Abstract
Objectives
To study the agreement between clinical axial spondyloarthritis (axSpA) diagnoses and fulfilment of the Assessment of SpondyloArthritis international Society (ASAS) axSpA and modified New York (mNY) classification criteria, and to compare disease/health status between axSpA subtypes.
Method
Patients with prevalent, clinical axSpA attending a rheumatology clinic were enrolled in a cross-sectional study. Assessments included physical evaluation, laboratory testing, questionnaires, and appropriate imaging, allowing classification. Standard axSpA outcome measures were compared between patients fulfilling mNY/radiographic versus non-radiographic axSpA (r-axSpA/nr-axSpA) criteria.
Results
Of 239 consecutively included patients, 141 fulfilled ASAS r-axSpA and/or mNY criteria, while 57 fulfilled nr-axSpA criteria. The agreement between r-axSpA and mNY criteria fulfilment was 94%. The positive predictive value (PPV) of a clinical ankylosing spondylitis (AS) diagnosis for mNY criteria fulfilment was 71%; the PPV of an undifferentiated axSpA (u-axSpA) diagnosis for fulfilment of nr-axSpA criteria was 30% and 40% for mNY criteria. Patients with r-axSpA/AS were older, more often men, and had longer disease duration, more uveitis, and worse spinal mobility than nr-axSpA patients, who had more enthesitis and dactylitis.
Conclusion
We found an overall good concordance between clinical axSpA diagnoses and classification criteria fulfilment, with 83% fulfilling ASAS axSpA and/or mNY criteria. Regarding axSpA subtypes, the concordance was weaker, and although the ICD-10 code for AS correctly identified patients meeting mNY criteria in 71% of cases, one-third of mNY-positive patients lacked an AS diagnosis. Moreover, clinical u-axSpA diagnoses could not serve as a proxy to identify nr-axSpA, highlighting the importance of thorough classification in research on axSpA subtypes.
The term axial spondyloarthritis (axSpA) was introduced by the Assessment of SpondyloArthritis international Society (ASAS) in 2009 and encompasses patients with non-radiographic (nr-axSpA) and radiographic axSpA (r-axSpA) (Citation1). The ASAS axSpA criteria were mainly developed to enable identification of patients in early disease, without structural changes in the sacroiliac (SI) joints, and therefore comprise magnetic resonance imaging (MRI) findings. ASAS classification criteria for peripheral spondyloarthritis also exist, but when axial symptoms are present, the axial classification system is recommended (Citation2).
The ASAS axSpA criteria use the same radiographic criterion as the modified New York (mNY) criteria for ankylosing spondylitis (AS) (Citation3), while the clinical criteria items differ somewhat (Supplementary Table 1). Criteria for AS and r-axSpA have consistently shown agreements above 90%, and are suggested to be used interchangeably (Citation4). Previously, the term undifferentiated spondyloarthritis (SpA) has often been used for patients without structural SI-joint changes, but also for patients with solely peripheral disease. With the ASAS criteria, such patients can be classified in a more structured way (Citation5–7).
No diagnostic criteria for axSpA exist (Citation8), and the main purpose of classification criteria is to create homogeneous patient groups for research. To diagnose a patient, the complete picture, including clinical, laboratory, and imaging findings, is evaluated and relevant differential diagnoses should be excluded (Citation9–12).
In the International Classification of Diseases, 10th revision (ICD-10), adequate diagnostic codes for some SpA subgroups are lacking. Specific codes exist for AS (M459), spinal enthesopathy (M460), and sacroiliitis (M461), as well as for reactive, psoriatic, and inflammatory bowel disease-related arthritis. Meanwhile, M468 and M469 are used for all other SpA patients, both axial and peripheral.
When comparing nr-axSpA to r-axSpA/AS, the disease burden has been reported to be similar, while r-axSpA/AS patients are more often men and smokers, and have longer disease duration and higher acute-phase reactant levels than nr-axSpA patients (Citation8, Citation13–17). Controversies regarding the ASAS axSpA classification criteria exist; in particular, the nr-axSpA entity has raised concerns over creating a too heterogeneous patient group for research purposes (Citation18–23). The risk of overdiagnosing nr-axSpA by human leucocyte antigen (HLA)-B27 positivity or changes on MRI has also been discussed (Citation21–23). Although there is some evidence of good agreement between fulfilment of ASAS axSpA criteria and older SpA criteria [i.e. the European Spondyloarthropathy Study Group (ESSG) and Amor criteria] (Citation8), knowledge regarding the ASAS classification subgroups from well-characterized axSpA cohorts is incomplete.
