Abstract
Objective
While considerable focus has been placed on pain due to inflammation in psoriatic arthritis (PsA), less is reported on pain despite inflammation control. Here, we aimed to investigate the occurrence/predictors of persistent pain, including non-inflammatory components, after starting anti-tumour necrosis factor (anti-TNF) therapy.
Method
Bionaïve PsA patients starting a first anti-TNF therapy 2004–2010 were identified (South Swedish Arthritis Treatment Group register; N = 351). Outcomes included unacceptable pain [visual analogue scale (VAS) pain > 40 mm], and unacceptable pain despite inflammation control (refractory pain; VAS pain > 40 mm + C-reactive protein < 10 mg/L + ≤ 1 swollen joint of 28), assessed at 0, 3, 6, and 12 months. Baseline predictors were estimated by logistic regression.
Results
Upon starting anti-TNF therapy, 85% of patients reported unacceptable pain, falling to 43% at 3 months and then remaining stable. After 12 months, refractory pain constituted 63% of all unacceptable pain. Higher baseline VAS pain/global, worse physical function and lower health-related quality-of-life were associated with a higher risk of unacceptable/refractory pain at 12 months. More swollen joints and higher evaluator’s global assessment were associated with a lower risk of 12-month refractory pain.
Conclusions
A substantial proportion of PsA patients reported unacceptable pain throughout the first anti-TNF treatment year. At 12 months, refractory pain constituted about two-thirds of this remaining pain load. More objective signs of inflammation at anti-TNF initiation were associated with less future refractory pain. This highlights insufficient effect of biologics in patients with inflammation-independent pain, warranting alternative treatments.
Pain is a common and debilitating symptom in inflammatory arthritides, and is associated with reduced quality of life, increased work disability, and depressive symptoms (Citation1–5). In several studies, pain is ranked as patients’ most prioritized domain for symptom relief (Citation2, Citation6, Citation7), and a lower pain level has been linked to an overall more acceptable symptom state (Citation8).
In inflammatory arthritides, pain has traditionally been considered a peripheral, nociceptive process, where inflamed synovium or entheses elicit pain (Citation9). However, it is well established that patients with inflammatory arthritides also display a higher prevalence of fibromyalgia compared to the general population (10–25% vs 2–6%, respectively) (Citation10–12), and this condition is generally regarded as non-inflammatory and thought to stem from central pain sensitization (Citation13).
It has previously been shown that a substantial proportion of patients with psoriatic arthritis (PsA) display features fulfilling classification criteria of comorbid fibromyalgia, with a pooled prevalence of 18% in a systematic review (Citation14). Moreover, widespread pain that does not necessarily meet the full set of fibromyalgia criteria may be twice as common as when the entire set of fibromyalgia criteria is fulfilled (Citation15). In relation to diverse pain mechanisms, several reports on rheumatoid arthritis (RA) have shown that pain deemed unacceptable by patients persists in a substantial portion of patients despite inflammation control (Citation16, Citation17). This supports the picture of a multifaceted spectrum regarding persistent pain in PsA, where both inflammatory and non-inflammatory pain mechanisms are at play, although the magnitude and contribution of the different pain components have been less well studied.
During the past few decades, a growing number of biological and targeted synthetic disease-modifying anti-rheumatic drugs (bDMARDs/tsDMARDs), as well as an increasing focus on a treat-to-target approach, have substantially improved the possibilities for achieving inflammation control in PsA patients (Citation18). Yet, a considerable pain burden seems to persist (Citation19), and the extent to which the load of unresolved pain has a pattern indicative of a non-inflammatory mechanism in biologic-treated PsA patients, as well as which characteristics could predict persistent non-inflammatory pain, remain unclear.
The aims of this study were to examine persistent pain, including pain indicative of a non-inflammatory mechanism, in bionaïve PsA patients regarding: (i) the occurrence during the year following the start of a first anti-tumour necrosis factor (anti-TNF) therapy; (ii) the relationship to treatment response and remission; and (iii) predictors at the start of anti-TNF therapy.
Materials and methods
Patients
Bionaïve patients aged ≥ 18 years starting their first anti-TNF therapy between 2004 and 2010, residing in Skåne county (population 1.3 million), and having a PsA diagnosis according to the treating physician, were identified in the prospective, observational South Swedish Arthritis Treatment Group (SSATG) register (n = 351). SSATG is a quality control register with a structured clinical protocol to monitor biological treatments of patients with rheumatic diseases, including PsA, involving 12 rheumatology units in southern Sweden (Citation20), functioning as an integral part of the national Swedish Rheumatology Quality register (SRQ) (Citation21). The coverage of SSATG for the Skåne region has previously been estimated to be 90–95% (Citation22).
Outcomes
Persistent pain was analysed using two outcome measures: (i) unacceptable pain, based on the patient acceptable symptom state (PASS) with the established definition of pain > 40 mm on a visual analogue scale (VAS; 0–100 mm) (Citation23); and (ii) refractory pain, defined as unacceptable pain with concomitant inflammation control, the latter captured by C-reactive protein (CRP) < 10 mg/L combined with a swollen joint count including 28 joints (SJC28) ≤ 1 Refractory pain was thus analysed as VAS pain > 40 + CRP < 10 + SJC ≤ 1, as in earlier RA reports (Citation16, Citation17).
