 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objectives
To assess sleep quality, and its associations with physical function, cardiorespiratory fitness, and spinal mobility, in axial spondyloarthritis (axSpA) patients.
Method
Baseline data from the Exercise for Spondyloarthritis trial were used. Assessments included [Pittsburgh Sleep Quality Index (PSQI), 0–21, 21 = worst], performance-based physical function [Ankylosing Spondylitis Performance Index (ASPI), seconds, higher = worse], patient-reported physical function [Bath Ankylosing Spondylitis Functional Index (BASFI), 0–10, 10 = worst], cardiorespiratory fitness [peak oxygen uptake (O2peak), mL/kg/min, lower = worse], and spinal mobility [Bath Ankylosing Spondylitis Metrology Index (BASMI), 0–10, 10 = worst]. Associations were examined in separate models using multiple linear regression.
Results
Ninety-nine patients with axSpA were included, 53% female, mean age 46 years, and 72% with high disease activity (ASDAS-C-reactive protein ≥ 2.1), of whom 84 (85%) had reduced sleep quality. Sleep disturbance was most frequently reported (65%), followed by poor subjective sleep quality (53%), daytime dysfunction (41%), and increased sleep latency (41%). Positive associations were observed between PSQI and ASPI [β = 0.10, 95% confidence interval (CI) 0.01, 0.19] and PSQI and BASFI (β = 0.85, 95% CI 0.51, 1.20), and there was an inverse association between PSQI and O2peak (β = −0.14, 95% CI −0.27, −0.01), adjusted for age and sex. There was no association between PSQI and BASMI.
Conclusion
Reduced sleep quality was common in axSpA patients with moderate to high disease activity. Better sleep quality was associated with better physical function and higher cardiorespiratory fitness. There was no association between sleep quality and spinal mobility.
Trial registration
ClinicalTrials.gov NCT02356874
Axial spondyloarthritis (axSpA) is an autoimmune inflammatory rheumatic disease that primarily affects the axial skeleton, and encompasses both radiographic (also termed ankylosing spondylitis) and non-radiographic axSpA (Citation1). The disease usually debuts in early adulthood, with an equal sex distribution among patients with non-radiographic axSpA, although more men tend to develop radiographic axSpA. The leading symptoms are inflammatory back pain and morning stiffness (Citation1). Patients also report reduced sleep quality (Citation2). The importance of sleep in axSpA is being increasingly recognized, and since 2021 sleep has been included as an important domain for clinical trials in the Assessment of SpondyloArthritis international Society (ASAS)–Outcome Measures in Rheumatology (OMERACT) core domain set for axSpA (Citation3, Citation4).
Systematic reviews report that between 35% and 90% of patients with axSpA have reduced sleep quality, and that sleep problems are more prevalent than in the general population (Citation5, Citation6). Furthermore, a meta-analysis indicated that patients with radiographic axSpA experience poorer subjective sleep quality, poorer sleep efficiency, and longer sleep latency, more frequently have sleep disturbances, and use more sleep medication compared to the general population (Citation6). Adequate sleep is essential to maintain good health, both for optimal cognitive function and for the immune system (Citation7, Citation8). The immune system and sleep are bidirectionally linked, and prolonged sleep deficiency can lead to chronic, systemic low-grade inflammation (Citation7). Hence, for individuals with inflammatory rheumatic diseases, reduced sleep quality can increase systemic inflammation and thereby amplify the effects of an already overactive immune system (Citation7). Moreover, in axSpA, reduced sleep quality is identified as a predictor for the development of widespread pain and a risk factor for poor quality of life (Citation9, Citation10).
In the general population, reduced sleep quality is known to be related to older age, female sex, stressors, depression, pain, and circadian factors (Citation8), while maintaining a healthy lifestyle, such as taking regular exercise, is known to improve sleep (Citation11). For individuals with axSpA, reduced sleep quality is reported to correlate with high disease activity, poor patient-reported physical function, and pain (Citation5). Furthermore, the onset of pharmacological treatment with tumour necrosis factor-α inhibitors (TNFi) targeted at reducing inflammation has been shown to improve sleep quality (Citation5). However, not all patients are candidates for TNFi treatment or achieve satisfactory clinical response (Citation12).
