Abstract
Objectives
This study assessed the position of apremilast in the treatment pathway of psoriasis (PsO) and psoriatic arthritis (PsA) in Finnish clinical practice, compared the characteristics of apremilast and biologic therapy users, evaluated persistence with apremilast and identified factors influencing treatment discontinuation.
Method
This retrospective study used data from Finnish national health registries. The target group was identified based on L40* diagnosis and medication records between 2015 and 2018. Treatment persistence was analysed using Kaplan–Meier curves and Cox regression.
Results
Of eligible patients (PsO 31 202; PsA 12 386), 1% (n = 471) used apremilast and 10% (n = 4214) biologics, apremilast users being older (mean age 55.9 vs 52.4 years, p < 0.001) with a higher Charlson comorbidity score (0.71 vs 0.54, p < 0.001). Most patients switched to apremilast from conventional synthetic therapy (PsO 75%; PsA 76%); 47% of patients remained on apremilast during the observation period (PsO 58%; PsA 42%). Most patients discontinuing apremilast switched to biologics (PsO 51%; PsA 51%). Apremilast persistence increased with age (p = 0.042) and was higher in PsO than in PsA (median 14 vs 11 months; p = 0.005). Compared to prior conventional synthetic therapy, prior biologic therapy decreased persistence (hazard ratio for discontinuation 2.15, 95% confidence interval 1.42–3.25).
Conclusion
In Finnish clinical practice, apremilast is mainly used between conventional synthetic therapy and biologics, with at least as high treatment persistence as reported in previous studies. Apremilast users were older with higher comorbidity burden than biologics users.
Psoriatic diseases are a group of heterogeneous chronic inflammatory diseases causing mainly skin (psoriasis, PsO) and joint (psoriatic arthritis, PsA) disorders, and are associated with high rates of cardiovascular and other comorbidities (Citation1). While the cause of psoriatic diseases is unclear, they are thought to have an autoimmune disease mechanism with genetic and environmental susceptibility factors (Citation2). Recently, an association between a genetic polymorphism in inflammasome-related genes and PsA was found (Citation3). Since psoriasis is a complex disease, patient preference, disease severity, and comorbidities should be considered when selecting the treatment.
While targeted biologic therapies have transformed the treatment landscape for psoriatic diseases, more research is required regarding different treatment strategies. Tumour necrosis factor (TNF) inhibitors were the first licensed biologics in psoriatic diseases and are widely used in clinical practice (Citation4). More recently, biologics with alternative mechanisms of action have been approved. For example, interleukin (IL)-12/23, IL-17A, and IL-23 inhibitors have been shown to be effective in the treatment of PsO and PsA.
In addition to biologics, another relatively new therapy available for PsA and PsO is apremilast (Citation5). Apremilast is a small-molecule inhibitor of phosphodiesterase-4 with an intracellular mechanism of action that increases levels of cyclic adenosine monophosphate (cAMP) expressed by immune cells. The increase in cAMP concentration favours the activation of protein kinase A (PKA), which, in turn, phosphorylates and indirectly inhibits nuclear factor-κB (NF-κB). This intracellular signalling promotes the synthesis of anti-inflammatory signals and inhibits the production of inflammatory mediators. In Finland, apremilast is reimbursed for PsO and PsA as a second line therapy when conventional synthetic therapy is insufficient, contraindicated, or otherwise inappropriate (Citation6). Apremilast is associated with low infection risk (Citation7), does not require follow-up blood counts or liver enzymes, and is administered orally. In clinical trials, apremilast had lower rates of efficacy than biologics (Citation8, Citation9). However, no data comparing apremilast and biologics in the same population are available and few studies have described apremilast use in real-world settings (Citation10–15). The national Finnish guideline for psoriasis, also including psoriasis arthritis, is evidence based and recommends apremilast, similarly to biologics, as one of the secondary systemic treatments after conventional synthetic disease-modifying anti-rheumatic drugs (DMARDs). The guideline emphasizes individualized treatment according to the activity of the disease and its manifestations (Citation16).