In epidemiological research, it is essential to know the agreement between clinical diagnoses and the fulfilment of relevant classification criteria. In addition, studies of subtypes of axSpA are important, especially regarding the more newly defined nr-axSpA entity, to improve knowledge of disease phenotypes and disease development (Citation11, Citation24, Citation25).
The main aim of this work was to study the agreement between clinical axSpA diagnoses (according to ICD-10 codes) and fulfilment of the ASAS axSpA and mNY classification criteria in a clinical cohort of well-characterized axSpA patients from a defined area. Secondary aims were to compare disease and health status between patients (i) fulfilling classification criteria for nr-axSpA versus r-axSpA/AS; and (ii) fulfilling versus not fulfilling ASAS axSpA or mNY criteria.
Method
The SPARTAKUS (SPondylARtrit TvÄrsnittsKohort Universitetssjukhuset i Skåne) study is an observational, clinical, cross-sectional study of axSpA. All patients, irrespective of disease duration, residing in a defined geographical area of southern Sweden and with at least one outpatient visit to the Department of Rheumatology, Skåne University Hospital, in 2011–2014, with an ICD-10 diagnosis suggestive of axSpA (M459, M460, M461, M468, M469), were identified (Citation17, Citation26, Citation27). All patients with ICD-10 codes M459, M460, and M461 were directly invited to enrol in the study, while patients with ICD-10 codes M468 and M469 also had to report back pain lasting for ≥ 3 months with onset before the age of 45 years to be eligible (Citation1).
At enrolment in SPARTAKUS, all patients attended a structured study visit according to a predefined protocol, encompassing a thorough medical history; patient questionnaires; clinical examinations by a rheumatologist, physiotherapist, and occupational therapist; and sampling of blood [for HLA-B27, C-reactive protein (CRP), etc.], urine, and faeces. Current medication, regarding conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs), biological disease-modifying anti-rheumatic drugs (bDMARDs), non-steroidal anti-inflammatory drugs (NSAIDs), and glucocorticoids, was recorded. The following imaging algorithm was used. First, all previously performed plain radiographs and/or MRI scans of the SI joints were re-examined and scored. If previous SI-joint radiographs were either missing or negative for fulfilment of the mNY criteria, a new radiograph was performed and scored. For patients without radiographic changes in the SI joints, and where nr-axSpA could not be confirmed based on HLA-B27 positivity and/or previous MRI findings and clinical characteristics, a new SI-joint MRI was performed and scored. For both plain X-rays and MRI examinations, a 24 month limit before the enrolment in SPARTAKUS was set for accepting earlier negative examinations (while positive examinations were accepted regardless of time-point). All radiographs and MRIs were evaluated and scored by one experienced musculoskeletal radiologist (coauthor MG).
Disease activity was assessed with the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) (Citation28) and the Ankylosing Spondylitis Disease Activity Score using C-reactive protein (ASDAS-CRP) (Citation29). The Maastricht Ankylosing Spondylitis Enthesitis Score (MASES, modified to also include bilateral plantar fascia: 0‒15 sites) was used to assess enthesitis, and the entheseal sites were evaluated as being tender (1) or not tender (0) (Citation30). The Bath Ankylosing Spondylitis Functional Index (BASFI) was used to assess physical function (Citation31) and the Bath Ankylosing Spondylitis Metrology Index (BASMI) for assessing spinal mobility (Citation32). Visual analogue scales (VASs) ranging from 0 mm (best) to 100 mm (worst) were used to assess pain, fatigue, and patient’s and evaluator’s global assessment of health. Health-related quality of life was assessed by the generic EuroQol 5-Dimensions (EQ-5D) instrument (Citation33), rendering utility values anchored at 0 (death) and 1 (full health).
For the present work, the first 239 consecutively included patients in the SPARTAKUS cohort were studied. The clinical ICD-10 diagnosis applied as the main diagnosis by the patient’s treating rheumatologist at the last outpatient visit prior to the SPARTAKUS enrolment was registered as the patient’s clinical SpA diagnosis. For the current analyses, patients were grouped into two clinical categories: AS (ICD-10 diagnosis M459) and undifferentiated axial spondyloarthritis (u-axSpA; ICD-10 diagnoses M461, M468, and M469). No patient with the ICD-10 code M460 was identified. As outlined above, patients with M468 and M469 also had to report back pain lasting for ≥ 3 months before the age of 45 years in a telephone interview preceding enrolment, since these ICD codes are often also used for peripheral SpA, without axial involvement.
The study was approved by the Regional Ethical Review Board, Lund University, Sweden (Dnr. 2015/436). Oral and written information was provided and informed written consent was obtained from all patients in compliance with the Declaration of Helsinki.
