Abstract
Objectives
To translate the Assessment of SpondyloArthritis international Society (ASAS) Health Index (HI) Environmental Factors Item Set (EFIS) into Swedish and culturally adapt it for a Swedish context, and to assess the construct validity of the Swedish version of the ASAS HI and test–retest reliability in ASAS HI and EFIS in Swedish patients with ankylosing spondylitis (AS).
Method
Translation and cross-cultural adaptation of the EFIS were carried out according to a forward–backward procedure consisting of five steps. The construct validity of the ASAS HI was tested using Spearman correlation with standard health outcomes for axial spondyloarthritis (axSpA). Reliability was analysed by internal consistency with the Cronbach’s alpha coefficient for ASAS HI, and test–retest reliability with intraclass correlation coefficients (ICCs) for ASAS HI and kappa agreement for the individual items of EFIS.
Results
The translation of EFIS showed acceptable face and content validity. ASAS HI showed an acceptable internal consistency (Cronbach’s alpha 0.79), and excellent test–retest reliability (ICC 0.87). Test–retest reliability for EFIS showed varied results, with kappa agreement for the individual items ranging from poor (−0.027) to good (0.80).
Conclusions
The Swedish version of ASAS HI proved to be valid and reliable and is recommended for assessing the impact of AS on global functioning and health. A Swedish version of EFIS has been produced and uploaded on the ASAS website. The EFIS proved to have acceptable face and content validity, and may contribute to the contextual interpretation of the ASAS HI.
Axial spondyloarthritis (axSpA) is a chronic inflammatory rheumatic disease. Inflammatory back pain is the most common onset symptom, but peripheral joints and entheses may also be affected (Citation1). Ankylosing spondylitis (AS), the prototype of axSpA, is characterized by inflammation and pathological new bone formation in the axial skeleton, peripheral arthritis, and enthesitis. Signs of sacroiliitis on plain radiographs are mandatory in diagnosing AS, but nowadays magnetic resonance imaging (MRI) of the sacroiliac joints is the recommended method in patients with sign and symptoms suspicious for axSpA (Citation2). The first symptoms often occur around 25 years of age and seldom after 40 years of age (Citation3). Patients with similar symptoms and signs of AS without radiographic changes at the sacroiliac joints can be classified as having non-radiographic axial spondyloarthritis (nr-axSpA) (Citation4).
Patients with axSpA suffer from pain, stiffness, and fatigue, causing limitations in activities and social participation (Citation5–7). Since axSpA usually starts early in life, the lifetime impact of the disease on functioning and health can be substantial. We previously found that both physical and mental health were affected in axSpA compared with controls (Citation8). Physical and mental health are often evaluated by the generic self-reported questionnaire Short Form-36 (SF-36), which is a validated and widely used instrument (Citation9). However, there is also a need to study overall functioning and health using disease-specific instruments, including factors raised by the patients as being important for their well-being.
An extensive global collaboration among rheumatologists from all over the world, and patient organizations in Canada, the UK, and the USA, resulted in the Assessments of SpondyloArthritis international Society Health Index (ASAS HI). The ASAS HI is a unidimensional questionnaire measuring global functioning and health across many aspects of health that are typical and relevant for patients with axSpA. It is based on the core set of AS (Citation10), which was derived from the International Classification of Functioning and Health and endorses the biopsychosocial framework of health (Citation11). The Assessment of SpondyloArthritis international Society–Outcomes Measures in Rheumatology (ASAS-OMERACT) core outcome set for axSpA includes overall functioning and health as a mandatory domain for all trials, independent of the therapy investigated (Citation12). The ASAS HI is selected as the mandatory outcome instrument for this domain and should be used in all studies (Citation13). The ASAS HI is also accompanied by a separate multidimensional item set assessing relevant contextual environmental factors – the Environmental Factors Item Set (EFIS) (Citation14, Citation15). Environmental factors are defined as elements external to the individual that can influence functioning either as a facilitator or as a barrier (Citation16). These two instruments have been translated and cross-culturally adapted into 15 languages (Citation14). However, only the ASAS HI, but not the EFIS, has been translated into Swedish, although not evaluated regarding its validity and reliability in Swedish patients with AS.