Design
The occurrence of unacceptable pain and refractory pain during the year following initiation of a first anti-TNF therapy was assessed at 0, 3, 6, and 12 months’ follow-up in the cohort as a whole, as well as separately for patients with no missing values for the pain outcomes at any of the follow-up time-points. Furthermore, unacceptable pain and refractory pain at the 3 month follow-up were analysed in relation to the European Alliance of Associations for Rheumatology (EULAR) 3 month treatment response and also compared between patients in EULAR remission and those not in remission at 3 months. Finally, baseline predictors of unacceptable and refractory pain at 12 months were assessed.
Covariates
Baseline variables included in the predictor models were chosen based on subject matter knowledge, and comprised age, sex, disease duration, tender joint count including 28 joints (TJC28), SJC28, CRP, erythrocyte sedimentation rate (ESR), VAS pain (0–100 mm), patient’s global assessment of disease activity (VAS global; 0–100 mm), evaluator’s global assessment of disease activity (EGA; Likert scale 0–4), Disease Activity Score based on 28-joint count (DAS28), Health Assessment Questionnaire (HAQ) score, health-related quality of life [EuroQol 5 Dimensions (EQ-5D)], ongoing methotrexate (MTX) use (yes/no), concurrent corticosteroid use (yes/no), concurrent non-steroidal anti-inflammatory drug (NSAID) use (yes/no), and concurrent use of analgesics of other kind (yes/no).
Statistics
Within-group differences in the proportions of patients with unacceptable/refractory pain between different time-points were assessed by McNemar’s test, while differences in pain outcomes between EULAR response groups and between patients in remission and those not in remission were analysed by the chi-squared test. Baseline predictors of unacceptable pain and refractory pain were first estimated by univariate logistic regression. Each univariably significant predictor was then analysed in a separate multivariate model, and potential confounders in regard to that predictor were chosen based on subject matter knowledge. To avoid multicollinearity, potential confounders showing a correlation of >0.4/<−0.4 (Spearman) with the studied predictor were not introduced in that model. SPSS statistics software, version 27 (IBM Corp., Armonk, NY, USA) was used, and p-values < 0.05 were considered significant.
Sensitivity analyses
In a sensitivity analysis, the occurrence and development of refractory pain were assessed when exchanging the criterion ≤ 1 swollen joint out of 28 for ≤ 1 swollen joint of 66, so that refractory pain was defined as VAS pain > 40 + CRP < 10 + SJC ≤ 1 (out of 66).
Moreover, for unacceptable/refractory pain in relation to 3 month treatment response, analyses were performed with Psoriatic Arthritis Response Criteria (PsARC) in addition to EULAR response/EULAR remission (Citation24).
Baseline predictors of 12 month refractory pain were assessed in two sensitivity analyses: first, limited to patients not displaying refractory pain at the start of anti-TNF therapy; and secondly, with alteration of the criterion of ≤1 swollen joint so that this was based on 66 joints, as described above.
Results
We identified 351 bionaïve PsA patients starting a first anti-TNF therapy between 2004 and 2010 (48% women; mean ± sd age 47 ± 12 years). The mean disease duration was 10 ± 9 years and 63% had ongoing MTX. The pain level reported at baseline was substantial, with a mean VAS pain of 64 ± 20. NSAIDs and other analgesics (continuously or on demand) were used by 67% and 62% of patients, respectively. Etanercept was the most frequently initiated anti-TNF therapy (54%) (). Out of 351 included patients, 101 did not retain the initiated anti-TNF therapy throughout the first 12 months (49 owing to treatment failure, 45 to adverse events, and seven to other causes). For baseline characteristics of retainers versus non-retainers, see Online Supplementary Table S1
Table 1. Baseline characteristics for bionaïve patients with psoriatic arthritis starting a first anti-tumour necrosis factor(anti-TNF) therapy in 2004–2010.
Persistent pain during the first treatment year
At the start of a first anti-TNF therapy, 85% of PsA patients reported unacceptable pain (VAS pain >40), which declined to 43% after 3 months and then remained stable during the rest of the study period, being 39% at 12 months (). In contrast, the fraction of patients displaying refractory pain (unacceptable pain despite inflammation control; VAS pain > 40 + CRP < 10 + SJC ≤ 1) was largely unchanged throughout the first treatment year: 24% at anti-TNF start, 27% at 3 months, and 25% at 12 months. At the 12 month follow-up, refractory pain made up 63% of all remaining unacceptable pain (). Comparable results were found in a sensitivity analysis, using 66/68-joint count instead of 28-joint count for assessing refractory pain in a subsample, where such data were available (Online Supplementary Figure 1S).