Statistical analyses
Demographics, disease, health, and treatment characteristics were compared between patients with nr-axSpA and r-axSpA/AS, as well as between patients fulfilling versus not fulfilling any axSpA classification criteria (ASAS axSpA or mNY classification criteria), using the Student’s t-test, Mann–Whitney U-test, chi-squared test, or Fisher’s exact test, as appropriate.
A p-value < 0.05 was considered statistically significant. All analyses were performed with SPSS version 26 for Windows (IBM Corp., Armonk, NY, USA).
Results
Classification criteria fulfilment
Of the 239 patients included in the present analyses, 198 (83%) fulfilled the ASAS axSpA and/or the mNY criteria, of whom 192 met the ASAS axSpA criteria (for radiographic or non-radiographic axSpA). A majority of patients (143 out of 239; 60%) demonstrated radiographic SI-joint changes meeting the mNY definition of sacroiliitis, of whom 141 also fulfilled the ASAS r-axSpA criteria and/or mNY criteria for AS (r-axSpA, n = 135; mNY, n = 132). Two patients with radiographic changes did not fulfil any of the assessed axSpA criteria. Fifty-seven patients (24%) fulfilled the ASAS nr-axSpA criteria ().
Figure 1. Concordance between clinical International Classification of Diseases, 10th Revision (ICD-10) diagnoses and fulfilment of classification criteria. *M45.9; **M46.1, M46.8, M46.9 (no patient with M46.0 was identified); †Assessment of SpondyloArthritis international Society (ASAS) axial spondyloarthritis (axSpA) and/or modified New York (mNY) criteria for ankylosing spondylitis (AS). u-axSpA, undifferentiated axial spondyloarthritis; r-axSpA, radiographic axial spondyloarthritis; nr-axSpA, non-radiographic axial spondyloarthritis.
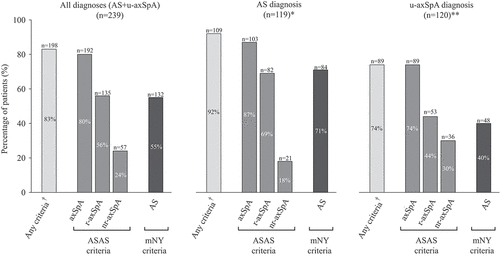
The concordance between the ASAS r-axSpA criteria and the mNY criteria was good, with 94% (224 of 239 patients) fulfilling either both or neither of these sets of criteria. Six patients fulfilled the mNY criteria for AS but not the ASAS r-axSpA criteria. Reasons for not fulfilling the ASAS r-axSpA criteria were lack of reporting back pain with a duration of ≥ 3 months (n = 5) or symptom onset after 45 years of age (n = 1). Conversely, nine patients fulfilled the ASAS r-axSpA criteria but not the mNY criteria, since they neither reported back pain to have been improved by exercise and not relieved by rest nor fulfilled the clinical requirements for limited spinal mobility.
Beyond the 239 patients included in the present analyses, an additional 11 subjects were enrolled in SPARTAKUS at this stage. For eight of these patients, the treating rheumatologist had changed the clinical ICD-10 diagnosis from M459, M461, M468, or M469 to another diagnosis between the time-point when the subjects were identified for enrolment in the SPARTAKUS cohort and the actual enrolment visit, rendering them unsuitable for inclusion in the present analyses. For further information on these patients, who were excluded owing to changed diagnoses (n = 8), see supplementary Table 2. Moreover, three patients did not perform all of the required imaging examinations, and therefore it was not possible to classify them adequately.
Characteristics of all the included patients fulfilling any axSpA criteria and those who did not are presented in . The 41 patients (17%) not fulfilling any of the assessed axSpA criteria were more often women, had less inflammatory back pain (even if back pain remained common), and few were HLA-B27 positive (10%). VAS pain, global health and fatigue, as well as the Bath Ankylosing Spondylitis indices, did not differ between patients who fulfilled any axSpA criteria and those who did not. At the same time, patients not fulfilling any axSpA criteria reported worse enthesitis scores and worse quality of life. Two patients who did not fulfil any axSpA classification criteria demonstrated X-ray changes in the SI joints meeting the mNY criteria definition of sacroiliitis, and both patients reported a history of uveitis. However, one reported disease onset after 45 years of age, the other was HLA-B27 negative, and neither of them met the clinical requirements of the assessed classification criteria. The majority (28 of 41) of the patients not fulfilling any axSpA criteria fulfilled the ASAS peripheral SpA classification criteria. Among those, one patient had SI-joint bone marrow oedema on MRI consistent with the ASAS criteria definition, leaving 13 patients (5%) who did not fulfil any criteria, either for axial or for peripheral SpA.
Table 1. Characteristics of patients fulfilling Assessment of SpondyloArthritis international Society (ASAS) axial spondyloarthritis (axSpA) and/or modified New York criteria versus patients not fulfilling any such criteria.