The aims of this project were to translate the EFIS into Swedish and culturally adapt it for a Swedish context, and to assess the construct validity of the Swedish version of ASAS HI and test–retest reliability in ASAS HI and EFIS in Swedish patients with AS.
Method
ASAS HI and EFIS
The ASAS HI and the EFIS contain 17 and nine items, respectively, with a dichotomous response option indicating ‘I agree’ and ‘I do not agree’ (see Supplementary material 1). The total sum of the ASAS HI ranges from 0 to 17, with a lower score indicating better health status. The sum score is calculated according to the user manual presented on the ASAS homepage (https://www.asas-group.org/wp-content/uploads/2021/11/asas_hi_english_um.pdf) and it may be calculated with up to 20% of values missing. Cut-off values for ASAS HI scores to define health status are ≤ 5 for good health, < 5 to < 12 for moderate health, and ≥ 12 for poor health (Citation11, Citation17). The EFIS does not provide a sum score because of its multidimensional nature, in which the items can act as both facilitators and barriers (Citation15).
Translation and cross-cultural adaptation
Translation and cross-cultural adaptation of the EFIS were carried out according to the forward–backward procedure previously used to translate this instrument (Citation14) and described in detail by Beaton et al (Citation18) The procedure consists of five steps: initial translation, synthesis of the translations, back-translation, expert committee review, and a field test with cognitive debriefing.
An initial forward translation of the instrument (EFIS) from the original language (English) to the target language (Swedish) was carried out by three independent bilingual translators with Swedish as their mother tongue. Two translators provided a clinical perspective, and one translator was a professional translator with no medical or clinical background. The translators together synthesized the results of the translations and produced one common translation. Based on this common translation, two back-translations into the original language (English) were produced by two bilingual translators blinded to the original version. One translator was provided a clinical perspective and one translator was a professional translator with no medical or clinical background. Back-translation is a process of validity checking to make sure that the translated version reflects the same content as the original version. An expert committee including all of the translators, i.e. health professionals, methodologists, and language professionals, reviewed all reports to reach consensus on discrepancies and produced a prefinal version. Decisions were made in collaboration with one of the original developers to achieve equivalence between the original version and the target version regarding semantic equivalence, idiomatic equivalence, experiential equivalence, and conceptual equivalence. The last step was the field test to test the prefinal Swedish version in a small group of relevant patients in order to test alternative wording and to check the understandability, interpretation, and cultural relevance of the translation. Each patient completed the prefinal version and was interviewed according to a standardized operating procedure (Supplementary material 2). The patients completed the EFIS in the presence of an interviewer and the completion time for the EFIS was recorded. After this, the patients underwent a structured interview focusing on skipped or missing items, clarity and relevance, and problematic items. The aim of the cognitive debriefing interview was to test the relevance, acceptability, and comprehensiveness of the translated EFIS. The patients were asked to comment on the items of the questionnaire, the instructions, and the response format. The information retrieved from the cognitive debriefing was documented in a semi-structured written report.
Participants
Two separate cohorts were used. First, 10 adult patients with axSpA, i.e. AS (60%) and nr-axSpA (40%), were recruited to the field test with cognitive debriefing of the translated version of EFIS. The patients were recruited by convenience sampling from the Rheumatology Clinic, Sahlgrenska University Hospital, Gothenburg, Sweden, with the aim of representing the clinical spectrum of axSpA regarding diagnosis, age, and gender. Inclusion criteria were a diagnosis of axSpA according to the ASAS classification criteria for axSpA (Citation4) or the modified New York (mNY) criteria for AS (Citation1), and age > 18 years. Exclusion criteria were impaired cognitive function, and an inability to speak or read Swedish at the level required to answer the questionnaires.