Figure 1. Occurrence of unacceptable and refractory pain during the first year after starting a first anti-tumour necrosis factor (anti-TNF) therapy in bionaïve patients with psoriatic arthritis in the South Swedish Arthritis Treatment Group register (2004–2010). (A) All patients with complete data for both pain outcomes at the respective time-point. (B) Patients with complete outcome data for both pain outcomes at all follow-up time-points (N = 109). In (B), bars with added p-values represent comparisons of the proportions of patients between anti-TNF start and 12 month follow-up for the indicated pain states. Unacceptable pain: visual analogue scale (VAS) pain > 40 mm; refractory pain: VAS pain > 40 mm + C-reactive protein < 10 mg/L + ≤ 1 swollen joint (of 28)
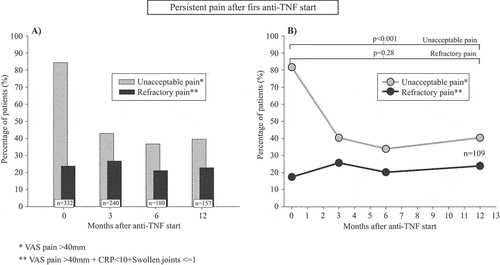
When restricting the analysis to patients with complete data for both unacceptable and refractory pain at all follow-up time-points, very similar results were displayed. The proportion of patients with unacceptable pain decreased from 82% at anti-TNF start to 40% at 3 months, and then remained stable during the rest of the study period, being 40% at 12 months (p < 0.001 for the change from baseline to 12 months). Also here, refractory pain remained fairly stable during the study period: 17% at treatment start, 26% at 3 months, and 24% at 12 months (p = 0.28 for the change from baseline to 12 months). Refractory pain constituted 58% of all remaining unacceptable pain at 12 months ().
Transition between pain groups
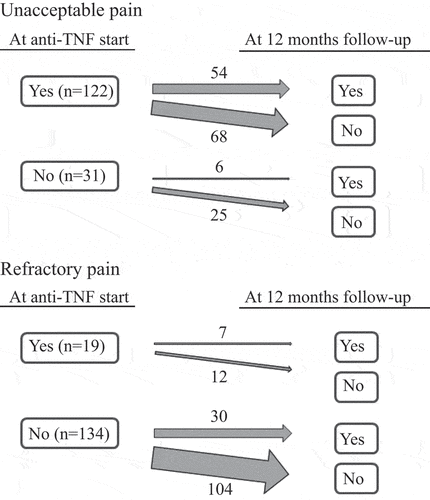
The transition between pain groups (on the patient level) between starting anti-TNF therapy and the 12 month follow-up for patients with complete pain outcome data on both occasions is shown in . Out of patients displaying unacceptable pain at 12 months, 90% already had unacceptable pain at anti-TNF initiation. Conversely, among patients demonstrating refractory pain at 12 months, 81% did not show this pain pattern at the start of therapy.
Figure 2. Transition between pain groups regarding unacceptable and refractory pain from starting a first anti-tumour necrosis factor (anti-TNF) to the 12-month follow-up for bionaïve patients with psoriatic arthritis in the South Swedish Arthritis Treatment Group register (2004–2010) with complete data for unacceptable and refractory pain at both time-points (N = 153).
Persistent pain in relation to EULAR 3 month treatment response and remission
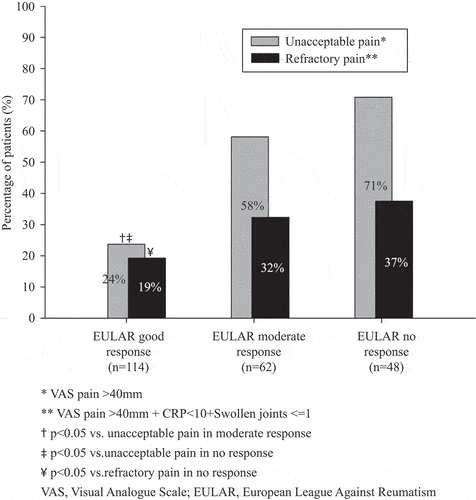
The fraction of bionaïve PsA patients displaying unacceptable pain 3 months after starting a first anti-TNF therapy was linked to EULAR 3 month treatment response (24% of EULAR good responders vs 71% of non-responders; p < 0.001). Regarding refractory pain, the relationship was significant but less pronounced (19% of good responders vs 37% of non-responders; p = 0.016) (). Among EULAR 3 month good responders, refractory pain at the 3 month follow-up constituted 81% of all remaining unacceptable pain, whereas the corresponding figure for non-responders was 52%.
Figure 3. Occurrence of unacceptable and refractory pain 3 months after starting a first anti-tumour necrosis factor (anti-TNF) therapy in bionaïve patients with psoriatic arthritis in the South Swedish Arthritis Treatment Group register (2004–2010). Data were stratified on European Alliance of Associations for Rheumatology (EULAR) 3 month response and are shown for patients with complete data for unacceptable and refractory pain as well as Disease Activity Score based on 28-joint count (DAS28) at both anti-TNF start and the 3 month follow-up (N = 224).
Moreover, a lower proportion of patients reaching EULAR-defined remission at 3 months reported unacceptable pain at the 3 month follow-up compared to patients not in remission (20% vs 62%; p < 0.001). Concerning refractory pain, the link was significant but less marked (17% of patients in remission vs 34% of those not in remission; p = 0.003). Refractory pain made up 85% of all 3 month unacceptable pain among patients in remission.
