relapse and patients with a new diagnosis.
Treatment and PTX3 levels
Patients who had received induction treatment at inclusion (n = 71) had significantly higher plasma PTX3 levels than treatment-naïve patients (n = 8) (3.15 vs 1.35 ng/mL, p = 0.03, Wilcoxon rank-sum test). No significant difference in urinary PTX3 levels between these groups was found.
Plasma PTX3 levels correlated significantly with the cumulative GC dose given at baseline (ρ = 0.37, p < 0.001). In contrast, there was a negative correlation between CRP and the cumulative GC dose (ρ = −0.31, p < 0.01). There was also a significant correlation between the GC dose at baseline and plasma PTX3 levels (ρ = 0.43, p < 0.0001). However, a significant correlation between uPTX3/Cr and the GC dose at baseline or the cumulative GC dose at baseline was not found.
We found no significant correlation between plasma PTX3 or CRP levels and GC dose at 6 months.
There was no significant difference in plasma or urinary PTX3 levels between patients who had received CYC at baseline and those who had not, and no correlation was found between CYC dose given at baseline and plasma PTX3 levels. No difference was found between patients who had received treatment with methotrexate (n = 7) at baseline and those who had not. As only very few patients had received RTX (n = 2) and MMF (n = 2), no analysis was conducted for comparison for these groups.
Predictive role of PTX3
ROC curve analysis was performed to assess whether PTX3 could be a potential prognostic marker for kidney function (eGFR) at the 6 month follow-up. The area under the curve was 0.62 (data not shown).
Discussion
The identification of novel inflammatory markers that more closely reflect disease activity and specific organ involvement in patients with AAV is an unmet need. In this study, we found increased plasma PTX3 levels in active AAV compared to inactive disease and healthy controls, and a decrease following immunosuppressive therapy. Similarly, urinary PTX3 levels were higher in patients with active disease than in those in remission, and correlated with disease activity (indicated by BVAS). Our findings are consistent with the results of our previous cross-sectional study, where we found higher levels of circulating PTX3 as well as PTX3 expressed on circulating microparticles in patients with active AAV disease compared to inactive disease and controls (Citation25). In this study, we could also show that circulating PTX3 levels did not correlate with CRP, nor was there a correlation between CRP levels and BVAS at baseline.
PTX3 is a long pentraxin that is produced by various cells in response to inflammatory signals and therefore is believed to reflect local tissue inflammatory activity and immune responses (Citation12, Citation13). PTX3 is stored in granules in neutrophils and released to NETs on stimulation (Citation20). PTX3 has been shown to increase in inflammatory diseases, and also in other inflammatory conditions such as infections and myocardial infarction (Citation29–31). The fact that neutrophils and NET formation play an important role in the pathogenesis of AAV suggests that PTX3 may be a potential biomarker in these diseases.
In contrast to levels of the commonly used inflammatory marker CRP, plasma PTX3 correlated with disease activity (BVAS), implying that PTX3 may be a more dependable biomarker for monitoring disease activity in AAV. Our results are also in line with previous reports on diabetic nephropathy and systemic lupus erythematous (SLE), which showed a better correlation between the disease activity and the plasma PTX3 levels compared to CRP (Citation32, Citation33). Furthermore, several studies on patients with various systemic inflammatory and cardiovascular diseases have failed to show a significant correlation between CRP and PTX3 (Citation30, Citation33–38), including a previous cross-sectional study of 43 AAV patients (Citation23).
We are not convinced that PTX3 is a better disease marker than BVAS, which, so far, has been the gold standard for assessing disease activity in AAV in research and clinical trials. However, BVAS is rarely used in clinical settings, which would make more readily available biomarkers more useful. However, as reliable biomarkers in AAV are lacking, our study suggests that PTX3 may have a role here. Our results show that it correlates with disease activity and, furthermore, is a better disease activity marker than the commonly used CRP.
Besides the association with disease activity, there was a correlation between both plasma and urinary PTX3 levels and albuminuria and kidney function. Furthermore, higher plasma and urinary PTX3 levels were seen among patients with kidney involvement, also suggesting a potential role in AAV kidney pathogenesis. A possible explanation for elevated plasma PTX3 levels in patients with impaired kidney function is retention caused by decreased kidney clearance. PTX3 (42 kDa) forms multimers that are approximately 440 kDa in size (Citation16), which could affect its clearance. This has been suggested by previous studies demonstrating elevated plasma PTX3 levels in patients with chronic kidney disease (Citation39, Citation40). However, our results show that urinary PTX3 levels were elevated in patients with kidney involvement. No correlation was found between plasma PTX3 levels and urinary PTX3 levels. The reason for this is unclear and, furthermore, urine samples were only available in a subset of patients. Even though our results indicate that both plasma and urinary PTX3 may be potentially useful and possibly independent disease activity markers in AAV, further longitudinal studies are required to determine the optimal use of PTX3 in plasma and urine as a potential biomarker reflecting disease activity in AAV patients with or without kidney involvement. In addition, even though the baseline plasma PTX3 level did reflect disease activity, it did not seem to predict damage, as reflected by kidney function, in the short 6 month follow-up period.