In addition to pharmacological treatment, regular exercise and physiotherapy are recommended as cornerstones in the management of axSpA (Citation13). Exercise has beneficial effects on cardiorespiratory fitness, physical function, spinal mobility, pain, and disease activity in patients with axSpA (Citation14), but the impact of exercise on sleep has been less well explored. Previous research has mainly addressed the association between sleep quality and patient-reported physical function in patients with radiographic axSpA (Citation5). Hence, there is a lack of knowledge regarding the impact of performance-based physical function, physical fitness, and spinal mobility on sleep quality. The primary aim of this study was to examine the prevalence of reduced sleep quality and to identify aspects of sleep that are affected in a group of patients with axSpA with moderate to high disease activity. The second aim was to assess the association between sleep quality and physical function, cardiorespiratory fitness, and spinal mobility in this patient group.
Method
Design
This cross-sectional study was a secondary analysis using baseline data from the Exercise for SpondyloArthritis (ESpA) trial, a multicentre randomized controlled trial (RCT). The primary aim of ESpA was to compare the effects of three months supervised high-intensity cardiorespiratory and strength exercise with no intervention on disease activity (Citation15). The ESpA trial was prospectively registered in ClinicalTrials.gov (NCT02356874), and approved by the Regional Committee for medical and health Ethics of South East Norway (2015/86) and the Regional Ethical Review Board Gothenburg (032-16). All procedures adhered to the Declaration of Helsinki (Citation16). Participants provided written informed consent before inclusion in the trial. Reporting was guided by the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) checklist for cross-sectional studies (Citation17).
Participants
Participants were recruited between November 2015 and September 2016 from three outpatient rheumatology departments in Norway (Diakonhjemmet Hospital, Martina Hansens Hospital, and University Hospital in Northern Norway) and one in Sweden (Sahlgrenska University Hospital). Patients with axSpA according to the ASAS classification criteria (Citation18), aged 18–70 years, with stable medication for >3 months, high disease activity [Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) ≥ 3.5 (Citation19)], and no regular cardiorespiratory or strength exercise habits (>1 h per week for the past 6 months) at prescreening were eligible. Exclusion criteria were established or symptoms of cardiovascular disease, other comorbidities involving reduced exercise capacity, inability to participate in weekly supervised sessions, and pregnancy.
Assessments
One assessor conducted all of the clinical examinations in the following order: laboratory evaluation of C-reactive protein (CRP), assessment of spinal mobility, performance-based physical function (added after the inclusion of the first 34 participants), and cardiorespiratory fitness. In addition, the patients completed a questionnaire addressing sociodemographic information, disease-related health, physical function, and sleep.
Sleep quality was assessed with the Pittsburgh Sleep Quality Index (PSQI) (Citation20), a generic questionnaire commonly used in clinical and research settings (Citation21) that is widely used in patients with rheumatic diseases (Citation22). It is considered to be valid as it discriminates patients with sleeping problems and controls (Citation20). PSQI assesses sleep quality during the past month and includes 19 items that are grouped into seven component scores: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. Each component is scored 0–3, and a score ≥2 indicates reduced sleep quality for this component. The seven component scores are summed into the PSQI global score (0–21, 21 = severe sleep difficulties), with a score >5 differentiating between those with normal and reduced sleep quality (Citation20, Citation21). In heterogeneous populations, a PSQI global cut off score of >7 has been suggested to discriminate between those with normal and reduced sleep quality (Citation21).
Physical function was assessed with two measures: performance-based function was tested with Ankylosing Spondylitis Performance Index (ASPI) (Citation23), and patient-reported function was assessed with the Bath Ankylosing Spondylitis Functional Index (BASFI) (Citation24). ASPI consists of three tests: bending and picking up six pens from the floor, getting up from the floor from lying supine (three attempts, mean time), and putting on a pair of socks. The total time needed to complete the three tests is reported in seconds (longer time = worse) (Citation25). BASFI includes eight items regarding activities of daily living and two items addressing participation. Each item is answered on an 11-point numeric rating scale (NRS) and the mean gives the BASFI score (0–10, 10 = worst) (Citation26).