Also, when using PsARC as the response measure, the difference between responders and non-responders at 3 months was numerically more pronounced for 3 month unacceptable pain (34% vs 65%; p = 0.004) than for refractory pain (18% vs 38%; p = 0.036), in a subsample with available 66/68-joint count data.
Factors associated with persistent pain
Unacceptable pain
In univariate analysis, higher baseline levels of VAS pain and VAS global, as well as higher HAQ scores, were associated with a higher risk of having unacceptable pain at 12 months, whereas higher EQ-5D utility and ongoing MTX at anti-TNF initiation were associated with lower risk of having 12 month unacceptable pain (). Estimates for these variables remained significant after adjustments (in multivariate analyses): VAS pain [odds ratio (OR) 1.3, 95% confidence interval (CI) 1.1–1.5, per 10-unit increase], VAS global (OR 1.4, 95% CI 1.2–1.7, per 10-unit increase), HAQ (OR 3.6, 95% CI 1.8–7.2, per unit increase), EQ-5D (OR 0.2, 95% CI 0.1–0.7, per unit increase), and ongoing MTX (OR 0.4, 95% CI 0.2–0.9, for yes vs no).
Table 2. Baseline predictors of unacceptable pain† 12 months after starting a first anti-tumour necrosis factor (anti-TNF) therapy in bionaïve patients with psoriaticarthritis initiating therapy in 2004–2010.
Refractory pain
Higher levels of VAS pain and VAS global, as well as higher HAQ scores, were associated with a higher risk of having refractory pain 12 months after starting anti-TNF therapy, in univariate analysis. Conversely, higher levels of EQ-5D, SJC, and EGA, as well as ongoing MTX, were linked to a lower risk of having 12 month refractory pain (). After subsequent adjustment (in multivariate analyses), significance was no longer shown regarding ongoing MTX, but estimates remained significant for VAS pain (OR 1.3, 95% CI 1.0–1.6, per 10-unit increase), VAS global (OR 1.4, 95% CI 1.1–1.8, per 10-unit increase), HAQ (OR 4.2, 95% CI 1.8–9.5, per unit increase), EQ-5D (OR 0.2, 95% CI 0.0–0.7, per unit increase), SJC (OR 0.9, 95% CI 0.8–1.0, per unit increase), and EGA (OR 0.4, 95% CI 0.2–0.7, per unit increase).
Table 3. Baseline predictors of refractory pain† 12 months after starting a first anti-tumour necrosis factor(anti-TNF) therapy in bionaïve patients with psoriaticarthritis initiating therapy in 2004–2010.
When limiting the analysis to patients without refractory pain at the start of anti-TNF treatment, we found the same predictors as in the main analysis, both univariably and multivariably, apart from ongoing MTX and SJC (the latter just barely lost significance: p = 0.06) (Supplementary Table S2). Also, in a second sensitivity analysis, when basing the refractory pain definition on 66/68-joint count, similar estimates to those in the main analysis were found (Supplementary Table S3).
Regarding adjustments in the multivariate models for unacceptable/refractory pain, VAS pain, VAS global, HAQ, and EQ-5D were adjusted for basic demographics (sex, age, and disease duration) and signs of ongoing inflammation (SJC and CRP). Regarding ongoing MTX, adjustment was performed for the same factors, but also for VAS pain (as a constituent of the outcome variables). VAS pain, VAS global, HAQ, and EQ-5D were all too highly correlated to be introduced for adjustment in the same models. EGA was adjusted for sex, age, disease duration, CRP, and VAS pain (but not for SJC owing to too high correlation), whereas SJC was adjusted for sex, age, disease duration, and CRP. Covariates and model-of-fit measures for the different multivariate models are shown in Supplementary Table S4.
Discussion
Main findings
In this study of PsA patients initiating a first anti-TNF therapy, we show that almost 40% reported unacceptable pain at 12 months and that nearly two-thirds of this remaining pain load was attributed to a pain pattern indicative of a non-inflammatory mechanism (refractory pain). Moreover, unacceptable pain at 3 months was strongly linked to EULAR 3 month response, and for good responders refractory pain constituted the main component of the persisting unacceptable pain. Higher baseline HAQ scores, higher levels of VAS pain/global, and lower EQ-5D utility were associated with a higher risk of unacceptable pain and refractory pain at 12 months. Conversely, more swollen joints and higher EGA were associated with a lower risk of 12 month refractory pain.
Previous research
Refractory pain is a novel way of attempting to capture persistent non-inflammatory pain in PsA. Prevalence estimates of around 25% are well in accordance with previous estimates regarding comorbid fibromyalgia in PsA (16–27%) (Citation10, Citation15, Citation25), and with estimates of neuropathic pain (25–28%) using the PainDETECT instrument (Citation20, Citation26). Yet, although refractory pain is likely to catch most PsA patients with comorbid fibromyalgia, it may miss those who at the same time are active in their PsA. Furthermore, some patients meeting refractory pain criteria may still have ongoing inflammation, with pain stemming from enthesitis and lacking a substantial elevation of inflammatory markers (Citation27).