In this study, we describe apremilast use among over 43 000 patients with PsO and/or PsA in Finland, and compare the patient characteristics of apremilast and biologic therapy users. In addition, apremilast treatment persistence and factors predicting the drug discontinuation were identified.
Method
This retrospective register-based cohort study included data derived through linkage of national population-based registers: the Care Register for Healthcare in Finland (CRHF) and the Prescription Centre. The CRHF is controlled by the National Institute for Health and Welfare of Finland and collects comprehensive data on the activities of Health Care Centres, hospitals, and other institutions providing inpatient and outpatient care, and on the patients treated in them. The Prescription Centre registry includes all prescriptions and dispensing events made by community pharmacies. This study was approved by the National Institute for Health and Welfare of Finland (permission number THL/1680/5.05.00/2019).
Study population
We included individuals in primary, secondary, or tertiary care (inpatient or outpatient care) with an International Classification of Diseases, 10th Revision (ICD-10) code indicating psoriatic diseases (L40.0, L40.1, L40.2, L40.3, L40.4, L40.5, L40.8, and L40.9) as a primary or secondary diagnosis, recorded in the CRHF between 1 January 2015 and 31 December 2018. Medications in the prescription registry dispensed during the same period were then linked to eligible patients.
The study population was divided into PsA and PsO cohorts: patients with ICD-10 codes L.40.5 or L40* + M07.0, M07.1, M07.2, M07.3, or M09.0 were included in the PsA cohort and all other patients were included in the PsO cohort. Individuals with at least one record of apremilast [Anatomical Therapeutic Chemical (ATC) code L04AA32] dispensed from a Community Pharmacy were defined as apremilast users. Similarly, patients on biologic therapy (TNF inhibitors and interleukin inhibitors) were identified based on dispensed medication.
The validity of psoriasis diagnoses has been previously studied in Finland (Citation17). The positive predictive value of ICD-10 code L40.0 for psoriasis compared to a chart review of electronic medical records was 88.0%. For patients using systemic treatment for psoriasis, the validity is likely to be even higher. Patients requiring systemic treatment for psoriasis are entitled to higher than regular reimbursement for medication in Finland. The certificate needed for reimbursement can be written only by a dermatologist, rheumatologist, or resident working at relevant specialist clinics. For the more expensive drugs such as biologics or apremilast, a similar certificate from a specialist is needed even for the regular reimbursement. Therefore, it is likely that only very few, if any, patients will use these medications without a visit to a specialist clinic for the confirmation of the diagnosis and reimbursement application.
Assessment of treatment lines
Treatment lines before and after apremilast initiation were classified as follows: conventional synthetic therapy (methotrexate, sulfasalazine, acitretin, leflunomide, and cyclosporine), biologic therapy [divided into TNF inhibitors (etanercept, adalimumab, certolizumab, and golimumab) and interleukin inhibitors (ustekinumab, secukinumab, brodalumab, guselkumab, and ixekizumab)], januse kinase (JAK) inhibitors (for PsA), non-systemic treatment (including topical treatment as well as no documented previous treatment), and other medication (azathioprine, hydroxychloroquine, or sodium aurothiomalate). Only treatment lines related to medication were considered, and they may be combined with ultraviolet (UV) therapies.
Comorbidities
Comorbidities based on both primary and secondary diagnoses (ICD-10 codes) from specialist inpatient and outpatient care were assessed during the observation period (2015–2018). Comorbidities were categorized as Acute coronary syndrome, cancer, cardiac diseases, cerebrovascular disease, chronic obstructive pulmonary disease (COPD), diabetes, infections, renal disease, and thromboembolism (Supplementary Table). A Charlson comorbidity score without adjustment for age was calculated for each patient (Citation18).
Apremilast treatment duration
Duration of apremilast treatment (persistence) was defined as the time between apremilast initiation (dispensed for the first time from a pharmacy) and initiation of the next treatment line (e.g. conventional synthetic therapy or biologic therapy), when the patient stopped using apremilast, or end of follow-up. Initiation of conventional synthetic therapy was considered as initiation of the next treatment only in cases were apremilast was discontinued. Otherwise, conventional synthetic therapy was interpreted as an add-on therapy to apremilast. A patient was assumed to have stopped using apremilast if no apremilast was dispensed to the patient for a period of 6 months. As we did not have access to information on prescribed dose or days’ supply dispensed, we assessed persistence with apremilast based on dispensation dates without considering the dispensed quantity.