Cardiorespiratory fitness was assessed by an indirect maximal test on a treadmill with a modified Balke protocol for estimation of peak oxygen uptake (O2peak) (mL/kg/min) (Citation27). The test includes walking at an individually adapted speed starting at 2.5% inclination, with an increase in inclination by 1.5% every minute. If 15% inclination was reached, the speed was increased by 0.3 km/h every minute. The test was stopped when the participants could not further increase their workload and/or reported a perceived exertion between very hard (17) and maximal exertion (20) on the Borg scale (Citation28). The estimated
O2peak was calculated based on the American College of Sports Medicine’s formula for graded walking (lower = worse) (Citation29).
Mobility was assessed with the Bath Ankylosing Spondylitis Metrology Index (BASMI), which consists of four measures of spinal mobility and one measure of hip mobility (Citation30). The measures were collected and recorded following the recommendations from ASAS (Citation26). The formula for BASMI linear was used to compute the total score (0–10, 10 = worst) (Citation31).
Disease activity was assessed with the Ankylosing Spondylitis Disease Activity Score based on C-reactive protein (ASDAS-CRP) (Citation32) and the BASDAI (0–10, 10 = worst) (Citation33). The ASDAS is a composite score of CRP and four patient-reported variables, all reported on an 11-point NRS: neck/back/ hip pain, peripheral joint pain, duration of morning stiffness, and patient global assessment (Citation32). The ASDAS was calculated based on the formula including CRP, and a cut-off at <2.1 was used to differentiate between low and high disease activity (Citation34). A history of extra-musculoskeletal manifestations was collected by the questionnaire.
Sample size
The estimation of sample size was not performed for sleep quality in the present study. The sample size was determined by the power calculation for the primary outcome, disease activity, in the ESpA trial (Citation15). Based on a pilot study, exercise had a mean treatment effect of 0.7 points in the ASDAS-CRP (Citation35); with 80% power and a 5% significance level, an estimated non-compliance of 10%, and a dropout rate of 20%, it was calculated that 100 patients were needed.
Statistical analyses
Patient characteristics are presented as mean and standard deviation (sd), median and interquartile range (IQR) or counts and percentages. Differences between subgroups of patients were examined with the independent sample t-test, Mann–Whitney U test, or chi-squared test, as appropriate. Mean differences are presented with the 95% confidence interval (CI). Missing data were handled with either complete case analysis or single-item imputations with the simple mean with the average value within a component (PSQI) or the average of the entire measure (BASFI).
Multiple linear regression analyses were applied to evaluate the association between PSQI (dependent variable) and each of the independent variables of interest: ASPI, BASFI, O2peak, and BASMI. Covariate variables were selected based on theoretical relevance. First, unadjusted estimates were determined: in Model 1 age and sex (femaleref/male) were included as covariates; and in Model 2 age, sex, disease activity (ASDAS lowref/high disease activity), and use of TNFi (noref/yes) were included. Results from the multiple linear regression models are presented as unadjusted and adjusted estimates with the beta (β) value, 95% CI, and p-value. The models were assessed for normality assumptions with propability plots, histograms, and scatterplots of regression standardized residuals. All analyses were replicated with unimputed data of PSQI global score and BASFI, and compared with the primary analyses. Statistical analyses were performed using SPSS for Windows, version 27.0. A p-value < 0.05 was considered statistically significant.
Results
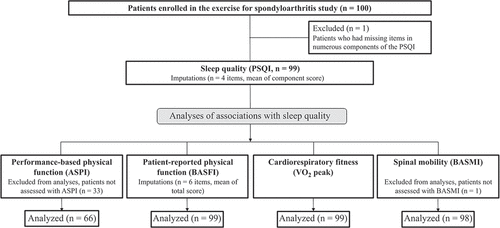
In total, 100 patients with axSpA were enrolled in the ESpA trial. Out of these, 99 patients had data on sleep quality (PSQI) and were included in the present study. shows an overview of participants included in the different analyses and imputations performed. ASPI was assessed in 66 participants (67%), and there were no statistically significant differences in age, sex, axSpA subgroup, disease activity, or use of TNFi between patients with ASPI data and those who were included before this outcome measure was added to the data collection (data not shown).
Figure 1. Flow diagram of participants included in the different analyses and performed imputations. ASPI: Ankylosing Spondylitis Performance Index, BASFI: Bath Ankylosing Spondylitis Functional Index, BASMI: Bath Ankylosing Spondylitis Metrology Index, PSQI: Pittsburgh Sleep Quality index, VO2peak: peak oxygen uptake.
