Abstract
Objective
Patients with anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) require immunosuppressive therapy for disease control and relapse prevention and may be at risk for severe coronavirus disease 2019 (COVID-19). The study objective was to analyse risk factors and outcomes of COVID-19 in well-characterized AAV patients.
Method
Data were retrieved from March 2020 to May 2021 from medical records of AAV cohorts in Stockholm and Uppsala, Sweden. COVID-19 was confirmed by positive PCR test or by ELISA. Severe COVID-19 was defined as need for non-invasive ventilation, intensive care unit care, and/or death. Age, gender, ANCA antibody type, ongoing immunosuppressive medication, and estimated glomerular filtration rate were recorded.
Results
The cohort comprised 310 AAV patients, of whom 29 (9%) were diagnosed with COVID-19. Four deaths were attributed to COVID-19. Fifteen patients (52%) were on prednisolone in the COVID-19 group and 130 (46%) in the non-COVID group, with significantly higher doses in COVID-19 patients (p < 0.01). Ongoing induction therapy was more prevalent in the COVID-19 group (p < 0.01). Severe COVID-19 was diagnosed in 9/29 (31%). Significant risk factors for severe COVID-19 were impaired kidney function (p = 0.01) and more intense immunosuppressive therapy (p = 0.02), with a trend for age (p = 0.07). Maintenance therapy with rituximab was not associated with severe COVID-19.
Conclusions
Our findings highlight risks and suggest that more attention should be given to optimal AAV treatment in a pandemic situation. They also emphasize the need for continued shielding, mitigation strategies, and effective vaccination of AAV patients.
Coronavirus disease 2019 (COVID-19) was declared a pandemic by the World Health Organization in March 2020. Lung involvement is the most common disease manifestation, but extrapulmonary manifestations are prevalent (Citation1, Citation2). Age, male gender, and chronic kidney disease (CKD) were soon identified as significant risk factors for hospital admission, critical illness, and mortality (Citation3). Data regarding a more detailed risk profile and granular treatment aspects of patients with anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV), a disease mainly affecting the respiratory tract and kidneys, have remained limited.
There has been much concern regarding ongoing immunosuppressive therapy in patients during the pandemic paired with a potential heightened vulnerability for severe infection on top of underlying immune-mediated diseases, age, and associated comorbidities. The COVID-19 Global Rheumatology Alliance reported outcomes in 3729 severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2)-infected patients, in which older age, male gender, CKD, and other comorbidities were associated with COVID-19-related death. Factors such as moderate/high disease activity, a diagnosis of vasculitis, and treatment with rituximab (RTX) (compared to methotrexate) were associated with mortality (Citation4). In another study examining biologics specifically, enrolling 1116 patients, RTX remained the only factor associated with hospitalization risk in a multivariate analysis (Citation5). Whether RTX maintenance therapy had similar COVID-19-associated risks compared to RTX induction was not determined in these studies.
The aim of the current study was to investigate the consequences of COVID-19 in a large cohort of AAV patients, with regard to incidence, underlying risk factors, treatment, need for hospitalization, intensive care unit (ICU) care, or death. An additional aim was to compare the risks in active patients with those in patients in remission regarding RTX induction versus maintenance treatment.
Method
In a collaborative project between the Departments of Nephrology and Rheumatology at Karolinska University Hospital in Stockholm and the Department of Rheumatology at Uppsala University Hospital, AAV patients were previously included in large research cohorts. The occurrence and consequences of COVID-19 were retrieved from medical records or the Swedish Rheumatology Registry from March 2020 to January 2021 (Karolinska) and to May 2021 (Uppsala) for living patients in these cohorts (n = 310). Patients diagnosed with granulomatosis with polyangiitis (n = 232), microscopic polyangiitis (n = 56), or eosinophilic granulomatosis with polyangiitis (n = 22) were included. The eligibility criteria were the Chapel Hill Consensus Conference 2012 definitions, and a current or prior positive test for proteinase-3 (PR3)- or myeloperoxidase (MPO)-ANCA or the European Medicines Agency (EMA) classification (Citation6, Citation7). The Swedish Ethical Review Authorities approved the study.