Concordance between clinical ICD-10 diagnoses and classification criteria fulfilment
Out of 119 patients with a clinical ICD-10 diagnosis of AS (M459), 103 (87%) fulfilled any ASAS axSpA criteria, with 82 (69%) meeting the ASAS r-axSpA criteria and 84 (71%) the mNY criteria. Out of 120 patients with a clinical ICD-10 diagnosis of undifferentiated axSpA (M461, n = 11; M468, n = 96; M469, n = 13), 36 (30%) fulfilled the ASAS criteria for nr-axSpA, while higher proportions fulfilled the ASAS criteria for r-axSpA (n = 53; 44%) or the mNY criteria for AS (n = 48; 40%). For further information, see . The positive predictive value (PPV) of having received a clinical AS diagnosis for fulfilment of mNY criteria for AS was 71%. The corresponding PPV of having received a clinical u-axSpA diagnosis for fulfilment of the ASAS criteria for nr-axSpA was 30%. Conversely, the negative predictive value (NPV) of not having received a clinical AS diagnosis for not meeting the mNY criteria for AS was 60%, while the corresponding NPV of no clinical u-axSpA diagnosis for non-fulfilment of the nr-axSpA criteria was 82%. The clinical diagnoses, fulfilment of classification criteria, and predictive values for clinical diagnoses are presented in .
Table 2. Clinical diagnoses and predictive values of clinical diagnoses of ankylosing spondylitis (AS) (M459) and undifferentiated axial spondyloarthritis (u-axSpA) (M461, M468, M469) for classification criteria fulfilment.
Comparison of r-axSpA/AS and nr-axSpA
Comparisons of characteristics for patients fulfilling ASAS r-axSpA and/or mNY criteria of AS combined versus patients meeting ASAS nr-axSpA criteria are presented in . Patients with r-axSpA/AS were older, more often men, had longer disease duration, and more often had a history of uveitis and worse spinal mobility than patients with nr-axSpA, who, in turn, had higher enthesitis scores and more dactylitis. No other disease and health status assessments differed between the groups.
Table 3. Characteristics of the radiographic axial spondyloarthritis/ankolysing spondylitis (r-axSpA/AS) and non-radiographic axial spondyloarthritis (nr-axSpA) patients.
Discussion
The current cross-sectional analysis of an axSpA cohort, diagnosed at a rheumatology clinic, showed good overall concordance between clinical ICD-10 diagnoses and axSpA classification criteria fulfilment, with 83% fulfilling ASAS axSpA and/or mNY criteria, while 95% met any SpA criteria (ASAS axSpA, mNY, or ASAS peripheral SpA). However, regarding the clinical diagnoses of AS or u-axSpA, the concordance with classification criteria fulfilment for subtypes of axSpA was weaker. The ICD-10 code for AS only correctly identified patients fulfilling the mNY criteria in 71% of cases, while one-third of subjects meeting mNY criteria were not identified by the ICD-10 code for AS. Furthermore, nr-axSpA, as a classification entity, could not be sufficiently identified by applying the u-axSpA ICD-10 codes as proxy, since only 30% of u-axSpA patients fulfilled the classification criteria for nr-axSpA. In this study, with a rather long mean disease duration, a substantial group of u-axSpA patients fulfilled the mNY criteria. One explanation for this could be that radiographic examinations were not systematically performed during clinical follow-up and therefore some clinical diagnoses were potentially not updated when the disease progressed from non-radiographic to radiographic axSpA. Another problem is that radiographs obtained within clinical practice are usually not scored according to the mNY criteria.
That the ASAS axSpA classification criteria show good agreement with clinical diagnoses in a cohort of established axSpA disease is in accordance with the ASAS-COMOSPA study (Citation8). However, most prior studies have been performed in patients with chronic back pain and suspected axSpA, with diverging results regarding agreement between clinical diagnoses and classification criteria fulfilment (Citation34–39).
Radiographic sacroiliitis was seen in a majority (60%) of the SPARTAKUS patients fulfilling axSpA classification criteria, similarly to another study on patients with established disease from the US Corrona Registry (Citation40).
We found an overall concordance between r-axSpA and AS of 94%, in agreement with a study comparing SpA patients with back pain for 3 months and the presence of radiographic changes in the SI joints from six different SpA and back pain cohorts (Citation4). The most common reason for AS patients not fulfilling the r-axSpA criteria in our study was lack of reporting back pain lasting for ≥ 3 months, while in the above-mentioned study age at disease onset was the most common reason (Citation4). Notably, in our study, patients with ICD-10 codes M468 and M469 had to report back pain for ≥ 3 months before the age of 45 years to be eligible.