Patient characteristics
Of the 99 included patients, 52 (53%) were female, the mean age was 46 years, and 69 (70%) had radiographic axSpA (). A total of 71 (72%) had high disease activity (ASDAS-CRP), and 44 (44%) were being treated with TNFi.
Table 1. Demographic and clinical features in patients with axial spondyloarthritis (axSpA).
Sleep quality
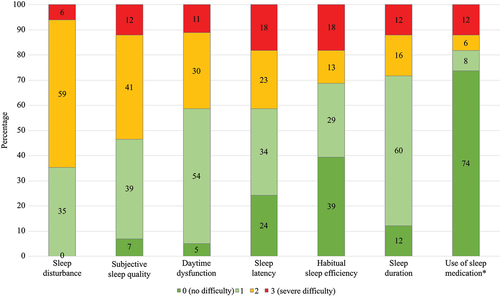
The mean ± sd PSQI was 9.1 ± 3.7, and 84 (85%) were categorized with reduced sleep quality (PSQI > 5). presents the seven components of sleep included in the PSQI questionnaire and the proportion of the patients’ scores within each component. Sleep disturbance was the most frequently reported aspect of sleep quality, in 64 (65%), followed by poor subjective sleep quality in 53 (54%), daytime dysfunction in 41 (41%), sleep latency in 41 (41%), habitual sleep efficiency in 31 (31%), sleep duration in 28 (28%), and use of sleep medication in 18 (18%).
Figure 2. Bar graphs illustrating the proportion of patients with axial spondyloarthritis (n = 99) scores in the different aspects of sleep quality included in the Pittsburgh sleep quality index. In each aspect, the score ranges from zero to three, and a score ≥2 indicates reduced sleep quality. *0 = not during the past month, 1 = less than once a week, 2 = once or twice per week, 3 = three or more times a week.
Associations between sleep quality, physical function, cardiorespiratory fitness, and spinal mobility in patients with axSpA
shows differences between patients with normal and reduced sleep quality (PSQI > 7). Patients with normal sleep quality had statistically significantly better performance-based physical function, patient-reported physical function, and cardiorespiratory fitness than patients with reduced sleep quality. There was a tendency for a larger proportion of patients with non-radiographic axSpA (77%) compared to radiographic axSpA (55%) to be categorized with reduced sleep quality (p = 0.07).
Table 2. Comparison of axial spondyloarthritis (axSpA) patients with normal sleep quality versus axSpA patients with reduced sleep quality.
shows the associations between sleep quality (PSQI) and performance-based physical function (ASPI), patient-reported physical function (BASFI), cardiorespiratory fitness (O2peak), and spinal mobility (BASMI). Both univariate and multivariate regression analyses identified ASPI, BASFI, and
O2peak as being statistically significantly associated with PSQI. Adjusted for age and sex, for each second less needed in ASPI, the PSQI score was reduced (β = 0.10, 95% CI 0.01, 0.19); for each lower score in BASFI, the PSQI score was reduced (β = 0.85, 95% CI 0.51, 1.20); and for each unit higher
O2peak, the PSQI score was reduced (β = −0.14, 95% CI −0.27, −0.01). These results indicate that better sleep quality is associated with better performance-based physical function, better patient-reported physical function, and higher cardiorespiratory fitness. When adding disease activity and use of immunosuppressive medication into the models, the results did not substantially change, except for the association with
O2peak, which was no longer statistically significant (p = 0.08). No statistically significant associations between PSQI and BASMI were found in either univariate or multivariate regression analyses. Replicated analyses with unimputed data in PSQI and BASFI showed identical results to the primary analyses (data not shown).
Table 3. Association between sleep quality and performance-based physical function, patient-reported physical function, cardiorespiratory fitness, and spinal mobility in patients with axial spondyloarthritis.
Discussion
The results of this study demonstrated that sleep problems are highly prevalent and that several aspects of sleep quality, such as sleep disturbances, daytime dysfunction, and sleep latency, are frequently reported in axSpA patients with moderate to high disease activity who do not engage in regular exercise. The extent and magnitude of reduced sleep quality found in this study require attention, as unmanaged sleep problems can amplify the systemic inflammation of the disease and pain sensitivity, and cause poor quality of life for individuals living with axSpA (Citation7, Citation9, Citation10). Our findings emphasize the need to routinely assess sleep quality in axSpA patients with moderate to high disease activity. Furthermore, the results showed that better physical function and higher cardiorespiratory fitness were significantly associated with better sleep quality, whereas there was no association between spinal mobility and sleep quality. The results indicate that exercise aimed at improving physical function and cardiorespiratory fitness may be important for axSpA patients with sleeping problems.