In a previous report from our group, RA patients treated with biologics/triple therapy displayed a refractory pain prevalence at 1 year of around half that seen in the current study (Citation17). However, in that report there was a markedly shorter disease duration than in the present study (0.6 vs 10.3 years). Since pain sensitization develops over time (Citation13), this may have influenced prevalence estimates. Moreover, the difference in refractory pain could also reflect differences in pain development between PsA and RA, congruent with earlier evidence that indicated a higher frequency of central pain sensitization in PsA (Citation20).
In line with a report by Altawil et al on MTX-treated RA patients, persistent pain in our study was also substantial among EULAR good responders (Citation28), a finding congruent with results presented by Lee et al, who demonstrated the persistence of significant pain in patients with DAS28 remission (Citation29). Furthermore, our findings show that among EULAR good responders, refractory pain dominated the remaining unacceptable pain load markedly, signalling that as inflammation is brought under control, unmet needs regarding non-inflammatory pain components are substantial and more visible.
When evaluating unacceptable pain, an important focus is how to capture this pain state. Here, we used the validated measure of PASS for pain, i.e. VAS pain > 40 mm, as described by Tubach et al (Citation23), but a lower cut-off for so-called significant pain (VAS pain > 20 mm) has been suggested (Citation30), resulting in higher prevalence estimates when it is applied (Citation28). Furthermore, although refractory pain as defined here has been established as a measure of pain despite inflammation control, other instruments have been proposed to capture non-inflammatory pain. These include the continuous DAS28-P (a modified DAS28 based on patients’ global assessment and tender joint count) (Citation31), as well as the painDETECT questionnaire (mainly designed to capture neuropathic pain as a proxy for sensitization) (Citation20). To allow for a straightforward clinical interpretation, in this study we aimed at using a dichotomous cut-off reflecting pain deemed acceptable/unacceptable by patients, and used PASS combined with inflammation control.
From the analysis of pain group transitions, three groups of patients with non-inflammatory pain could be hypothesized: (i) patients who at the start of anti-TNF treatment already had detectable non-inflammatory pain features in addition to their inflammatory pain, which could have made a good therapeutic response less likely (Citation32); (ii) patients who had non-inflammatory pain at the start of anti-TNF therapy, but where these pain features were not revealed until inflammation was brought under control (Citation13); and (iii) patients without non-inflammatory pain at anti-TNF initiation, where such pain features were generated through pain sensitization during the first treatment year. Future studies should aim to further disentangle the patterns and mechanisms of non-inflammatory pain in PsA.
Reports on predictors of remaining pain in PsA are scarce, but the current results regarding more initial pain and worse physical function (by HAQ) as predictors of future persistent pain are in line with several studies on RA, both in early disease (Citation28, Citation33, Citation34) and at the start of biological therapy (Citation35). Our results are also consistent with previously reported risk factors of fibromyalgia in the general population (Citation11).
It is not surprising that worse physical function is strongly associated with pain outcomes after 12 months, since these two domains have been proven to be interrelated (Citation36)]. Notably, more swollen joints and higher EGA at the start of anti-TNF therapy were associated with a significantly lower risk of 12 month refractory pain (and numerically less unacceptable pain), suggesting that patients with more initial inflammation may be less prone to present with unresolved pain indicative of a non-inflammatory mechanism later on.
Ongoing MTX at anti-TNF initiation was associated with significantly less unacceptable pain at 12 months (and numerically less refractory pain), although combination treatment with MTX has previously not shown consistently better effects on overall disease activity in PsA, compared to anti-TNF monotherapy (Citation18). However, a recent report from the European Spondyloarthritis collaboration found a higher probability of reaching remission when combining a monoclonal anti-TNF therapy with a conventional synthetic DMARD in PsA (Citation37), and one could speculate whether a similar benefit from combination therapy (anti-TNF + MTX) may be present for pain. In addition, a systematic review summarized that combination therapy may, in some settings, have a better effect on structural damage and severe skin disease in PsA (Citation38), two domains that could potentially influence pain patterns.
In contrast to several earlier reports on risk factors for fibromyalgia in the general population (Citation11, Citation39), female sex was not associated with refractory pain in the present study, results that were also confirmed in sensitivity analyses including only patients without refractory pain at the start of anti-TNF therapy. This may suggest that non-inflammatory pain features in PsA – a disease characterized by its inflammatory, nociceptive pain process – constitute a different pain phenotype compared to patients with primary fibromyalgia without an equally obvious nociceptive trigger (Citation14). Another possible explanation is that some persisting pain may have an inflammatory origin not captured by the refractory pain definition, such as axial disease, which has been shown to be more common in men (Citation40).
Strengths and limitations
Strengths of the current study include the longitudinal follow-up in a register with good coverage from a defined catchment area and an established measure of unacceptable pain.