Secondly, another 61 adults with AS taking part in the Long-term Outcome Ankylosing Spondylitis (LOAS) study were recruited consecutively to assess the validity and reliability of ASAS HI and the test–retest reliability of EFIS. The LOAS study is an ongoing long-term follow-up of the cohort of patents with AS from Western Sweden, described in previous studies (Citation19, Citation20). Inclusion criteria were a diagnosis of AS according to the mNY criteria for AS (Citation1) and age > 18 years. Exclusion criteria were psoriasis, inflammatory bowel disease, impaired cognitive function, and an inability to speak and/or read Swedish at the level required to answer the questionnaires.
Assessments
Demographic information including age, gender, and disease duration was collected in both cohorts. People recruited to the field test also reported information regarding diagnosis, working status, and years of formal education, while the validity and reliability test included the other health outcomes: the Ankylosing Spondylitis Disease Activity Score–C-reactive protein (ASDAS-CRP) sum score (Citation21, Citation22), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) (Citation23), Bath Ankylosing Spondylitis Functional Index (BASFI) (Citation24), 3-level EuroQoL 5 Dimensions Questionnaire (EQ-5D-3L) pooled index, based on the Swedish experience-based value sets for the EQ-5D-3L (Citation25) and the EQ-5D visual analogue scale (VAS) (Citation26), and the 36-item Short Form Health Survey (SF-36) physical component summary (PCS) and Mental component summary (MCS) scores (Citation27).
Statistics
Construct validity was assessed through Spearman’s correlation analysis with the other health outcomes. Based on the constructs of ASAS HI and the other health outcomes, an a priori hypothesis was formulated in which the summary scores of the ASAS HI were hypothesized to have a good correlation with the ASDAS-CRP, BASDAI, BASFI, EQ-5D, and SF-36. The level of correlation is based on predefined thresholds: low ≤ 0.3, moderate 0.3–0.49, good 0.5–0.79, and very good ≥ 0.8 (Citation28).
Reliability was analysed by internal consistency for ASAS HI and test–retest reliability for both ASAS HI and EFIS. Internal consistency was evaluated with the Cronbach’s α coefficient. Acceptable internal consistency was defined as α ≥ 0.7 (Citation29). To evaluate test–retest reliability, the patients were invited to complete the same questionnaires again after approximately 1 week. This period of time was considered to be sufficiently short to assume that the variable being measured had not changed. The intraclass correlation coefficient (ICC) was used for the sum score of ASAS HI, and kappa agreement was calculated for the individual items on EFIS. An ICC ≥ 0.80 was considered to indicate excellent reliability. Kappa values < 0.20 indicated poor, 0.21–0.40 fair, 0.41–0.60 moderate, 0.61–0.80 good, and 0.81–1.0 very good reliability (Citation30).
Ethical considerations
Ethical approval was obtained from the Swedish Ethical Review Board (2021-05448-01) for both the translation and cross-cultural adaptation of the EFIS and the assessment of validity and reliability. Written informed consent was obtained from all respondents prior to their participation, in accordance with the standards of the Declaration of Helsinki of 1975/83.
The LOAS study has also been approved by the Swedish Ethical Review Board (2021-03484).
Results
Translation and cross-cultural adaptation of the EFIS
The EFIS was successfully translated into Swedish. The first four steps (Citation18) were performed with minor adjustments by the translators. Through consensus, the expert committee produced a prefinal version that was tested among patients. Demographic characteristics of the patients recruited to the field test with cognitive debriefing are presented in .
Table 1. Description of the patients participating in the field test.
In the field test, the EFIS was experienced as easy to complete, relevant, and easy to understand. There were no skipped or missing items, and the mean time to complete the EFIS was 1 min and 23s, ranging from 46s to 2 min and 52s. However, several patients requested questions about work and suggested separating work-life from family-life as these may differ. Still, they acknowledged the difficulties in doing this since not all patients are engaged in work. Some patients also raised a concern regarding answering the questionnaire when living alone. The wording of item 2 was found to be difficult and was therefore reworded. Several patients also pointed out that three items (Citation2, Citation3, and Citation8) used double negatives, which may cause confusion. However, they did not find the questions difficult to answer and therefore no adjustments were made. The Swedish version of EFIS is attached in Supplementary material 3; it is available free to use and can be downloaded from the ASAS homepage, section clinical instruments (http://www.asas-group.org/clinical-instruments.php?id=03).