Retrieved data included age, gender, diagnosis, and antibody type (PR3 or MPO), as well as ongoing immunosuppressive medication at the onset of COVID-19, or at last follow-up during the study period in non-COVID individuals. Usage and doses of corticosteroids, disease-modifying anti-rheumatic drugs (DMARDs), cyclophosphamide (CYC), and RTX were tabulated.
Kidney involvement (ever), lung involvement (ever), and estimated glomerular filtration rate (eGFR) at the onset of COVID-19 or last follow-up (Citation8) were recorded. Active disease was specified as the recent onset of induction therapy with high-dose corticosteroids, RTX, and/or CYC. Patients on maintenance therapy were regarded as inactive based on their latest visit and a Birmingham Vasculitis Activity Score (BVAS) = 0.
Diagnosis of COVID-19
COVID-19 was confirmed either by a positive upper airway polymerase chain reaction (PCR) test or by serology [enzyme-linked immunosorbent assay (ELISA)]. Severe COVID-19 was defined as requiring non-invasive ventilation, ICU care, and/or death.
Statistics
Statistical analysis was performed using GraphPad Prism (version 9.1 for Windows). Data are presented as mean ± standard deviation (for parametric data) or median and interquartile range (IQR) (for non-parametric data). Differences in continuous variables between groups were assessed by the Student’s t-test (parametric) or Mann–Whitney unpaired t-test (non-parametric). The chi-squared test was used to compare categorical variables, presented as count (%). Significant differences were statistically defined by p values ≤ 0.05.
Results
The study included 157 females and 153 males, of whom 38 females and 39 males were in the Uppsala cohort. Median age at data retrieval was 67 years (IQR 53.5–76.0 years). In total, 29 patients (9%) developed COVID-19 and were diagnosed either by PCR (n = 19) or by ELISA (n = 9). In one case in the early pandemic phase, no COVID-19 testing was performed and the diagnosis was based on the clinical features and pulmonary radiology.
The COVID-19 patients were younger [median age 60 years (IQR 40.5–72.5 years)] than the non-COVID-19 group [median age 68 years (IQR 55.0–77.0 years)] (p = 0.02).
Previous lung and kidney involvement was recorded in 170 patients and 169 patients (55%), respectively, but did not differ between patients who developed COVID-19 and those who did not (ns). Neither gender nor AAV subtype differed between groups ().
Table 1. Characteristics of the patients diagnosed with COVID-19 and non-COVID-19 patients.
Development of severe COVID-19
Of the 29 patients with COVID-19, 13 were hospitalized and nine (31%) developed severe COVID-19. The median age of patients with severe COVID-19 was higher than that of those with non-severe disease, but this difference was not statistically significant. Four patients died of COVID-19-related complications (14%) (). The approximate duration of disease until death ranged from 11 to 27 days.
Table 2. Characteristics comparing non-severe and severe COVID-19 infection.
Corticosteroids and immunosuppressive therapy as risk factors
Prednisolone treatment was ongoing in 15 out of 29 COVID-19 patients (52%) compared to 130 out of 281 (46%) in the non-COVID-19 group.
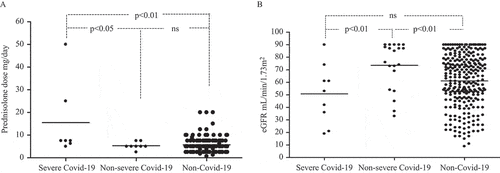
Prednisolone doses were significantly higher in the COVID-19 patients [median dose 6.25 mg (IQR 2.5–50 mg)] than in the non-COVID-19 group [median 5 mg (IQR 0.625–20 mg)] (p < 0.01) ( and ).
Figure 1. (A) Prednisolone dose (mg/day) in anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) patients with severe coronavirus disease 2019 (COVID-19) (n = 9) and non-severe COVID-19 (n = 20), and the rest of the cohort (n = 281); (B) estimated glomerular filtration rate (eGFR) (mL/min/1.73 m2) in AAV patients with severe COVID-19 (n = 9) and non-severe COVID-19 (n = 20), and the rest of the cohort (n = 281).