Statistical analysis
Continuous variables were summarized using standard statistical measures, i.e. number of observations, mean, and standard deviation (sd). Pearson’s chi-squared test was used to compare categorical variables and analysis of variance (ANOVA) to compare continuous variables. Apremilast persistence was analysed using Kaplan–Meier curves and multivariate Cox regression analysis. In these analyses, apremilast discontinuation was the outcome event. Patients were censored at loss to follow-up or the end of the study period. The following variables were used in the multivariate Cox regression analyses: gender, age, diagnosis (PsO vs PsA), Charlson comorbidity score, and previous therapy. p-Values less than 0.05 were considered statistically significant. Statistical analysis was performed using Python version 3.7.4 with Pandas and other standard statistical packages. Missing values were encoded as missing and no data imputation was performed.
Results
Patient characteristics
Of 43 588 eligible patients, 28.4% (n = 12 386) were diagnosed with PsA and 71.6% (n = 31 202) with PsO only (). Mean ± sd age was 55.8 ± 17.9 years; 51% of patients were male. The most common comorbidities were infectious disease (51% of all patients), cardiac disease (38%), and diabetes (17%).
Table 1. Patient characteristics and comparison of the sub-cohorts.
We identified 471 (1.1%) apremilast users and 4214 (9.7%) biologics users. In both groups, approximately 70% (apremilast 318/471; biologics 2812/4214) were diagnosed with PsA. Compared with biologics users, apremilast users were older, more likely to be female, and had a higher comorbidity burden. Mean ± sd Charlson comorbidity score was higher in apremilast users without adjustment for age (0.71 ± 0.92 vs 0.54 ± 0.85, respectively).
Treatment lines before and after apremilast
Immediately before apremilast initiation, 75% (114/153) of PsO patients had received conventional synthetic therapy and 5% (8/153) had received biologic therapy; 20% (30/153) initiated apremilast without earlier systemic treatment (). Among PsA patients, 76% (241/318), 12% (37/318), and 5% (16/318) had received conventional synthetic therapies, biologics, and other systemic medications before initiating apremilast, respectively; 8% (24/318) of PsA patients did not use any systemic treatment before apremilast use.
Figure 1. Treatment lines before initiation and after discontinuation of apremilast among patients with psoriatic arthritis (PsA) and patients with psoriasis (PsO). (Other medication: azathioprine, hydroxychloroquine, or sodium aurothiomalate). TNF, tumour necrosis factor.
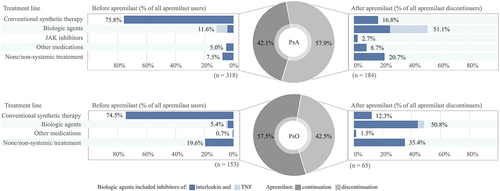
Among apremilast users, 58% (88/153) of those with PsO and 42% (134/318) of those with PsA remained on apremilast until the end of the observation period. The median follow-up time of apremilast use was 18 months (1st quartile 10 months, 3rd quartile 26 months). Among PsO patients discontinuing apremilast, the majority switched to interleukin inhibitors [46% (30/65)] or conventional synthetic therapy [12% (8/65)]. In addition, for 35% (23/65) of PsO patients, no systemic therapy was found after apremilast discontinuation. Among PsA patients discontinuing apremilast, 26% (48/184), 25% (46/184), and 17% (31/184) switched to TNF inhibitors, interleukin inhibitors, and conventional synthetic therapy, respectively. For 21% (38/184) of PsA patients, no systemic therapy was found after apremilast discontinuation.
Approximately 18% (8/45) of those PsO or PsA patients who used biologics before apremilast initiation used conventional synthetic therapy as add-on therapy with biologics. Correspondingly, the conventional synthetic therapy was combined with apremilast for 30% (141/471) of patients. After apremilast discontinuation, conventional synthetic therapy was combined with biologics/JAK inhibitors for 26% (34/132) of patients.