This study also has limitations. The subcriterion of ≤ 1 swollen joint for inflammation control would, in PsA, optimally be based on 66 joints instead of 28 joints. Unfortunately, 66/68-joint data were available only in a subgroup of patients, but when performing analyses on this group, occurrence and predictors of refractory pain did not differ in any major way from the main analysis. Another limitation was the lack of information on enthesitis, an important PsA feature that could contribute to pain without raising inflammatory markers (Citation41, Citation42). In addition, CRP is a less sensitive inflammation marker in PsA than in RA (Citation43, Citation44), which is why the addition of the criterion SJC ≤ 1 (to CRP < 10 mg/L) to ensure inflammation control was central. Furthermore, DAS28 is a widely used measure of disease activity in PsA in clinical practice and EULAR-defined treatment response is often used for treatment evaluation (Citation24, Citation45). However, other indices to assess disease activity, such as the Disease Activity index for PSoriatic Arthritis (DAPSA) and minimal disease activity (MDA), are increasingly being used, but were less common during the current study period (Citation45, Citation46). Since data on skin involvement were not available, MDA could not be assessed. We also preferred a response measure not containing pain (like DAPSA). Yet, when using PsARC in a subset of patients with available 66/68-joint count data, the results were similar to those of EULAR response/remission.
Furthermore, this is a real-life setting with missing data due to termination of initiated therapy and/or lack of registration. This could have influenced prevalence estimates and predictor results, although we also assessed the occurrence of unacceptable/refractory pain in patients with complete data at all follow-ups and found similar results.
Finally, we lacked access to certain variables that have previously been shown to predict remaining pain in inflammatory arthritides, such as smoking, body mass index, sleeping disturbances, and emotional coping (Citation47–49), and also to information on depression and anxiety disorders (Citation33, Citation47, Citation50). In addition, we lacked data on alcohol use, education, work disability, and catastrophizing, factors previously associated with fibromyalgia in the general population (Citation11, Citation39).
Conclusion
In this study, 40% of bionaïve PsA patients reported unacceptable pain 1 year after starting their first anti-TNF therapy. Refractory pain, indicative of a non-inflammatory mechanism, constituted almost two-thirds of the persistent unacceptable pain, and for EULAR 3 month good responders this proportion was even higher. Worse baseline levels of patient-reported outcomes, such as pain, global health, physical function, and health-related quality of life, were associated with a higher risk of future refractory pain, whereas more objective signs of inflammation were associated with a lower risk. The results highlight insufficient effects of biologics on a subset of patients with inflammation-independent pain, warranting an increased focus on early detection and alternative treatment strategies for pain management.
Ethics and consent
This study was approved by the Regional Ethical Review Board of Lund University (2014/754). Data collection was part of routine care and patients have consented to participate in the register used for the research.
Supplemental Material
Download MS Word (205.4 KB)Acknowledgements
We are indebted to all colleagues and staff in the South Swedish Arthritis Treatment Group for their cooperation and for supplying the data.
Disclosure statement
JKW has received speaker’s bureau fees from AbbVie and Amgen, and research support unrelated to the current work from AbbVie, Amgen, Eli Lilly, Novartisa, and Pfizer. TO has performed consulting tasks for Eli Lilly and Merck Sharp & Dohme unrelated to the present work. All of the other authors report no conflicts of interest.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/03009742.2023.2258644
Additional information
Funding
References
- Conaghan PG, Alten R, Deodhar A, Sullivan E, Blackburn S, Tian H, et al. Relationship of pain and fatigue with health-related quality of life and work in patients with psoriatic arthritis on TNFi: results of a multi-national real-world study. RMD Open 2020;6:e001240.
- Gudu T, Gossec L. Quality of life in psoriatic arthritis. Expert Rev Clin Immunol 2018;14:405–17.
- Olofsson T, Söderling JK, Gülfe A, Kristensen LE, Wallman JK. Patient-reported outcomes more important than objective inflammatory markers for sick leave in biologics-treated rheumatoid arthritis patients. Arthritis Care Res 2018;70:1712–16.
- Olofsson T, Petersson IF, Eriksson JK, Englund M, Simard JF, Nilsson JA, et al. Predictors of work disability during the first 3 years after diagnosis in a national rheumatoid arthritis inception cohort. Ann Rheum Dis 2014;73:845–53.
- Rathbun AM, Harrold LR, Reed GW. Temporal associations between the different domains of rheumatoid arthritis disease activity and the onset of patient-reported depressive symptoms. Clin Rheumatol 2015;34:653–63.
- Geenen R, Overman CL, Christensen R, Åsenlöf P, Capela S, Huisinga KL, et al. EULAR recommendations for the health professional’s approach to pain management in inflammatory arthritis and osteoarthritis. Ann Rheum Dis 2018;77:797–807.
- ten Klooster PM, Veehof MM, Taal E, van Riel PL, van de Laar MA. Changes in priorities for improvement in patients with rheumatoid arthritis during 1 year of anti-tumour necrosis factor treatment. Ann Rheum Dis 2007;66:1485–90.
- Puyraimond-Zemmour D, Etcheto A, Fautrel B, Balanescu A, de Wit M, Heiberg T, et al. Associations between five important domains of health and the patient acceptable symptom state in rheumatoid arthritis and psoriatic arthritis: a cross-sectional study of 977 patients. Arthritis Care Res 2017;69:1504–9.