Construct validity and test–retest reliability of the Swedish version of ASAS HI and test–retest reliability for EFIS
Sixty-one patients in the LOAS study were asked to participate in the reliability study. All patients had already filled in a set of questionnaires including the ASAS HI and EFIS as part of the LOAS study and were asked to fill in the two questionnaires ASAS HI and EFIS again after 1 week. All 61 patients agreed to participate, of whom 53 (86.9%) refilled and returned the ASAS HI and EFIS. A description of the patients is presented in .
Table 2. Description of patients participating in the validity and reliability test of the Assessment of SpondyloArthritis international Society Health Index (ASAS HI) and test–retest reliability of the Environmental Factors Item Set (EFIS).
Interpretability of the ASAS HI
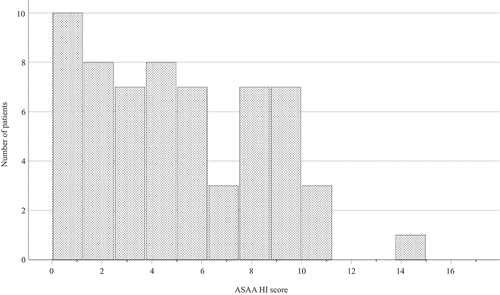
The mean ± sd total score was 4.9 ± 3.4. The distribution of the ASAS HI scores showed a non-normal distribution with a potential floor effect, as five out of 61 (8%) scored “I do not agree” on all items. The majority, 33 out of 61 (54%), scored good health ≤ 5 and one scored poor health > 12 ().
Construct validity of the ASAS HI
ASAS HI scores showed a good correlation with the ASDAS-CRP, BASDAI, BASFI, EQ-5D, and SF-36, confirming expected correlations ().
Table 3. Spearman correlations between Assessment of SpondyloArthritis international Society Health Index (ASAS HI) scores and the other health outcomes.
Internal consistency
ASAS HI scores showed an acceptable Cronbach’s αof 0.79. Item 14 showed zero variance and was therefore removed from the analysis.
Test–retest reliability
ASAS HI scores showed excellent test–retest reliability (ICC 0.87, 95% confidence interval 0.77–0.92, p < 0.001) (). The Bland–Altman plot showed one outlier but otherwise good agreement, and there was no systematic error between the assessments ().
Figure 2. Bland–Altman plot of the distribution of Assessment of SpondyloArthritis international Society Health Index (ASAS HI) scores.
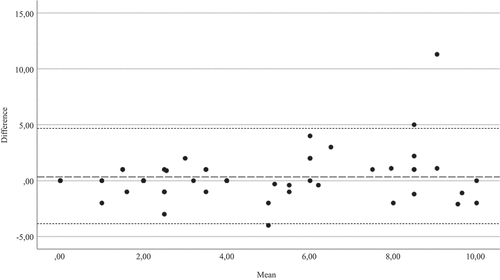
Table 4. Intraclass correlation coefficient (ICC) for the Assessment of SpondyloArthritis international Society Health Index ASAS HI (score) and kappa agreement for the Environmental Factors Item Set (EFIS) (discrete items).
Of the individual items on the ASAS EFIS, items 1 and 9 showed good reliability; items 4, 5, 6, and 7 showed moderate reliability; while items 2, 3, and 8 showed poor or fair reliability. Kappa agreement ranged from −0.027 (se 0.020, p = 0.84) to 0.80 (se 0.098, p < 0.001) ().