Sleep is one of the most important aspects of health for patients with axSpA (Citation2), and the present study showed that in a group with moderate to high disease activity, a total of 85% had reduced sleep quality. The prevalence of sleep problems in previous studies that have assessed sleep with PSQI in patients with axSpA varies between 37% and 90% (Citation5, Citation36–39). Hence, the proportion of patients with axSpA reporting reduced sleep quality in the present study is among the highest that have been reported. This may partly be explained by differences in study samples, as only patients with moderate to high disease activity are included in the present study. Notably, sleep problems are common in Scandinavia, and the prevalence of insomnia is among the highest in Europe (Citation8). A cross-sectional study found that 44–50% of the Norwegian population had reduced sleep quality when sleep was assessed with the same questionnaire as used in the current study (Citation40). Hence, consistent with previous research (Citation5), our results indicate that reduced sleep quality is common in patients with axSpA.
The results showed that all the measured aspects of sleep were affected in the patients with axSpA. A high proportion of the included patients reported sleep disturbances, low subjective sleep quality, increased sleep latency, and daytime dysfunction. Being woken by back pain in the night is one of the key features of axSpA (Citation1), but the results from the present study show that reduced sleep quality is not limited to sleep disturbances. Our results align with a meta-analysis reporting that patients with axSpA have more sleep disturbances, lower subjective sleep quality, lower sleep efficiency, and higher use of sleep medication than healthy controls (Citation6).
In the present study, we found that better sleep quality was associated with better physical function and higher cardiorespiratory fitness, suggesting that physical function and cardiorespiratory fitness may have potential roles as modifiable factors for sleep quality in patients with axSpA. Our finding supports previous studies, reporting that better patient-reported physical function is associated with better sleep quality (Citation5, Citation36, Citation37, Citation39, Citation41, Citation42). To the best of our knowledge, the present study is the first to demonstrate associations between sleep quality and clinical examinations of performance-based physical function and cardiorespiratory fitness. We found that the association between sleep quality and performance-based physical function was weaker than with patient-reported physical function. This may be explained by the fact that the patient-reported physical function includes items of vitality and participation in daily life, items that are included in the sleep quality questionnaire, and hence patient-reported physical function and sleep quality cover some of the same aspects. In addition, patients seem to incorporate pain in their assessment of perceived physical function to a greater extent than in performance-based physical function (Citation43, Citation44). Our finding that both patient-reported and performance-based physical function are independently associated with sleep quality adds support for the role of physical function for sleep quality in axSpA.
Our finding that higher cardiorespiratory fitness was associated with better sleep quality is in line with the findings in a large cross-sectional study of healthy adults, reporting that higher cardiorespiratory fitness was associated with fewer insomnia symptoms (Citation45). Furthermore, a meta-analysis reported that regular exercise has beneficial effects on sleep quality in the general population (Citation11). This is partly supported by a cross-sectional study of patients with axSpA, showing that patients with low levels of physical activity reported more sleep disturbance than those reporting high levels of physical activity (Citation41). Hence, the associations between sleep quality and cardiorespiratory fitness found in the present study may be partly explained by physical activity level and exercise habits. In patients with axSpA, regular exercise has demonstrated benefits on cardiorespiratory fitness and physical function, and has the potential to improve pain, fatigue, and inflammation (Citation15, Citation46, Citation47), factors closely related to sleep quality (Citation5, Citation7). However, there is scarce knowledge about the effects of regular exercise on sleep quality in patients with axSpA, although some evidence suggests that exercise may improve sleep quality (Citation48, Citation49). More studies examining possible causal relationships between exercise and sleep quality in axSpA are needed.