In total, 182 patients (65%) in the non-COVID-19 group and 19 (65.5%) in the COVID-19 group were on other ongoing disease-modifying drugs (ns). A higher proportion of patients with COVID-19 (4/29, 14%) were on induction therapy (CYC and/or RTX) compared to non-COVID-19 patients (7/281, 2.5%) (p < 0.01), but there was no difference regarding the use of CYC only (ns) ().
The range of AAV disease duration prior to COVID-19 infection was 4–339 months (median 118 months).
In patients with severe COVID-19, seven out of nine (78%) had prednisolone treatment compared to eight out of 20 (40%) in the non-severe group. The median prednisolone dose was higher in patients with severe COVID-19 [7.5 mg (IQR 5–50 mg] compared with those with non-severe disease [5 mg (IQR 2.5–5 mg)] (p = 0.02) ().
In the severe COVID-19 group, seven out of nine patients were on DMARDs, compared with 12 out of 20 in the non-severe group (ns). Three patients (33%) were on induction therapy versus one patient (5%) in the non-severe group (p = 0.08). Two severe patients were treated with CYC versus none in the non-severe group (p = 0.05). There was no difference when comparing severity versus non-severity in patients on RTX maintenance therapy in doses ranging from 500 to 1000 mg every 6 months ().
Kidney function and COVID-19
The median eGFR was 64 mL/min/1.73 m2 and did not differ between patients with and those without COVID-19 (ns) (). However, patients with severe COVID-19 had a lower eGFR prior to infection [median 53 mL/min/1.73 m2 (IQR 28.5–67.5 mL/min/1.73 m2) compared to non-severe cases [median 80.5 mL/min/1.73 m2 (IQR 61.5–89.0 mL/min/1.73 m2)] (p = 0.01) ().
Discussion
In this study, we report a high incidence of severe COVID-19 in patients with AAV, adding to previous data (Citation9), with a high mortality rate in affected individuals. Risk factors for severe COVID-19 were impaired kidney function, more intense immunosuppressive therapy, which may also reflect an active AAV disease state as a potential risk, and a trend for age. There were fewer older AAV patients with COVID-19, suggesting that they were better at shielding. RTX as maintenance therapy, however, did not increase the risk of severe COVID-19, which is a novel and reassuring finding. Neither gender nor AAV subtype or antibody type was associated with severe disease.
Prednisolone doses were significantly higher in individuals with severe COVID-19 compared with non-severe patients, which can probably be explained by higher doses during ongoing or recent induction therapy. A higher proportion of patients with COVID-19 was also on other induction therapy (RTX and/or CYC), compared to non-COVID-19 patients.
Treatment data and outcome for 694 COVID-19 patients with inflammatory rheumatic and musculoskeletal diseases from a French registry reported that older age, comorbidities including CKD, and use of prednisone ≥ 10 mg/day or equivalent, mycophenolate mofetil, or RTX were linked with COVID-19 severity, but methotrexate, tumour necrosis factor inhibitors, and interleukin-6 inhibitors were not (Citation10). Furthermore, the COVID-19 Global Rheumatology Alliance published a retrospective study, including 352 patients with AAV. Older age, higher disease activity, and treatment with CYC and RTX, compared to no DMARDs, were associated with severe infection. Among comorbidities, only CKD was associated with a higher risk of severe COVID-19. In the total cohort, a daily dose of prednisolone > 10 mg, compared to no prednisolone, was also associated with more severe COVID-19 (Citation11). The UKIVAS results published by Rutherford et al comprised 65 COVID-19-infected patients with vasculitis, including 55 patients with AAV. An increased risk of severe disease outcome was found in patients with comorbid respiratory disease and any dose of prednisolone at presentation (Citation12).
Previous lung involvement did not seem to predispose for more severe infection in our study, whereas impaired kidney function did. The latter has been shown to be an independent risk factor for severe and fatal COVID-19 (Citation3). Importantly, incomplete kidney function recovery after COVID-19 has also been reported in patients with glomerulonephritis and vasculitis, and thus warrants increased attention (Citation13).