Discussion
The aims of this project were to translate the EFIS into Swedish and culturally adapt it for a Swedish context, and to assess the validity of the Swedish version of ASAS HI and test–retest reliability in ASAS HI and EFIS. We found that the Swedish version of ASAS HI is a valid and reliable measure of function and health in Swedish patients with AS. The EFIS showed acceptable face and content validity, but the reliability showed varied results.
The EFIS was successfully translated into Swedish (Citation14). The patients found the content relevant, meaningful, and easy to complete, indicating an acceptable face and content validity. However, the patients pointed out that three items (item 2, 3, and 8) used double negatives, which constitute a potential cause of confusion. In the test of reliability, these three items showed poor or fair test–retest reliability, which confirmed this anticipation. However, as the original English version uses double negatives for these items, further adjustments were difficult. We suggest further discussions regarding the wording of these items to enhance clarity and reliability. The EFIS was originally developed to complement the ASAS HI and helps in the interpretation of the ASAS HI results by mediating the understanding of the interaction between health and environmental factors (Citation15). Although the reliability of the Swedish EFIS showed various results, individual items may still contribute to the interpretation of the ASAS HI and a general understanding of disability.
Concerning the measurement properties of ASAS HI, we found an excellent test–retest reliability, which is in line with the results of earlier studies evaluating ASAS HI in different languages (Citation17, Citation31–34). For construct validity, we found a good correlation with ASDAS-CRP, BASDAI, BASFI, EQ-5D, and SF-36, which also corresponds to earlier results (Citation17, Citation31–34). Furthermore, we found an acceptable internal consistency, slightly lower than the result of the other translation studies (Citation17, Citation31–34). In this evaluation, one item (item 14) was omitted from the analysis owing to zero variation in this question, i.e. all patients scored ‘I do not agree’ on the statement ‘I find it difficult to wash my hair’. We also found a slight floor effect in our study population, and when applying cut-off values (Citation17, Citation35), only one participant in our study reported poor health status (> 12 points), indicating a population with good or moderate health status. Compared to other evaluations (Citation17, Citation31–34), the population in our study had a lower average score on the ASAS HI, indicating a slightly better health status compared to the other studies, which may have influenced the result. The population was recruited from an ongoing longitudinal study in which the patients were participating as part of a follow-up visit 13 years after inclusion. This resulted in a relatively old population and no recently diagnosed patients, both of which may explain the lower ASAS HI scores.
Some limitations of this study need to be considered. The population for the field test includes both patients with AS and nrAxSpA, while the population for the test of validity and reliability was recruited from an ongoing longitudinal study and included only patients with AS. This may affect the generalizability to the whole axSpA population but also to the AS population, as the patients in an ongoing long-term study were asked to participate, rather than a random sample of patients. Furthermore, compared to corresponding studies (Citation17, Citation31–34), the patients constituted an older population with longer disease duration. However, this shows that ASAS HI is reliable also in an older population. When evaluating test–retest reliability, the stability of the health condition needs to be considered. A one week period in this population was not anticipated to cause a significant variability in health, but was considered a long enough interval to avoid recall bias when filling out the questionnaires.
Conclusion
Health and health-related quality of life are significant aspects raised by patients, and reliable and valid measures of these constructs are important. The ASAS HI and EFIS are internationally recognized, and the ASAS HI has been selected as the mandatory outcome measure to study health in people with axSpA. The Swedish version of ASAS HI was evaluated and proved to be valid and reliable for assessing the impact of AS on global functioning and health. A Swedish version of EFIS has now been produced and uploaded on the ASAS website to accompany the previously translated Swedish version of the ASAS HI. The EFIS showed acceptable face and content validity and may contribute to the contextual interpretation of the ASAS HI and to a general understanding of disability in patients with axSpA.
Author contributions
The study design and protocol were developed by HFd’E and CF in collaboration with UK. Translation was performed by CF, Ad’E, and HFd’E, and data collection was carried out by CF, MH, and AD. Data interpretation and analysis were carried out, and the first draft of the manuscript was written by CF and HFd’E. All authors were responsible for critical revision and finalizing of the manuscript. All authors read and approved the final manuscript.