Previously, urinary PTX3 has mainly been studied in relation to urinary tract infections and the results have indicated that PTX3 in urine may play a role in the innate immune defence against pathogens in the urinary tract (Citation41). Furthermore, elevated urinary PTX3 levels have been found in patients with kidney scars following pyelonephritis (Citation42). This suggests that there is increased local production of PTX3 during inflammation in the kidney and the urinary tract, independent of systemic PTX3 levels. Hence, urinary PTX3 may reflect local kidney inflammation better than the plasma levels. Supporting this hypothesis are findings demonstrating that PTX3 is expressed in kidney tissue in patients with immunoglobulin A nephropathy (IgAN), type 1 membranoproliferative glomerulonephritis, and lupus nephritis (LN) (Citation43, Citation44). PTX3 was shown to be expressed in the glomeruli, on infiltrating inflammatory cells in the peritubular capillaries, and in the kidney interstitium in IgAN (Citation43), as well as in the tubulointerstitial area in patients with active LN (Citation43, Citation44). Moreover, urinary PTX3 levels have been shown to be elevated in patients with active LN compared to those with inactive disease and healthy controls (Citation44).
Animal model studies have previously demonstrated that PTX3 may have a role in limiting acute and chronic kidney injury (Citation45). This may indicate that PTX3 influences several compartments within the kidney and potentially also has a protective role by controlling inflammation depending on the pathophysiological conditions. However, studies are needed to delineate these mechanisms in AAV, particularly in patients with kidney involvement where tissue samples are available for further analysis.
We found a positive correlation between plasma PTX3 levels and the cumulative dose of GCs at baseline. Similar results have been shown in patients with SLE, where a significant correlation was shown between PTX3 levels and the prednisolone dose (Citation33). One possible explanation is that patients requiring higher doses have more severe disease, and therefore plasma PTX3 may reflect disease activity better than the dose of GC given. In contrast, CRP was negatively correlated with the cumulative GC dose given at baseline, which suggests that CRP kinetics may differ by being more readily affected by GC treatment. Another possible explanation is that the high-dose GC treatment could increase the circulating PTX3 levels. In line with this hypothesis, PTX3 levels have been shown to increase with intravenous GC treatment and to be elevated in patients with Cushing’s syndrome. Doni et al also showed that GCs had different effects in different cell types, inducing PTX3 gene expression in non-haematopoietic cells and repressing it in haematopoietic cells (Citation46). Plasma PTX3 levels were also higher in patients who had received induction treatment than in those who were treatment naïve at sampling. These findings can probably be explained by more severe disease activity in this group compared to those who had not yet received treatment.
A strength of our study is having access to both baseline information and prospective follow-up samples combined with clinical data. Furthermore, patients with various organ manifestations, ranging from localized ENT symptoms to serious disease with multi-organ involvement, were included. Limitations include the lack of further follow-up after 6 months, which could have contributed to a better understanding of PTX3 as a potential predictor of relapse. Another limitation is that the majority of patients were not treatment naïve, as most had started induction treatment owing to pressing clinical needs, before inclusion in the study. We tried to address this by examining the effects of the administered GC doses and immunosuppressive therapy on PTX3 levels at inclusion.
Conclusion
Plasma and urinary PTX3 levels were significantly increased in active AAV disease, and in particular in patients with kidney involvement. Furthermore, both plasma and urinary PTX3 levels seem to reflect disease activity in active AAV better than the commonly used CRP. GC doses may possibly affect the PTX3 levels, but this relationship needs to be explored further. Plasma and urinary PTX3 may have a role in AAV pathogenesis, and PTX3 could be a potential new biomarker in AAV. Future studies should include long-term follow-up to assess whether PTX3 may also have a role in detecting AAV relapses at an early stage.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised international Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheumatol 2013;65:1–11.
- Yates M, Watts R. ANCA-associated vasculitis. Clin Med (London) 2017;17:60–4.
- Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med 1997;337:1512–23.