In accordance with a systematic review, we found no association between spinal mobility and sleep quality in patients with axSpA (Citation6). In contrast, a cross-sectional study reported that better spinal mobility was a predictor for reduced sleep quality in axSpA (Citation50). Discrepancies between the findings may partly be due to the use of different measures to assess sleep, and differences in subgroups of axSpA included, as Wadeley et al (Citation50) included 91% radiographic axSpA. Hence, associations between spinal mobility and sleep may be different in non-radiographic and radiographic axSpA. Despite this, the present study found that a larger proportion of patients with non-radiographic (77%) than radiographic axSpA (55%) were categorized with reduced sleep quality, indicating that factors other than spinal mobility are more important for sleep quality in axSpA.
Our findings should be interpreted in the context of limitations. Our study sample cannot be seen as representative for the total axSpA population, as only patients with moderate to high disease activity not engaging in regular exercise were eligible for inclusion. In addition, only 67% of the patients had data on performance-based physical function (ASPI). Furthermore, no a priori power calculation on sleep quality was performed for this study because it was a secondary analysis of baseline data of an RCT, and it cannot be ruled out that the sample size was too small to detect associations. Although we found several statistically significant associations indicating that we had enough power, there may be a risk that the results could be due to chance, as this was a secondary analysis and multiple statistical tests were performed.
However, in the present study, the statistical analyses were performed based on predefined research questions, and because of the relatively small sample size it was decided not to correct for multiple testing as this would had increased the likelihood of not detecting a significant correlation when it actually existed. In addition, missing data were imputed with average mean within a component (PSQI) or the mean of the total measure (BASFI). However, there were few missing data, and replicated analyses with unimputed data in PSQI and BASFI showed identical results to the primary analyses; therefore, imputation of missing data probably did not influence the results. Furthermore, the cross-sectional design is a limitation as it does not allow us to determine causal relationships between sleep and physical function and cardiorespiratory fitness. Strengths of the present study include the use of a widely used generic measure to measure sleep quality and the use of validated disease-specific measures of physical function, spinal mobility, and disease activity. However, the validity of the PSQI has not been examined in populations with axSpA. Furthermore, it is a strength that physical function was measured with both a performance-based test and a patient-reported questionnaire.
Conclusion
The results demonstrated that reduced sleep quality is highly prevalent, and encompasses multiple aspects of sleep, in a group of axSpA patients with moderate to high disease activity that does not engage in regular exercise. The large majority of the patients reported that they experienced sleep disturbances, and many reported increased sleep latency and daytime dysfunction due to reduced sleep quality. Better physical function and cardiorespiratory fitness were associated with increased sleep quality, while there was no association between spinal mobility and sleep quality. These findings indicate that interventions aimed at improving physical function and cardiorespiratory fitness may have a beneficial effect on sleep quality in axSpA.
Acknowledgements
The authors gratefully thank all participating patients, study personnel, and patient research partners.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data underlying this article are available from the corresponding author upon reasonable request https://www.diakonhjemmetsykehus.no/.
Additional information
Funding
References
- Navarro-Compán V, Sepriano A, El-Zorkany B, van der Heijde D. Axial spondyloarthritis. Ann Rheum Dis 2021;80:1511–21.
- Kiltz U, Essers I, Hiligsmann M, Braun J, Maksymowych WP, Taylor WJ, et al. Which aspects of health are most important for patients with spondyloarthritis? A best worst scaling based on the ASAS Health Index. Rheumatology (Oxford) 2016;55:1771–6.
- Navarro-Compán V, Boel A, Boonen A, Mease P, Landewé R, Kiltz U, et al. The ASAS-OMERACT core domain set for axial spondyloarthritis. Semin Arthritis Rheum 2021;51:1342–9.
- Editorial. A wake-up call for sleep in rheumatic diseases. Lancet Rheumatol. 2022;4: E739. doi:10.1016/S2665-9913(22)00311-3
- Leverment S, Clarke E, Wadeley A, Sengupta R. Prevalence and factors associated with disturbed sleep in patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis: a systematic review. Rheumatol Int 2017;37:257–71.
- Li Z, Fu T, Wang Y, Dong C, Shao X, Li L, et al. Sleep disturbances in ankylosing spondylitis: a systematic review and meta-analysis. Psychol Health Med 2019;24:911–24.
- Besedovsky L, Lange T, Haack M. The sleep-immune crosstalk in health and disease. Physiol Rev 2019;99:1325–80.