The present findings that one-third of patients classified as having AS had a clinical diagnosis of u-axSpA and that nr-axSpA could not be sufficiently captured using clinical diagnoses as proxy, indicate that in studies aiming to compare r-axSpA/AS and nr-axSpA, thorough classification according to defined classification criteria is essential.
In a Swedish validation study of ICD-10 diagnoses for AS and undifferentiated SpA in the Swedish National Patient Register, the results regarding fulfilment of different axSpA (ASAS, Amor, and mNY) classification criteria were similar to our findings (Citation41). There was an almost identical PPV (70%) of a clinical AS diagnosis for fulfilment of the mNY criteria as in our cohort, although they hypothesized this PPV to be in the lower range since patients lacking SI-joint X-rays were counted as not meeting the criteria. Moreover, fewer patients with an undifferentiated SpA diagnosis fulfilled the mNY criteria than in our study, which is possibly explained by our stricter selection of patients with axial disease requiring the presence of back pain for ≥ 3 months for patients with clinical diagnoses M468 and M469 (Citation41).
In another Swedish register study of patients with clinical AS diagnoses, the prevalence of AS was estimated to be 0.18% (Citation42). This may be an underestimation, since clinical u-axSpA diagnoses were not included in their case definition, and we found that 40% of patients with clinical u-axSpA diagnoses fulfilled the mNY criteria for AS.
The main function of classification criteria is to create well-defined, relatively homogeneous groups of patients for research (Citation9, Citation43). Therefore, classification criteria tend to exclude atypical patients, which may result in some patients becoming false negatives, i.e. they do not fulfil classification criteria despite having the diagnosis (Citation9). In our study, three patients demonstrated a typical clinical picture of axSpA, without fulfilling the classification criteria for either axSpA or AS.
Comparison of patients fulfilling versus not fulfilling ASAS axSpA or mNY criteria
Patients fulfilling any axSpA criteria (ASAS or mNY) were, as expected, more likely to display typical axSpA features, such as inflammatory back pain, HLA-B27 positivity, sacroiliitis/SI-joint bone marrow oedema, and uveitis, compared to patients not fulfilling any axSpA criteria, similarly to other studies (Citation8, Citation44). Most patients not fulfilling any axSpA criteria (predominantly women) in our study met the ASAS peripheral SpA criteria. In this group, enthesitis was more frequent, as also reported in the Esperanza cohort comparing patients fulfilling ASAS axSpA and peripheral SpA criteria (Citation44). Moreover, congruent with our results, Cheung et al found inflammatory back pain to be less common among patients not fulfilling any of the ASAS axSpA, ESSG, or Amor classification criteria (Citation45). However, non-fulfillers in their study also had less peripheral arthritis and lower CRP, which was not the case in our study (Citation45); these differences could at least partly be explained by different study designs and inclusion criteria.
Comparison of r-axSpA/AS and nr-axSpA
Not surprisingly, the average symptom duration in this population-based cohort (ranging from 3 to 61 years) was substantially longer than in inception cohorts of new-onset axSpA patients, but will thereby more adequately reflect the overall axSpA population. Notably, also in the nr-axSpA group, the symptom duration was up to 45 years, indicating that not all axSpA patients develop structural SI-joint damage. Our results support the theory that AS and nr-axSpA are subtypes of the same disease, but with somewhat different expressions (Citation43). The finding that the disease burden did not differ substantially between AS and nr-axSpA patients has been reported previously, both in cross-sectional settings (Citation8, Citation35, Citation40, Citation46, Citation47) and in the longitudinal DESIR inception cohort study (Citation15). In addition, the female predominance of the nr-axSpA group is in accordance with earlier studies and illustrates differences in disease phenotype between the sexes (Citation48, Citation49).
Strengths and limitations
A strength of this study is that all patients with a clinical diagnosis of axSpA, both AS and u-axSpA, residing in a defined area, and with clinical visits during a specific period of 4 years to the Department of Rheumatology, Skåne University Hospital, were invited to participate. This study design should make the findings reflect ‘real-world’ rheumatology for axSpA patients, and thereby allow the results to be comparable with most rheumatology clinics and generalizable to other population-based study settings. Furthermore, all participants were assessed thoroughly, according to a predefined protocol, by rheumatologists and physiotherapists with a special interest in axSpA, and were subjected to a detailed imaging algorithm for classification. The same experienced radiologist with a special interest in axSpA scored all radiographic examinations.
A limitation of this study is that not all patients had undergone MRI early in the disease, when inflammatory back pain was predominant. For many patients, disease onset was before MRI examinations were commonly used as a diagnostic tool for identifying sacroiliitis. In addition, certain MRI examinations of the SI joints were performed as part of the study protocol for classification purposes, with some patients already on potent pharmacological treatment. This may have underestimated signs of inflammation.