- Walton EW. Giant-cell granuloma of the respiratory tract (Wegener’s granulomatosis). Br Med J 1958;2:265–70.
- Nakazawa D, Masuda S, Tomaru U, Ishizu A. Pathogenesis and therapeutic interventions for ANCA-associated vasculitis. Nat Rev Rheumatol 2019;15:91–101.
- Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL, Werb Z, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med 2009;15:623–5.
- Schreiber A, Rousselle A, Becker JU, von Massenhausen A, Linkermann A, Kettritz R. Necroptosis controls NET generation and mediates complement activation, endothelial damage, and autoimmune vasculitis. Proc Natl Acad Sci U S A 2017;114:E9618–E25.
- Kettritz R. How anti-neutrophil cytoplasmic autoantibodies activate neutrophils. Clin Exp Immunol 2012;169:220–8.
- Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 2007;176:231–41.
- Wang H, Wang C, Zhao MH, Chen M. Neutrophil extracellular traps can activate alternative complement pathways. Clin Exp Immunol 2015;181:518–27.
- Chen M, Jayne DRW, Zhao MH. Complement in ANCA-associated vasculitis: mechanisms and implications for management. Nat Rev Nephrol 2017;13:359–67.
- Mantovani A, Garlanda C, Doni A, Bottazzi B. Pentraxins in innate immunity: from C-reactive protein to the long pentraxin PTX3. J Clin Immunol 2008;28:1–13.
- Deban L, Jaillon S, Garlanda C, Bottazzi B, Mantovani A. Pentraxins in innate immunity: lessons from PTX3. Cell Tissue Res 2011;343:237–49.
- Mantovani A, Garlanda C, Bottazzi B. Pentraxin 3, a non-redundant soluble pattern recognition receptor involved in innate immunity. Vaccine 2003;21:S43–7.
- Ma YJ, Garred P. Pentraxins in complement activation and regulation. Front Immunol 2018;9:3046.
- Bottazzi B, Vouret-Craviari V, Bastone A, De Gioia L, Matteucci C, Peri G, et al. Multimer formation and ligand recognition by the long pentraxin PTX3. Similarities and differences with the short pentraxins C-reactive protein and serum amyloid P component. J Biol Chem 1997;272:32817–23.
- Nauta AJ, Bottazzi B, Mantovani A, Salvatori G, Kishore U, Schwaeble WJ, et al. Biochemical and functional characterization of the interaction between pentraxin 3 and C1q. Eur J Immunol 2003;33:465–73.
- Ma YJ, Doni A, Hummelshoj T, Honore C, Bastone A, Mantovani A, et al. Synergy between ficolin-2 and pentraxin 3 boosts innate immune recognition and complement deposition. J Biol Chem 2009;284:28263–75.
- Deban L, Jarva H, Lehtinen MJ, Bottazzi B, Bastone A, Doni A, et al. Binding of the long pentraxin PTX3 to factor H: interacting domains and function in the regulation of complement activation. J Immunol 2008;181:8433–40.
- Jaillon S, Peri G, Delneste Y, Fremaux I, Doni A, Moalli F, et al. The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps. J Exp Med 2007;204:793–804.
- Daigo K, Takamatsu Y, Hamakubo T. The protective effect against extracellular histones afforded by long-pentraxin PTX3 as a regulator of NETs. Front Immunol 2016;7:344.
- Deban L, Russo RC, Sironi M, Moalli F, Scanziani M, Zambelli V, et al. Regulation of leukocyte recruitment by the long pentraxin PTX3. Nat Immunol 2010;11:328–34.
- Fazzini F, Peri G, Doni A, Dell’Antonio G, Dal Cin E, Bozzolo E, et al. PTX3 in small-vessel vasculitides: an independent indicator of disease activity produced at sites of inflammation. Arthritis Rheumatol 2001;44:2841–50.
- Ramirez GA, Rovere-Querini P, Blasi M, Sartorelli S, Di Chio MC, Baldini M, et al. PTX3 intercepts vascular inflammation in systemic immune-mediated diseases. Front Immunol 2019;10:1135.
- Manojlovic M, Juto A, Jonasdottir A, Colic J, Vojinovic J, Nordin A, et al. Microparticles expressing myeloperoxidase as potential biomarkers in anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitides (AAV). J Mol Med (Berlin) 2020;98:1279–86.
- Mukhtyar C, Lee R, Brown D, Carruthers D, Dasgupta B, Dubey S, et al. Modification and validation of the Birmingham Vasculitis Activity Score (version 3). Ann Rheum Dis 2009;68:1827–32.
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12.