- Riemann D, Baglioni C, Bassetti C, Bjorvatn B, Dolenc Groselj L, Ellis JG, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res 2017;26:675–700.
- Macfarlane GJ, Rotariu O, Jones GT, Pathan E, Dean LE. Determining factors related to poor quality of life in patients with axial spondyloarthritis: results from the British Society for Rheumatology Biologics Register (BSRBR-AS). Ann Rheum Dis 2020;79:202–8.
- Soni A, Nishtala R, Ng S, Barnett R, Chyou TY, Cavill C, et al. The natural history of chronic widespread pain in patients with axial spondyloarthritis: a cohort study with clinical and self-tracking data. Rheumatology (Oxford) 2022;62:2444–52.
- Kredlow MA, Capozzoli MC, Hearon BA, Calkins AW, Otto MW. The effects of physical activity on sleep: a meta-analytic review. J Behav Med 2015;38:427–49.
- Navarro-Compán V, Plasencia-Rodríguez C, de Miguel E, Diaz Del Campo P, Balsa A, Gratacós J. Switching biological disease-modifying antirheumatic drugs in patients with axial spondyloarthritis: results from a systematic literature review. RMD Open 2017;3:e000524.
- Ramiro S, Nikiphorou E, Sepriano A, Ortolan A, Webers C, Baraliakos X, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis 2023;82:19–34.
- Ortolan A, Webers C, Sepriano A, Falzon L, Baraliakos X, Landewé RB, et al. Efficacy and safety of non-pharmacological and non-biological interventions: a systematic literature review informing the 2022 update of the ASAS/EULAR recommendations for the management of axial spondyloarthritis. Ann Rheum Dis 2023;82:142–52.
- Sveaas SH, Bilberg A, Berg IJ, Provan SA, Rollefstad S, Semb AG, et al. High intensity exercise for 3 months reduces disease activity in axial spondyloarthritis (axSpA): a multicentre randomised trial of 100 patients. Br J Sports Med 2020;54:292–7.
- World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191–4.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–7.
- Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83.
- Kwon OC, Park MC. BASDAI cut-off values corresponding to ASDAS cut-off values. Rheumatology (Oxford) 2022;61:2369–74.
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213.
- Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh Sleep Quality Index as a screening tool for sleep dysfunction in clinical and non-clinical samples: a systematic review and meta-analysis. Sleep Med Rev 2016;25:52–73.
- Chu P, Ju YS, Hinze AM, Kim AHJ. Measures of sleep in rheumatologic diseases: sleep quality patient-reported outcomes in rheumatologic diseases. Arthritis Care Res (Hoboken) 2020;72:410–30.
- van Weely SF, Dekker J, Steultjens MP, van Denderen JC, Nurmohamed MT, Dijkmans BA, et al. Objective evaluation of physical functioning after tumor necrosis factor inhibitory therapy in patients with ankylosing spondylitis: a selection of 3 feasible performance-based tests. J Rheumatol 2015;42:623–9.
- Calin A, Garrett S, Whitelock H, Kennedy LG, O’Hea J, Mallorie P, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol 1994;21:2281–5.
- van Weely SF, van Denderen CJ, van der Horst-Bruinsma IE, Nurmohamed MT, Dijkmans BA, Dekker J, et al. Reproducibility of performance measures of physical function based on the BASFI, in ankylosing spondylitis. Rheumatology (Oxford) 2009;48:1254–60.
- Sieper J, Rudwaleit M, Baraliakos X, Brandt J, Braun J, Burgos-Vargas R, et al. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis 2009;68:ii1–44.
- Balke B, Ware RW. An experimental study of physical fitness of air force personnel. U S Armed Forces Med J 1959;10:675–88.
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14:377–81.
- American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription, 8th edn. Baltimore: Lippincott Williams & Wilkins, 2010.
- Jenkinson TR, Mallorie PA, Whitelock HC, Kennedy LG, Garrett SL, Calin A. Defining spinal mobility in ankylosing spondylitis (AS). The Bath AS Metrology Index. J Rheumatol 1994;21:1694–8.
- van der Heijde D, Deodhar A, Inman RD, Braun J, Hsu B, Mack M. Comparison of three methods for calculating the Bath Ankylosing Spondylitis Metrology Index in a randomized placebo-controlled study. Arthritis Care Res (Hoboken) 2012;64:1919–22.