The current study has both strengths and weaknesses. The study population comprises a large cohort with well-characterized patients and consistent data collection. However, we have not been able to gather information on, and correct for, other risk factors known to be associated with severe COVID-19, such as obesity and comorbidities other than CKD and previous lung involvement. Moreover, some cases may have been missed owing to the retrospective collection of data, and some patients who were deemed to be inactive based on their latest visit may have had subclinical disease. Finally, patients in Stockholm were included in 2020 through March 2021 and those in Uppsala up to May 2021. The vaccine rollout for risk groups such as AAV started in late March–April of 2021 in Sweden and it is therefore unlikely that vaccines had a substantial impact on the results of this study.
The novel and reassuring finding that RTX maintenance therapy was not associated with a more severe disease course suggests that adjusting immunosuppressive therapy is important when clinically possible. It is, however, well known that B-cell depletion suppresses the vaccine antibody response, hampering protection against COVID-19 infection (Citation14). Postponing maintenance therapy with RTX in stable inactive patients may therefore be considered to achieve better vaccine response.
Conclusion
In summary, patients with AAV are at risk of severe COVID-19 driven by known risk factors such as older age, but also impaired kidney function. Higher steroid doses and ongoing induction therapy are also significant risk factors, which raises questions about the optimal induction treatment in a pandemic situation. Our findings highlight the need for continued shielding and mitigation strategies, early recognition of COVID-19, and a strategy for prompt vaccination and booster doses in AAV patients.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med 2020;26:1017–32.
- Petrilli CM, Jones SA, Yang J, Rajagopalan H, O’Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 2020;369:m1966.
- Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430–6.
- Strangfeld A, Schafer M, Gianfrancesco MA, Lawson-Tovey S, Liew JW, Ljung L, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis 2021;80:930–42.
- Felten R, Duret PM, Bauer E, Sedmak N, Djossou JH, Bensalem M, et al. B-cell targeted therapy is associated with severe COVID-19 among patients with inflammatory arthritides: a 1-year multicentre study in 1116 successive patients receiving intravenous biologics. Ann Rheum Dis 2022;81:143–5.
- Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheumatol 2013;65:1–11.
- Watts R, Lane S, Hanslik T, Hauser T, Hellmich B, Koldingsnes W, et al. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis 2007;66:222–7.
- Nyman U, Grubb A, Larsson A, Hansson LO, Flodin M, Nordin G, et al. The revised Lund-Malmo GFR estimating equation outperforms MDRD and CKD-EPI across GFR, age and BMI intervals in a large Swedish population. Clin Chem Lab Med 2014;52:815–24.
- Kant S, Morris A, Ravi S, Floyd L, Gapud E, Antichos B, et al. The impact of COVID-19 pandemic on patients with ANCA associated vasculitis. J Nephrol 2021;34:185–90.
- FAI2R/SFR/SNFMI/SOFREMIP/CRI/IMIDIATE consortium and contributors. Severity of COVID-19 and survival in patients with rheumatic and inflammatory diseases: data from the French RMD COVID-19 cohort of 694 patients. Ann Rheum Dis. 2020;80:527–38.
- Sattui SE, Conway R, Putman MS, Seet AM, Gianfrancesco MA, Beins K, et al. Outcomes of COVID-19 in patients with primary systemic vasculitis or polymyalgia rheumatica from the COVID-19 Global Rheumatology Alliance physician registry: a retrospective cohort study. Lancet Rheumatol 2021;3:e855–e864.
- Rutherford MA, Scott J, Karabayas M, Antonelou M, Gopaluni S, Gray D, et al. UK and Ireland Vasculitis Rare Disease Group (UKIVAS). Risk factors for severe outcomes in patients with systemic vasculitis and COVID-19: a binational, registry-based cohort study. Arthritis Rheumatol 2021;73:1713–19.
- Waldman M, Soler MJ, Garcia-Carro C, Lightstone L, Turner-Stokes T, Griffith M, et al. Results from the IRoc-GN international registry of patients with COVID-19 and glomerular disease suggest close monitoring. Kidney Int 2021;99:227–37.
- Bruchfeld A, Kronbichler A, Alberici F, Fervenza FC, Jayne DRW, Segelmark M, et al. COVID-19 and ANCA-associated vasculitis: recommendations for vaccine preparedness and the use of rituximab. Nephrol Dial Transplant 2021;36:1758–60.