Apremilast persistence
Apremilast persistence was higher among patients with PsO than patients with PsA (median treatment duration 14 vs 11 months, respectively, p = 0.005) (). In addition, prior treatment line had a statistically significant effect on apremilast discontinuation. Median treatment duration was 7 months after biologic therapy, 6 months after other therapies (azathioprine, hydroxychloroquine, or sodium aurothiomalate), 12 months after conventional synthetic therapy, and 16 months without any previous systemic therapies (). The drug persistence in different age groups is depicted in ). In multivariate Cox regression analysis of apremilast discontinuation, a diagnosis of PsO [p = 0.020, hazard ratio (HR) 0.71, 95% confidence interval (CI) 0.53–0.95], and older age (p = 0.042, HR 0.99, 95% CI 0.98–1.00) were associated with higher apremilast persistence (). Conversely, prior biologic therapy (p < 0.001, HR 2.15, 95% CI 1.42–3.25), prior other therapies (p = 0.015, HR 2.09, 95% CI 1.15–3.78), and higher Charlson comorbidity score (p = 0.046, HR 1.16, 95% CI 1.00–1.35) were associated with lower apremilast persistence.
Figure 2. Kaplan–Meier analysis of apremilast persistence. (A) Patients with psoriatic arthritis (PsA) and patients with psoriasis (PsO); (B) treatment line before apremilast initiation (the most recent treatment before apremilast initiation); (C) age group. y-axis: probability of continuing apremilast treatment; x-axis: time from apremilast initiation to discontinuation (months).
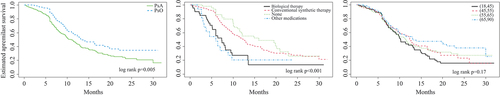
Table 2. Multivariate Cox regression analysis of apremilast discontinuation.
Discussion
Apremilast is a relatively new treatment option for patients with psoriatic diseases and is indicated in patients with PsO and PsA with an inadequate response or intolerance to a prior conventional synthetic therapy. In this retrospective registry study of 43 588 patients treated in Finnish clinical practice, 1% (n = 471) of patients with PsO or PsA were treated with apremilast and 10% (n = 4214) were treated with biologics.
Most patients (around 75%) moved to apremilast from conventional synthetic therapy. Compared with PsA patients, median treatment duration was higher among those with PsO (14 vs 11 months). In addition, prior treatment line, age, and Charlson comorbidity score were predictors of apremilast persistence. However, 42% of PsO patients and 58% of PsA patients discontinued apremilast during the observation period. The majority of these patients switched to a biologic therapy (PsO 51%; PsA 51%), indicating that apremilast is typically located between conventional synthetic therapy and biologic therapy in Finnish clinical practice. Of note, approximately one-third (143/471) of apremilast users received conventional synthetic therapy as add-on therapy and 16% switched to it in the next treatment line. This may reflect the diversity of the clinical scenarios in treating PsO and PsA patients.
Median time to apremilast discontinuation was higher for patients with PsO than for those with PsA (14 vs 11 months), suggesting that the more severe symptoms of PsA require a higher number of medications to find an optimal treatment balance. Overall, the observed persistence of apremilast treatment seems to be at least as high as in other published real-life studies (Citation10–15). However, direct comparisons are difficult owing to differences in the definitions of persistence. In our study, the permissible gap between two dispensations was 6 months, which is quite long and may lead to overestimation of persistence. Conversely, the use of shorter permissible gaps could lead to underestimation of persistence, as a recent US study showed that stopping and reinitiation of apremilast is common (Citation19). In addition, the treatment line before apremilast was a predictor of persistence, indicating that apremilast persistence is higher when it is initiated after less effective therapies. However, in contrast to our results, it has also been reported that previous treatment line does not predict apremilast treatment persistence (Citation12).