Only axSpA patients who had been seen at the rheumatology clinic were invited to the study, with possible selection bias towards patients with more severe disease. In addition, for patients with a clinical diagnosis of M468 or M469, back pain for ≥ 3 months and onset before the age of 45 years were required for participation, which may have excluded some with late onset of disease. In a previous study, almost 10% of axSpA patients had disease onset after 45 years of age (Citation50). However, if all patients with clinical diagnoses of M468 and M469 had been included, the predictive values may have been altered by many patients having only peripheral arthritis. Finally, the cross-sectional design of our study, where some patients had long disease duration, creates a risk of recall bias, as patients may not remember how back pain was experienced early in the disease course, and therefore underreport symptoms of inflammatory back pain.
Conclusion
We found an overall good agreement between clinical ICD-10 diagnoses of axSpA and classification criteria fulfilment, with 83% meeting ASAS axSpA or mNY criteria. However, the clinical ICD-10 diagnoses did not identify subtypes of axSpA in a sufficient way, and, in particular, nr-axSpA could not be discerned by ICD codes in a useful manner. This highlights the importance of thorough classification for research questions focusing on disease subtypes, such as comparing r-axSpA/AS and nr-axSpA or assessing the prevalence of axSpA subtypes. Moreover, patients classified as r-axSpA/AS were older, more often men, and had longer disease duration than those with nr-axSpA. Yet, since disease burden and health status were similar for both axSpA subtypes, the issue of greatest clinical importance for the patient group as a whole is to diagnose axSpA, preferably early, enabling proper management to prevent disease progression and impaired health-related quality of life.
Supplemental Material
Download PDF (436.7 KB)Acknowledgements
We thank all the patients and staff involved in the SPARTAKUS study. A special thanks to our research nurse Miriam Walsh-Ingelström for excellent study coordination.
Disclosure statement
TO has received interview fees from Eli Lilly and provided consultancy for Merck, Sharp & Dohme (MSD) (unrelated to the present work). MG has received consultancy fees from UCB Pharma, AbbVie, Novartis and Pfizer (unrelated to the present work). JKW has received consultancy fees from AbbVie; Amgen, Celgene; Eli Lilly and Novartis (unrelated to the present work). EM has received consultancy fees from Novartis (unrelated to the present work). EL and AJ have no competing interests.
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/03009742.2022.2064183.
Additional information
Funding
References
- Rudwaleit M, van der Heijde D, Landewe R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83.
- Rudwaleit M, van der Heijde D, Landewe R, Akkoc N, Brandt J, Chou CT, et al. The Assessment of SpondyloArthritis international Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis 2011;70:25–31.
- van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–8.
- Boel A, Molto A, van der Heijde D, Ciurea A, Dougados M, Gensler LS, et al. Do patients with axial spondyloarthritis with radiographic sacroiliitis fulfil both the modified New York criteria and the ASAS axial spondyloarthritis criteria? Results from eight cohorts. Ann Rheum Dis 2019;78:1545–9.
- Sieper J, van der Heijde D. Review: nonradiographic axial spondyloarthritis: new definition of an old disease? Arthritis Rheum 2013;65:543–51.
- Dougados M, van der Linden S, Juhlin R, Huitfeldt B, Amor B, Calin A, et al. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum 1991;34:1218–27.
- Baraliakos X, Braun J. Spondyloarthritides. Best Pract Res Clin Rheumatol 2011;25:825–42.
- Bakker P, Molto A, Etcheto A, Van den Bosch F, Landewe R, van Gaalen F, et al. The performance of different classification criteria sets for spondyloarthritis in the worldwide ASAS-COMOSPA study. Arthritis Res Ther 2017;19:96.
- Aggarwal R, Ringold S, Khanna D, Neogi T, Johnson SR, Miller A, et al. Distinctions between diagnostic and classification criteria? Arthritis Care Res (Hoboken) 2015;67:891–7.
- Sieper J, Braun J, Dougados M, Baeten D. Axial spondyloarthritis. Nat Rev Dis Primers 2015;1:15013.
- Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet 2017;390:73–84.
- Ritchlin C, Adamopoulos IE. Axial spondyloarthritis: new advances in diagnosis and management. BMJ 2021;372:m4447.
- Wallis D, Haroon N, Ayearst R, Carty A, Inman RD. Ankylosing spondylitis and nonradiographic axial spondyloarthritis: part of a common spectrum or distinct diseases? J Rheumatol 2013;40:2038–41.