- Lukas C, Landewé R, Sieper J, Dougados M, Davis J, Braun J, et al. Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis 2009;68:18–24.
- Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994;21:2286–91.
- Machado P, Landewé R, Lie E, Kvien TK, Braun J, Baker D, et al. Ankylosing Spondylitis Disease Activity Score (ASDAS): defining cut-off values for disease activity states and improvement scores. Ann Rheum Dis 2011;70:47–53.
- Sveaas SH, Berg IJ, Provan SA, Semb AG, Hagen KB, Vøllestad N, et al. Efficacy of high intensity exercise on disease activity and cardiovascular risk in active axial spondyloarthritis: a randomized controlled pilot study. PLoS One 2014;9:e108688.
- Karatas G, Bal A, Yuceege M, Firat H, Gurcay E, Ardic S, et al. Evaluation of sleep quality in patients with ankylosing spondylitis and efficacy of anti-TNF-α therapy on sleep problems: a polisomnographic study. Int J Rheum Dis 2018;21:1263–9.
- Nie A, Wang C, Song Y, Xie X, Yang H, Chen H. Prevalence and factors associated with disturbed sleep in outpatients with ankylosing spondylitis. Clin Rheumatol 2018;37:2161–8.
- Zhou W, Guo J, He M, Li J, Chen Y, Liu J, et al. Fatigue and contributing factors in Chinese patients with ankylosing spondylitis. Clin Rheumatol 2020;39:2337–44.
- Yüce E, Şentürk E, Sağaltıcı E, Şentürk İA, Aytekin E. Sleep quality and depression in patients with ankylosing spondylitis and their associations with clinical parameters: a cross-sectional, case-control study. Agri 2023;35:1–9.
- Pallesen S, Omvik S, Sivertsen B, Matthiesen SB, Bjorvatn B. Pittsburgh Sleep Quality Index [in Norwegian]. Tidsskrift Norsk Psykologiforening 2005;8:714–7.
- Urkmez B, Keskin Y. Relationship between sleep quality and physical activity level in patients with ankylosing spondylitis. Mod Rheumatol 2020;30:1053–9.
- Maatallah K, Makhlouf Y, Ferjani H, Cherif I, Nessib DB, Triki W, et al. Factors associated with the inflammatory process in pain in ankylosing spondylitis. Pan Afr Med J 2022;41:331.
- van Weely SF, van Denderen JC, Steultjens MP, van der Leeden M, Nurmohamed MT, Dekker J, et al. Moving instead of asking? Performance-based tests and BASFI-questionnaire measure different aspects of physical function in ankylosing spondylitis. Arthritis Res Ther 2012;14:R52.
- Fongen C, Dagfinrud H, Bilberg A, Pedersen E, Johansen MW, van Weely S, et al. Responsiveness and interpretability of 2 measures of physical function in patients with spondyloarthritis. Phys Ther 2020;100:728–38.
- Strand LB, Laugsand LE, Wisløff U, Nes BM, Vatten L, Janszky I. Insomnia symptoms and cardiorespiratory fitness in healthy individuals: the Nord-Trøndelag Health Study (HUNT). Sleep 2013;36:99–108.
- Sveaas SH, Smedslund G, Hagen KB, Dagfinrud H. Effect of cardiorespiratory and strength exercises on disease activity in patients with inflammatory rheumatic diseases: a systematic review and meta-analysis. Br J Sports Med 2017;51:1065–72.
- Metsios GS, Moe RH, Kitas GD. Exercise and inflammation. Best Pract Res Clin Rheumatol 2020;34:101504.
- Altan L, Bingöl U, Aslan M, Yurtkuran M. The effect of balneotherapy on patients with ankylosing spondylitis. Scand J Rheumatol 2006;35:283–9.
- Sveaas SH, Dagfinrud H, Berg IJ, Provan SA, Johansen MW, Pedersen E, et al. High-intensity exercise improves fatigue, sleep, and mood in patients with axial spondyloarthritis: secondary analysis of a randomized controlled trial. Phys Ther 2020;100:1323–32.
- Wadeley A, Clarke E, Leverment S, Sengupta R. Sleep in ankylosing spondylitis and non-radiographic axial spondyloarthritis: associations with disease activity, gender and mood. Clin Rheumatol 2018;37:1045–52.