Supplemental Material
Download PDF (609.3 KB)Acknowledgements
The authors thank all patients who participated in the study. We also thank the professional translators, Ulrika Stigeborn Hernelid at Lingualista and Jennifer Evans at Changeling Translations AB, and the Clinical Rheumatology Research Center (KRF) at the Rheumatology Clinic at Sahlgrenska University Hospital.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/03009742.2023.2266903.
Additional information
Funding
References
- van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–8.
- Vander Cruyssen B, Ribbens C, Boonen A, Mielants H, de Vlam K, Lenaerts J, et al. The epidemiology of ankylosing spondylitis and the commencement of anti-TNF therapy in daily rheumatology practice. Ann Rheum Dis 2007;66:1072–7.
- Feldtkeller E, Khan MA, van der Heijde D, van der Linden S, Braun J. Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol Int 2003;23:61–6.
- Rudwaleit M, van der Heijde D, Landewe R, Listing J, Akkoc N, Brandt J, et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83.
- van Echteld I, Cieza A, Boonen A, Stucki G, Zochling J, Braun J, et al. Identification of the most common problems by patients with ankylosing spondylitis using the international classification of functioning, disability and health. J Rheumatol 2006;33:2475–83.
- Ward MM. Health-related quality of life in ankylosing spondylitis: a survey of 175 patients. Arthritis Care Res 1999;12:247–55.
- Machado P, Landewe R, Braun J, Hermann KG, Baraliakos X, Baker D, et al. A stratified model for health outcomes in ankylosing spondylitis. Ann Rheum Dis 2011;70:1758–64.
- Law L, Beckman Rehnman J, Deminger A, Klingberg E, Jacobsson LTH, Forsblad-d’Elia H. Factors related to health-related quality of life in ankylosing spondylitis, overall and stratified by sex. Arthritis Res Ther 2018;20:284.
- Sullivan M, Karlsson J, Ware JE Jr. The Swedish SF-36 Health Survey – I. Evaluation of data quality, scaling assumptions, reliability and construct validity across general populations in Sweden. Soc Sci Med 1995;41:1349–58.
- Boonen A, Braun J, Bruinsma IEV, Huang F, Maksymowych W, Kostanjsek N, et al. ASAS/WHO ICF core sets for ankylosing spondylitis (AS): how to classify the impact of AS on functioning and health. Ann Rheum Dis 2010;69:102–7.
- Kiltz U, van der Heijde D, Boonen A, Cieza A, Stucki G, Khan MA, et al. Development of a health index in patients with ankylosing spondylitis (ASAS HI): final result of a global initiative based on the ICF guided by ASAS. Ann Rheum Dis 2015;74:830–5.
- Navarro-Compan V, Boel A, Boonen A, Mease P, Landewe R, Kiltz U, et al. The ASAS-OMERACT core domain set for axial spondyloarthritis. Semin Arthritis Rheum 2021;51:1342–9.
- Navarro-Compan V, Boel A, Boonen A, Mease PJ, Dougados M, Kiltz U, et al. Instrument selection for the ASAS core outcome set for axial spondyloarthritis. Ann Rheum Dis 2023;82:763–72.
- Kiltz U, van der Heijde D, Boonen A, Bautista-Molano W, Burgos-Vargas R, Chiowchanwisawakit P, et al. Measuring impairments of functioning and health in patients with axial spondyloarthritis by using the ASAS Health Index and the Environmental Item Set: translation and cross-cultural adaptation into 15 languages. RMD Open 2016;2:e000311.
- Kiltz U, Boonen A, van der Heijde D, Bautista-Molano W, Burgos Vargas R, Chiowchanwisawakit P, et al. Development of an environmental contextual factor item set relevant to global functioning and health in patients with axial spondyloarthritis. Rheumatology (Oxford) 2022;61:2054–62.