- Kiltz U, Baraliakos X, Karakostas P, Igelmann M, Kalthoff L, Klink C, et al. Do patients with non-radiographic axial spondylarthritis differ from patients with ankylosing spondylitis? Arthritis Care Res (Hoboken) 2012;64:1415–22.
- Lopez-Medina C, Molto A, Claudepierre P, Dougados M. Clinical manifestations, disease activity and disease burden of radiographic versus non-radiographic axial spondyloarthritis over 5 years of follow-up in the DESIR cohort. Ann Rheum Dis 2020;79:209–16.
- Wallman JK, Kapetanovic MC, Petersson IF, Geborek P, Kristensen LE. Comparison of non-radiographic axial spondyloarthritis and ankylosing spondylitis patients – baseline characteristics, treatment adherence, and development of clinical variables during three years of anti-TNF therapy in clinical practice. Arthritis Res Ther 2015;17:378.
- Mogard E, Olofsson T, Bergman S, Bremander A, Kristensen LE, Kvistgaard Olsen J, et al. Chronic pain and assessment of pain sensitivity in patients with axial spondyloarthritis: results from the SPARTAKUS cohort. J Rheumatol 2021 Nov;48:1672–9.
- van der Linden S, Akkoc N, Brown MA, Robinson PC, Khan MA. The ASAS criteria for axial spondyloarthritis: strengths, weaknesses, and proposals for a way forward. Curr Rheumatol Rep 2015;17:62.
- Akkoc N, Khan MA. ASAS classification criteria for axial spondyloarthritis: time to modify. Clin Rheumatol 2016;35:1415–23.
- Baraliakos X, Regel A, Kiltz U, Menne HJ, Dybowski F, Igelmann M, et al. Patients with fibromyalgia rarely fulfil classification criteria for axial spondyloarthritis. Rheumatology (Oxford) 2018;57:1541–7.
- Dubreuil M, Deodhar AA. Axial spondyloarthritis classification criteria: the debate continues. Curr Opin Rheumatol 2017;29:317–22.
- Hayward RJ, Machado PM. Classification criteria in axial spondyloarthritis: what have we learned; where are we going? Rheum Dis Clin North Am 2020;46:259–74.23.
- Ortolan A, Kiltz U, Doria A, Aggarwal A, Ramonda R. Do we believe in non-radiographic axial spondyloarthritis? A debate. Autoimmun Rev 2021;20:102703.
- Michelena X, Lopez-Medina C, Marzo-Ortega H. Non-radiographic versus radiographic axSpA: what’s in a name? Rheumatology (Oxford) 2020;59:18–24.
- Lubrano E, De Socio A, Perrotta FM. Unmet needs in axial spondyloarthritis. Clin Rev Allergy Immunol 2018;55:332–9.
- Olofsson T, Lindqvist E, Mogard E, Andreasson K, Marsal J, Geijer M, et al. Elevated faecal calprotectin is linked to worse disease status in axial spondyloarthritis: results from the SPARTAKUS cohort. Rheumatology (Oxford) 2019;58:1176–87.
- Wallman JK, Mogard E, Marsal J, Andreasson K, Joud A, Geijer M, et al. Irritable bowel syndrome symptoms in axial spondyloarthritis more common than among healthy controls: is it an overlooked comorbidity? Ann Rheum Dis 2020;79:159–61.
- Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994;21:2286–91.
- Lukas C, Landewe R, Sieper J, Dougados M, Davis J, Braun J, et al. Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis 2009;68:18–24.
- Heuft-Dorenbosch L, Spoorenberg A, van Tubergen A, Landewe R, van Ver Tempel H, Mielants H, et al. Assessment of enthesitis in ankylosing spondylitis. Ann Rheum Dis 2003;62:127–32.
- Calin A, Garrett S, Whitelock H, Kennedy LG, O’Hea J, Mallorie P, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol 1994;21:2281–5.
- Jenkinson TR, Mallorie PA, Whitelock HC, Kennedy LG, Garrett SL, Calin A. Defining spinal mobility in ankylosing spondylitis (AS). The Bath AS Metrology Index. J Rheumatol 1994;21:1694–8.
- Hurst NP, Jobanputra P, Hunter M, Lambert M, Lochhead A, Brown H. Validity of Euroqol–a generic health status instrument–in patients with rheumatoid arthritis. Economic and Health Outcomes Research Group. Br J Rheumatol 1994;33:655–62.
- Tomero E, Mulero J, de Miguel E, Fernandez-Espartero C, Gobbo M, Descalzo MA, et al. Performance of the Assessment of SpondyloArthritis international Society criteria for the classification of spondyloarthritis in early spondyloarthritis clinics participating in the ESPERANZA programme. Rheumatology (Oxford) 2014;53:353–60.