Compared with biologics users, apremilast users were older (56 vs 52 years) and had a higher comorbidity burden (mean Charlson comorbidity score 0.71 vs 0.54). Patients in our study had a higher overall comorbidity burden than patients in the APPRECIATE study (Citation15), where only four diseases (metabolic syndrome, hypertension, obesity, and depression) were prevalent in over 5% of patients. The documentation methods of comorbidities may differ between the studies; here, the comorbidities were characterized based on diagnoses in national healthcare registries. In the present study, comorbidities were more common in apremilast users than biologics users. One possible explanation for this disparity could be the different contraindications and monitoring requirements for apremilast and biologics, meaning that apremilast may be a more practical option for patients with a high burden of comorbidities. Moreover, apremilast users in our study were older than biologics users, suggesting that the older patients may prefer the oral dosing of apremilast to injected biologics. The finding that treatment persistence increased with age further supports this theory.
To our knowledge, our study is one of the largest to assess apremilast use in clinical practice. However, there are some limitations. For example, clinical measurements and procedures which are not coded are missing data. Also, CRHF registries contain records only from public healthcare and do not include records from private or occupational healthcare, which is a potential information bias. In addition, UV therapies and medication administered in hospital (e.g. infliximab) are insufficiently recorded in the utilized registries and are excluded from the analysis and results. The lack of inpatient hospital data may lead to a false interpretation of prior and subsequent treatment lines of apremilast. However, to our knowledge, infliximab, for example, is not widely used in Finnish clinical practice compared to self-administered biologics. Also, as the data on medication use in this study covered only the years 2015–2018, we did not have access to apremilast users’ complete medication history. Therefore, we could not assess whether ever having used or a higher number of prior biologic or targeted synthetic therapies used could predict worse persistence with apremilast. Moreover, data regarding emigration or deaths were not available for this study, and loss to follow-up due to these events could not be taken into account in the treatment persistence analyses. Consequently, we may have underestimated apremilast persistence, particularly in older age groups.
In the study, we defined apremilast persistence as the period between the first and last purchase at the pharmacy, which is limited by the assumption that a patient is exposed immediately after the dispensation. In addition, the study is limited to the period from the launch of apremilast in the Finnish market and characterizes patients and treatment schemes in the era of the apremilast. The study did not investigate health outcomes or safety.
Conclusion
In Finnish clinical practice, apremilast is mainly used between conventional synthetic therapy and biologics, and apremilast persistence is at least as high as reported in available international data (Citation10–15). In addition, compared to biologic therapies, apremilast appears to be used for older patients and patients with a higher burden of comorbidities.
Supplemental Material
Download MS Word (14.9 KB)Acknowledgements
The authors wish to thank Arto Vuori and Emmi Liukko for their help in contributing the data, and Claire Desborough for editorial support.
Disclosure statement
IK has received travel fees from Amgen, Celgene, Pfizer, and Roche outside the present study. AP has received grants from the Finnish Medical Foundation, the Finnish Foundation for Cardiovascular Research, and the Turku University Hospital Research Foundation; consulting fees from Pfizer, AbbVie, and Amgen; lecture fees from MSD, Pfizer, and Sanofi; and travel expenses from Bristol-Myers-Squib and Novartis. LP has received consulting fees and honoraria from Novartis Finland, UCB Pharma Finland, Bristol-Myers-Squibb, Jansen- Cilag, Boehringer-Ingelheim, Mylan Finland, Oriola, AbbVie, Gilead, Orion, Pfizer, and Sanofi-Genzyme; personal research grants from the Hospital District of Southwest Finland and Maire Lisko Foundation; and grants from the Urpo Huunonen Fund and Aino Taberman Fund. RH has received consulting and/or lecture fees from Novartis Finland, UCB Pharma OY Finland, Sanofi, Jansen Cilag, Leo Pharma, Roche, Pfizer, and AbbVie; a lecture fee from Roche; and travel expenses from Celgene, Janssen Cilag, Lilly, Sanofi, AbbVie, Shire, and Novartis. JR, SK, and HS-M are employed by Oriola. PL is employed by Amgen.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/03009742.2022.2151109
Additional information
Funding
References
- Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med 2017;376:957–70.
- Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker JNWN. Psoriasis. Lancet 2021;397:1301–15.