- Gordeev VS, Maksymowych WP, Evers SMAA, Ament A, Schachna L, Boonen A. Role of contextual factors in health-related quality of life in ankylosing spondylitis. Ann Rheum Dis 2010;69:108–12.
- Kiltz U, van der Heijde D, Boonen A, Akkoc N, Bautista-Molano W, Burgos-Vargas R, et al. Measurement properties of the ASAS health index: results of a global study in patients with axial and peripheral spondyloarthritis. Ann Rheum Dis 2018;77:1311–17.
- Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976) 2000;25:3186–91.
- Klingberg E, Geijer M, Gothlin J, Mellstrom D, Lorentzon M, Hilme E, et al. Vertebral fractures in ankylosing spondylitis are associated with lower bone mineral density in both central and peripheral skeleton. J Rheumatol 2012;39:1987–95.
- Klingberg E, Lorentzon M, Mellstrom D, Geijer M, Gothlin J, Hilme E, et al. Osteoporosis in ankylosing spondylitis - prevalence, risk factors and methods of assessment. Arthritis Res Ther 2012;14:R108.
- van der Heijde D, Lie E, Kvien TK, Sieper J, Van den Bosch F, Listing J, et al. ASDAS, a highly discriminatory ASAS-endorsed disease activity score in patients with ankylosing spondylitis. Ann Rheum Dis 2009;68:1811–18.
- Machado P, Landewe R, Lie E, Kvien TK, Braun J, Baker D, et al. Ankylosing Spondylitis Disease Activity Score (ASDAS): defining cut-off values for disease activity states and improvement scores. Ann Rheum Dis 2011;70:47–53.
- Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing-spondylitis - the Bath Ankylosing-Spondylitis Disease-Activity Index. J Rheumatol 1994;21:2286–91.
- Calin A, Garrett S, Whitelock H, Kennedy LG, Ohea J, Mallorie P, et al. A new approach to defining functional ability in ankylosing-spondylitis - the development of the Bath Ankylosing-Spondylitis Functional Index. J Rheumatol 1994;21:2281–5.
- Burstrom K, Sun S, Gerdtham UG, Henriksson M, Johannesson M, Levin LA, et al. Swedish experience-based value sets for EQ-5D health states. Qual Life Res 2014;23:431–42.
- EuroQol G. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy (New York) 1990;16:199–208.
- Ware JE Jr, Sherbourne CD, The MOS. 36-item Short-Form Health Survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83.
- Fleiss JL. The design and analysis of clinical experiments. New York: Wiley, 1999.
- Terwee CB, Bot SD, de Boer MR, van der Windt DA, Knol DL, Dekker J, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol 2007;60:34–42.
- Altman DG. Practical statistics for medical research. 1st ed. London: Chapman and Hall, 1991:xii, 611.
- Rodrigues-Manica S, Cruz E, Ramiro S, Sousa S, Aguiar R, Sepriano A, et al. The European Portuguese version of the ASAS health index for patients with spondyloarthritis: measurement properties. Acta Reumatol Port 2020;45:26–33.
- Choi JH, Kim TJ, Shin K, Choi CB, Kim JH, Kim SH, et al. The reliability and validity of a Korean translation of the ASAS health index and environmental factors in Korean patients with axial spondyloarthritis. J Korean Med Sci 2014;29:334–7.
- Bautista-Molano W, Landewe RBM, Kiltz U, Valle-Onate R, van der Heijde D. Validation and reliability of translation of the ASAS Health Index in a Colombian Spanish-speaking population with spondyloarthritis. Clin Rheumatol 2018;37:3063–8.
- Di Carlo M, Lato V, Carotti M, Salaffi F. Clinimetric properties of the ASAS Health Index in a cohort of Italian patients with axial spondyloarthritis. Health Qual Life Outcomes 2016;14:78.
- Akgul O, Bodur H, Ataman S, Yurdakul FG, Capkin E, Gurer G, et al. Clinical performance of ASAS Health Index in patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis: real-world evidence from Multicenter Nationwide Registry. Rheumatol Int 2020;40:1793–801.