- Schaible HG, von Banchet GS, Boettger MK, Bräuer R, Gajda M, Richter F, et al. The role of proinflammatory cytokines in the generation and maintenance of joint pain. Ann N Y Acad Sci 2010;1193:60–9.
- Mease PJ. Fibromyalgia, a missed comorbidity in spondyloarthritis: prevalence and impact on assessment and treatment. Curr Opin Rheumatol 2017;29:304–10.
- Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum 1995;38:19–28.
- Heidari F, Afshari M, Moosazadeh M. Prevalence of fibromyalgia in general population and patients, a systematic review and meta-analysis. Rheumatol Int 2017;37:1527–39.
- Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011;152:S2–15.
- Zhao SS, Duffield SJ, Goodson NJ. The prevalence and impact of comorbid fibromyalgia in inflammatory arthritis. Best Pract Res Clin Rheumatol 2019;33:101423.
- Højgaard P, Ellegaard K, Nielsen SM, Christensen R, Guldberg-Møller J, Ballegaard C, et al. Pain mechanisms and ultrasonic inflammatory activity as prognostic factors in patients with psoriatic arthritis: a prospective cohort study. Arthritis Care Res 2019;71:798–810.
- Olofsson T, Wallman JK, Jöud A, Schelin MEC, Ernestam S, van Vollenhoven R, et al. Pain over 2 years after start of biological versus conventional combination treatment in early rheumatoid arthritis: results from the randomised controlled SWEFOT trial. Arthritis Care Res 2021;73:1312–21.
- Lourdudoss C, Di Giuseppe D, Wolk A, Westerlind H, Klareskog L, Alfredsson L, et al. Dietary intake of polyunsaturated fatty acids and pain in spite of inflammatory control among methotrexate treated early rheumatoid arthritis patients. Arthritis Care Res 2018;70:205–12.
- Gossec L, Baraliakos X, Kerschbaumer A, de Wit M, McInnes I, Dougados M, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 2020;79:700–12.
- Rifbjerg-Madsen S, Christensen AW, Christensen L, Hetland ML, Bliddal H, Kristensen LE. Pain and pain mechanisms in patients with inflammatory arthritis: a Danish nationwide cross-sectional DANBIO registry survey. PLoS One 2017;12:e0180014.
- Geborek P, Crnkic M, Petersson IF, Saxne T. Etanercept, infliximab, and leflunomide in established rheumatoid arthritis: clinical experience using a structured follow up programme in southern Sweden. Ann Rheum Dis 2002;61:793–8.
- Wadström H, Eriksson JK, Neovius M, Askling J. How good is the coverage and how accurate are exposure data in the Swedish biologics register (ARTIS)? Scand J Rheumatol 2015;44:22–8.
- Geborek P, Nitelius E, Noltorp S, Petri H, Jacobsson L, sLarsson L, et al. Population based studies of biological antirheumatic drug use in southern Sweden: comparison with pharmaceutical sales. Ann Rheum Dis 2005;64:1805–7.
- Tubach F, Ravaud P, Martin-Mola E, Awada H, Bellamy N, Bombardier C, et al. Minimum clinically important improvement and patient acceptable symptom state in pain and function in rheumatoid arthritis, ankylosing spondylitis, chronic back pain, hand osteoarthritis, and hip and knee osteoarthritis: results from a prospective multinational study. Arthritis Care Res 2012;64:1699–707.
- Gladman DD, Mease PJ, Healy P, Helliwell PS, Fitzgerald O, Cauli A, et al. Outcome measures in psoriatic arthritis. J Rheumatol 2007;34:1159–66.
- Lampa J. Pain without inflammation in rheumatic diseases. Best Pract Res Clin Rheumatol 2019;33:31703790.
- Di Carlo M, Muto P, Benfaremo D, Luchetti MM, Atzeni F, Salaffi F. The neuropathic pain features in psoriatic arthritis: a cross-sectional evaluation of prevalence and associated factors. J Rheumatol 2020;47:1198–203.
- Marchesoni A, De Marco G, Merashli M, McKenna F, Tinazzi I, Marzo-Ortega H, et al. The problem in differentiation between psoriatic related polyenthesitis and fibromyalgia. Rheumatology 2018;57:32–40.
- Altawil R, Saevarsdottir S, Wedrén S, Alfredsson L, Klareskog L, Lampa J. Remaining pain in early rheumatoid arthritis patients treated with methotrexate. Arthritis Care Res 2016;68:1061–8.
- Lee YC, Cui J, Lu B, Iannaccone CK, Shadick NA, Weinblatt ME, et al. Pain persists in DAS28 rheumatoid arthritis remission but not in ACR/EULAR remission: a longitudinal observational study. Arthritis Res Ther 2011;13:R83.
- Wolfe F, Michaud K. Assessment of pain in rheumatoid arthritis: minimal clinically significant difference, predictors, and the effect of anti-tumor necrosis factor therapy. J Rheumatol 2007;34:1674–83.