- Nordin A, Jensen-Urstad K, Bjornadal L, Pettersson S, Larsson A, Svenungsson E. Ischemic arterial events and atherosclerosis in patients with systemic sclerosis: a population-based case-control study. Arthritis Res Ther 2013;15:R87.
- Huang XL, Zhang L, Duan Y, Wang YJ, Wang J. Association of pentraxin 3 with autoimmune diseases: a systematic review and meta-analysis. Arch Med Res 2016;47:223–31.
- Peri G, Introna M, Corradi D, Iacuitti G, Signorini S, Avanzini F, et al. PTX3, A prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans. Circulation 2000;102:636–41.
- Hamed S, Behnes M, Pauly D, Lepiorz D, Barre M, Becher T, et al. Diagnostic value of Pentraxin-3 in patients with sepsis and septic shock in accordance with latest sepsis-3 definitions. BMC Infect Dis 2017;17:554.
- Uzun S, Ozari M, Gursu M, Karadag S, Behlul A, Sari S, et al. Changes in the inflammatory markers with advancing stages of diabetic nephropathy and the role of Pentraxin-3. Renal Fail 2016;38:1193–8.
- Assandri R, Monari M, Colombo A, Dossi A, Montanelli A. Pentraxin 3 plasma levels and disease activity in systemic lupus erythematosus. Autoimmune Dis 2015;2015:354014.
- Assandri R, Montanelli A. Pentraxin 3 and biopsy status in celiac patients. Gastroenterol Hepatol Bed Bench 2018;11:225–32.
- Dagna L, Salvo F, Tiraboschi M, Bozzolo EP, Franchini S, Doglioni C, et al. Pentraxin-3 as a marker of disease activity in Takayasu arteritis. Ann Intern Med 2011;155:425–33.
- Deniz T, Kizilgul M, Uzunlulu M, Oguz A, Isbilen B. Levels of pentraxin 3 and relationship with disease activity in patients with ankylosing spondylitis. Acta Reumatol Port 2014;39:137–42.
- Nisihara R, Skare TL, Zeni JO, Rasera H, Lidani K, Messias-Reason I. Plasma levels of pentraxin 3 in patients with spondyloarthritis. Biomarkers 2018;23:14–17.
- Shirai Y, Okazaki Y, Inoue Y, Tamura Y, Yasuoka H, Takeuchi T, et al. Elevated levels of pentraxin 3 in systemic sclerosis: associations with vascular manifestations and defective vasculogenesis. Arthritis Rheumatol 2015;67:498–507.
- Sjoberg B, Qureshi AR, Heimburger O, Stenvinkel P, Lind L, Larsson A, et al. Association between levels of pentraxin 3 and incidence of chronic kidney disease in the elderly. J Intern Med 2016;279:173–9.
- Tong M, Carrero JJ, Qureshi AR, Anderstam B, Heimburger O, Barany P, et al. Plasma pentraxin 3 in patients with chronic kidney disease: associations with renal function, protein-energy wasting, cardiovascular disease, and mortality. Clin J Am Soc Nephrol 2007;2:889–97.
- Jaillon S, Moalli F, Ragnarsdottir B, Bonavita E, Puthia M, Riva F, et al. The humoral pattern recognition molecule PTX3 is a key component of innate immunity against urinary tract infection. Immunity 2014;40:621–32.
- Becerir T, Yuksel S, Evrengul H, Ergin A, Enli Y. Urinary excretion of Pentraxin-3 correlates with the presence of renal scar following acute pyelonephritis in children. Int Urol Nephrol 2019;51:571–7.
- Bussolati B, Peri G, Salvidio G, Verzola D, Mantovani A, Camussi G. The long pentraxin PTX3 is synthesized in IgA glomerulonephritis and activates mesangial cells. J Immunol 2003;170:1466–72.
- Pang Y, Tan Y, Li Y, Zhang J, Guo Y, Guo Z, et al. Pentraxin 3 is closely associated with tubulointerstitial injury in lupus nephritis: a large multicenter cross-sectional study. Medicine (Baltimore) 2016;95:e2520.
- Xiao Y, Yang N, Zhang Q, Wang Y, Yang S, Liu Z. Pentraxin 3 inhibits acute renal injury-induced interstitial fibrosis through suppression of IL-6/Stat3 pathway. Inflammation 2014;37:1895–901.
- Doni A, Mantovani G, Porta C, Tuckermann J, Reichardt HM, Kleiman A, et al. Cell-specific regulation of PTX3 by glucocorticoid hormones in hematopoietic and nonhematopoietic cells. J Biol Chem 2008;283:29983–92.