- Sepriano A, Landewe R, van der Heijde D, Sieper J, Akkoc N, Brandt J, et al. Predictive validity of the ASAS classification criteria for axial and peripheral spondyloarthritis after follow-up in the ASAS cohort: a final analysis. Ann Rheum Dis 2016;75:1034–42.
- Meghnathi B, Saraux A, Dougados M, Molto A. Evaluation of the predictive validity of the ASAS axial spondyloarthritis criteria in the DESIR cohort. Clin Exp Rheumatol 2019;37:797–802.
- Ez-Zaitouni Z, Bakker PAC, van Lunteren M, Berg IJ, Landewe R, van Oosterhout M, et al. Presence of multiple spondyloarthritis (SpA) features is important but not sufficient for a diagnosis of axial spondyloarthritis: data from the SPondyloArthritis Caught Early (SPACE) cohort. Ann Rheum Dis 2017;76:1086–92.
- Deodhar A, Mease PJ, Reveille JD, Curtis JR, Chen S, Malhotra K, et al. Frequency of axial spondyloarthritis diagnosis among patients seen by us rheumatologists for evaluation of chronic back pain. Arthritis Rheumatol 2016;68:1669–76.
- Kiil RM, Mistegaard CE, Jurik AG, Christiansen AA, Hendricks O, Schiottz-Christensen B, et al. Diagnosing axial spondyloarthritis by multidiciplinary team conference at 3.5 years’ follow-up in a cohort of patients with disease features according to the ASAS criteria. E4Scand J Rheumatol. 2021;15:1–9.
- Mease PJ, Heijde DVD, Karki C, Palmer JB, Liu M, Pandurengan R, et al. Characterization of patients with ankylosing spondylitis and nonradiographic axial spondyloarthritis in the US-based Corrona registry. Arthritis Care Res (Hoboken) 2018;70:1661–70.
- Lindstrom U, Exarchou S, Sigurdardottir V, Sundstrom B, Askling J, Eriksson JK, et al. Validity of ankylosing spondylitis and undifferentiated spondyloarthritis diagnoses in the Swedish national patient register. Scand J Rheumatol 2015;44:369–76.
- Exarchou S, Lindstrom U, Askling J, Eriksson JK, Forsblad-d’Elia H, Neovius M, et al. The prevalence of clinically diagnosed ankylosing spondylitis and its clinical manifestations: a nationwide register study. Arthritis Res Ther 2015;17:118.
- Braun J, Baraliakos X, Kiltz U, Heldmann F, Sieper J. Classification and diagnosis of axial spondyloarthritis – what is the clinically relevant difference? J Rheumatol 2015;42:31–8.
- Del Rio-martinez P, Navarro-Compan V, Diaz-Miguel C, Almodovar R, Mulero J, De Miguel E, et al. Similarities and differences between patients fulfilling axial and peripheral ASAS criteria for spondyloarthritis: results from the Esperanza cohort. Semin Arthritis Rheum 2016;45:400–3.
- Cheung PP, Paternotte S, Burki V, Durnez A, Elhai M, Fabreguet I, et al. Performance of the assessment in SpondyloArthritis international Society classification for axial and peripheral spondyloarthritis in an established clinical cohort: comparison with criteria sets of Amor and the European Spondylarthropathy Study Group. J Rheumatol 2012;39:816–21.
- Bubova K, Forejtova S, Zegzulkova K, Gregova M, Husakova M, Filkova M, et al. Cross-sectional study of patients with axial spondyloarthritis fulfilling imaging arm of ASAS classification criteria: baseline clinical characteristics and subset differences in a single-centre cohort. BMJ Open 2019;9:e024713.
- Akasbi N, Siar N, Zoukal S, Kohen KE, Harzy T. Comparison of non-radiographic axial spondyloarthritis and ankylosing spondyltis from a single rheumatology hospital in Morocco. Curr Rheumatol Rev 2020;16:240–4.
- Ortolan A, van Lunteren M, Ramiro S, Ramonda R, Landewe RBM, Dagfinrud H, et al. Are gender-specific approaches needed in diagnosing early axial spondyloarthritis? Data from the SPondyloArthritis Caught Early cohort. Arthritis Res Ther 2018;20:218.
- Chimenti MS, Alten R, D’Agostino MA, Gremese E, Kiltz U, Lubrano E, et al. Sex-associated and gender-associated differences in the diagnosis and management of axial spondyloarthritis: addressing the unmet needs of female patients. RMD Open 2021;7:e001681.
- Bendahan LT, Machado NP, Mendes JG, Oliveira TL, Pinheiro MM. Performance of the classification criteria in patients with late-onset axial spondyloarthritis. Mod Rheumatol 2018;28:174–81.