- Joharatnam N, McWilliams DF, Wilson D, Wheeler M, Pande I, Walsh DA. A cross-sectional study of pain sensitivity, disease-activity assessment, mental health, and fibromyalgia status in rheumatoid arthritis. Arthritis Res Ther 2015;17:11.
- Iannone F, Nivuori M, Fornaro M, Venerito V, Cacciapaglia F, Lopalco G. Comorbid fibromyalgia impairs the effectiveness of biologic drugs in patients with psoriatic arthritis. Rheumatology 2020;59:1599–606.
- Lee YC, Lu B, Boire G, Haraoui BP, Hitchon CA, Pope JE, et al. Incidence and predictors of secondary fibromyalgia in an early arthritis cohort. Ann Rheum Dis 2013;72:949–54.
- Eberhard A, Bergman S, Mandl T, Olofsson T, Rydholm M, Jacobsson L, et al. Predictors of unacceptable pain and unacceptable pain with low inflammation in patients with rheumatoid arthritis over 5 years after diagnosis. Arthritis Res Ther 2021;23:16935.
- McWilliams DF, Walsh DA. Factors predicting pain and early discontinuation of tumour necrosis factor-α-inhibitors in people with rheumatoid arthritis: results from the British Society for Rheumatology biologics register. BMC Musculoskelet Disord 2016;17:337.
- Björk M, Gerdle B, Thyberg I, Peolsson M. Multivariate relationships between pain intensity and other aspects of health in rheumatoid arthritis–cross sectional and five year longitudinal analyses (the Swedish TIRA project). Disabil Rehabil 2008;30:1429–38
- Lindström U, Di Giuseppe D, Delcoigne B, Glintborg B, Möller B, Ciurea A, et al. Effectiveness and treatment retention of TNF inhibitors when used as monotherapy versus comedication with csDMARDs in 15 332 patients with psoriatic arthritis. Data from the EuroSpA collaboration. Ann Rheum Dis 2021;80:1410–18.
- Behrens F, Cañete JD, Olivieri I, van Kuijk AW, McHugh N, Combe B. Tumour necrosis factor inhibitor monotherapy vs combination with MTX in the treatment of PsA: a systematic review of the literature. Rheumatology 2015;54:915–26.
- Monden R, Rosmalen JGM, Wardenaar KJ, Creed F. Predictors of new onsets of irritable bowel syndrome, chronic fatigue syndrome and fibromyalgia: the lifelines study. Psychol Med 2020 Jun 17:1–9. doi:10.1017/S0033291720001774.
- Coates LC, van der Horst-Bruinsma IE, Lubrano E, Beaver S, Drane E, Ufuktepe B, et al. Sex-specific differences in patients with psoriatic arthritis: a systematic review. J Rheumatol 2023;50:488–96
- Kaeley GS, Eder L, Aydin SZ, Gutierrez M, Bakewell C. Enthesitis: a hallmark of psoriatic arthritis. Semin Arthritis Rheum 2018;48:35–43.
- Kasiem FR, Luime JJ, Vis M, Kok MR, Wervers K, Gerards AH, et al. Lessons learned from clinical phenotypes in early psoriatic arthritis: the real-world dutch south west early psoriatic arthritis study. Scand J Rheumatol 2021;50:124–31.
- Lindqvist UR, Alenius GM, Husmark T, Theander E, Holmström G, Larsson PT. Psoriatic arthritis group of the society for rheumatology. The Swedish early psoriatic arthritis register– 2-year followup: a comparison with early rheumatoid arthritis. J Rheumatol 2008;35:668–73.
- Punzi L, Podswiadek M, Oliviero F, Lonigro A, Modesti V, Ramonda R, et al. Laboratory findings in psoriatic arthritis. Reumatismo 2007;59:52–5
- Schoels MM, Aletaha D, Alasti F, Smolen JF. Disease activity in psoriatic arthritis (PsA): defining remission and treatment success using the DAPSA score. J Rheumatol 2007;34:1159–66.
- Gossec L, McGonagle D, Korotaeva T, Lubrano E, de Miguel E, Østergaard M, et al. Minimal disease activity as a treatment target in psoriatic arthritis: a review of the literature. J Rheumatol 2018;45:6–13.
- McWilliams DF, Zhang W, Mansell JS, Kiely PDW, Young A, Walsh DA. Predictors of change in bodily pain in early rheumatoid arthritis: an inception cohort study. Arthritis Care Res 2012;64:1505–13.
- Wolfe F, Häuser W, Hassett AL, Katz RS, Walitt BT. The development of fibromyalgia – I: Examination of rates and predictors in patients with rheumatoid arthritis (RA). Pain 2011;152:291–9.
- Dobkin PL, Liu A, Abrahamowicz M, Carrier N, de Brum-Fernandes AJ, Cossette P, et al. Predictors of pain for patients with early inflammatory polyarthritis. Arthritis Care Res 2013;65:992–9.
- Michelsen B, Kristianslund EK, Sexton J, Hammer HB, Fagerli KM, Lie E, et al. Do depression and anxiety reduce the likelihood of remission in rheumatoid arthritis and psoriatic arthritis? Data from the prospective multicentre NOR-DMARD study. Ann Rheum Dis 2017;76:1906–10.