- Juneblad K, Kastbom A, Johansson L, Rantapää-Dahlqvist S, Söderkvist P, Alenius GM. Association between inflammasome-related polymorphisms and psoriatic arthritis. Scand J Rheumatol 2021;50:206–12.
- Brownstone ND, Hong J, Mosca M, Hadeler E, Liao W, Bhutani T, et al. Biologic treatments of psoriasis: an update for the clinician. Biol Targets Ther 2021;15:39–51.
- Carrascosa JM, Del-Alcazar E. Apremilast for psoriasis treatment. G Ital Dermatol Venereol 2020;155:421–33.
- Social Insurance Institution of Finland. 377 Apremilasti ja dimetyylifumaraatti (psoriaasi) - kela.fi [Internet]. [ cited 2022 Apr 20]. Available from: https://www.kela.fi/laake377
- Dommasch ED, Kim SC, Lee MP, Gagne JJ. Risk of serious infection in patients receiving systemic medications for the treatment of psoriasis. JAMA Dermatol 2019;155:1142–52.
- Sawyer LM, Cornic L, Lå L, Gibbons C, Møller AH, Jemec GB. Long-term efficacy of novel therapies in moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis of PASI response. J Eur Acad Dermatol Venereol 2019;33:355–66.
- Kawalec P, Holko P, Moćko P, Pilc A. Comparative effectiveness of abatacept, apremilast, secukinumab and ustekinumab treatment of psoriatic arthritis: a systematic review and network meta-analysis. Rheumatol Int 2018;38:189–201.
- Papadavid E, Rompoti N, Theodoropoulos K, Kokkalis G, Rigopoulos D. Real-world data on the efficacy and safety of apremilast in patients with moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol 2018;32:1173–9.
- Abignano G, Fadl N, Merashli M, Wenham C, Freeston J, McGonagle D, et al. Apremilast for the treatment of active psoriatic arthritis: a single-centre real-life experience. Rheumatology (Oxford) 2018;57:578–80.
- Del Alcázar E, Suárez-Pérez JA, Armesto S, Rivera R, Herrera-Acosta E, Herranz P, et al. Real-world effectiveness and safety of apremilast in psoriasis at 52 weeks: a retrospective, observational, multicentre study by the Spanish Psoriasis Group. J Eur Acad Dermatol Venereol 2020;34:2821–9.
- Kishimoto M, Komine M, Kamiya K, Sugai J, Ohtsuki M. Drug survival of apremilast in a real-world setting. J Dermatol 2019;46:615–7.
- Sbidian E, Billionnet C, Weill A, Maura G, Mezzarobba M. Persistence of apremilast in moderate-to-severe psoriasis: a real-world analysis of 14 147 apremilast- and methotrexate-naive patients in the French National Health Insurance database. Br J Dermatol 2020;182:690–7.
- Augustin M, Kleyn CE, Conrad C, Sator PG, Ståhle M, Eyerich K, et al. Characteristics and outcomes of patients treated with apremilast in the real world: results from the APPRECIATE study. J Eur Acad Dermatology Venereol 2021;35:123–34.
- Psoriasis and psoriatic arthritis. Current care guidelines. Working group set up by the Finnish Medical Society Duodecim, Finnish Dermatological Society and Finnish Society for Rheumatology. Helsinki: The Finnish Medical Society Duodecim, [ cited 2022 Apr 20]. Available from: www.kaypahoito.fi
- Haverinen S, Vihervaara A, Loyttyniemi E, Peltonen S, Koulu L, Tasanen K, et al. Validation of psoriasis diagnoses recorded in Finnish biobanks. Acta Derm Venereol 2020 Oct 7;100:adv00297. Epub ahead of print.
- Gasparini A. comorbidity: an R package for computing comorbidity scores. J Open Source Softw 2018;3:648.
- Feldman SR, Zhang J, Martinez DJ, Lopez-Gonzalez L, Marchlewicz EH, Shrady G, et al. Real-world biologic and apremilast treatment patterns and healthcare costs in moderate-to-severe plaque psoriasis. Dermatol Online J 2021;27:1–9.
