Dantrolene, a ryanodine receptor stabilizer, is a potential therapeutic drug against various diseases such as ventricular tachycardia, hepatic steatosis, and Alzheimer’s disease (Citation1–3). It may also serve as a therapeutic agent against autoimmune diseases (Citation4, Citation5). We previously reported that oral dantrolene administration significantly reduced serum anti-type II collagen (CII) immunoglobulin G (IgG) levels in mice with collagen-induced arthritis (CIA), consequently preventing CIA (Citation4). These results highlight the potential for dantrolene as an anti-rheumatic drug. Herein, we further explore whether dantrolene acts as an immunosuppressant or immunomodulator.
We evaluated serum total IgG and anti-CII IgG levels in CIA mice treated with dantrolene (Citation4). In brief, 100 µg of bovine CII and 200 µg of Mycobacterium tuberculosis were emulsified with complete Freund’s adjuvant, and the emulsion was injected subcutaneously into 7–8-week-old male wild-type DBA/1 mice; this was the first immunization. The emulsion consisting of Freund’s incomplete adjuvant and 100 µg of CII was injected on day 21 as the second immunization. Dantrolene (100 mg/kg/day) was administered 7 days before the first immunization and until 63 days after it. The mice were killed 63 days after the first immunization, and their sera were collected. Serum total IgG and anti-CII IgG levels were detected using an enzyme-linked immunosorbent assay (ELISA) kit (Mouse Total IgG Antibody Detection Kit, cat. no. 3023; Chondrex, Woodinville, WA, USA) and Mouse Anti-Bovine Type II Collagen IgG Antibody Assay Kit (cat. no. 2032; Chondrex, USA). The control group comprised mice that were injected with neither the adjuvant nor CII, and fed to the same age as the CIA mice.
We further assessed the impact of dantrolene on serum total IgG and anti-ovalbumin (OVA) IgG levels in mice immunized with OVA. In brief, 100 µg of OVA was emulsified with complete Freund’s adjuvant, and the emulsion was injected subcutaneously into 9–10-week-old female wild-type C57BL/6 mice as the first immunization. The emulsion consisting of Freund’s incomplete adjuvant and 100 µg of OVA was injected on day 21 as the second immunization. Dantrolene (100 mg/kg/day) was administered as mentioned above. Serum total IgG and anti-OVA IgG levels were detected 63 days after the first immunization using an ELISA kit (Mouse Total IgG Antibody Detection Kit, cat. no. 3023; Chondrex, USA) and Mouse Anti-OVA IgG Antibody Assay Kit (cat. no. 3011; Chondrex, USA). A Mann–Whitney U-test was performed to compare differences between two independent groups. A Kruskal–Wallis test with post-hoc Dunn’s test was conducted to compare the means of four groups.
Oral dantrolene administration significantly reduced serum anti-CII IgG levels in CIA mice (). Serum total IgG levels in CIA mice were significantly higher than those in control mice. However, dantrolene did not alter serum total IgG levels in either CIA or control mice; the serum total IgG levels of control and CIA mice were similar regardless of the presence or absence of dantrolene (). Dantrolene also decreased serum anti-OVA IgG levels in OVA-immunized mice without affecting their total IgG levels, suggesting that dantrolene’s immunomodulatory effects are broad ranging rather than narrowly defined ().
Figure 1. Serum levels of anti-bovine type II collagen (CII) immunoglobulin G (IgG) (A) and total IgG (B) on day 63 after the first immunization. All experiments were conducted in duplicate, and the values are shown as mean ± sd. **p < 0.01, ***p < 0.001; ns, not significant. n, number of animals in the group; DAN, dantrolene; CIA, collagen-induced arthritis.
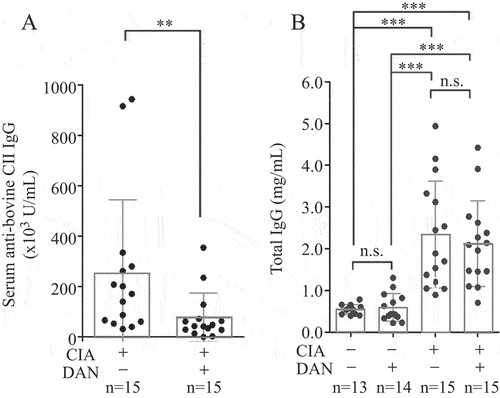
Figure 2. Serum levels of anti-ovalbumin (anti-OVA) immunoglobulin G (IgG) (A) and total IgG (B) on day 63 after the first immunization. Data were collected in a single experiment, and the values are shown as mean ± sd. *p < 0.05; ns, not significant. n, number of animals in the group; DAN, dantrolene.
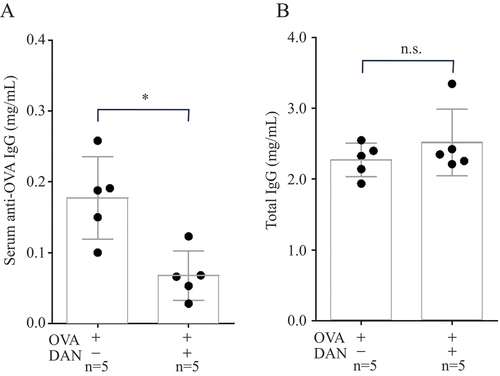
These results clearly demonstrate that dantrolene specifically reduces pathogenic IgG levels, indicating its potential as an immunomodulator with selective action on pathological immune cells.
Dantrolene has been widely used for treating malignant hyperthermia. Our previous study also confirmed the effectiveness of the intravenous dantrolene administration in suppressing and/or terminating sustained ventricular tachycardia or ventricular tachycardia storm in patients with heart failure (Citation1). Moreover, we are currently conducting a multicentre double-blind randomized controlled study (SHO-IN trial) to evaluate the efficacy and safety of long-term oral dantrolene administration in patients with chronic heart failure (Citation6). If the SHO-IN trial proves the safety of dantrolene, its long-term use may receive approval for an expanded indication of chronic diseases such as chronic heart failure. However, the molecular mechanisms underlying the pharmacological action of dantrolene on autoimmune diseases remain unclear, necessitating further studies to enable its clinical application as an immunomodulator for autoimmune diseases.
Acknowledgements
We thank Yoko Okamoto and Satomi Tateda for their technical assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
All data generated and/or analysed during this study are presented in this article.
Additional information
Funding
References
- Kobayashi S, Yamamoto T, Yoshiga Y, Okamura T, Kawano R, Yano M. Stabilizing tetrameric structure of ryanodine receptor cures lethal arrhythmia in heart failure. Circ Arrhythm Electrophysiol 2022;15:e011220.
- Nakamura Y, Yamamoto T, Xu X, Kobayashi S, Tanaka S, Tamitani M, et al. Enhancing calmodulin binding to ryanodine receptor is crucial to limit neuronal cell loss in Alzheimer disease. Sci Rep 2021;11:7289.
- Tamitani M, Yamamoto T, Yamamoto N, Fujisawa K, Tanaka S, Nakamura Y, et al. Dantrolene prevents hepatic steatosis by reducing cytoplasmic Ca2+ level and ER stress. Biochem Biophys Rep 2020;23:100787.
- Nawata T, Sakai H, Honda T, Otsuka M, Fujita H, Uchinoumi H, et al. Dantrolene, a stabilizer of the ryanodine receptor, prevents collagen-induced arthritis. Biochem Biophys Res Commun 2022;624:141–5.
- Osipchuk NC, Soulika AM, Fomina AF. Modulation of ryanodine receptors activity alters the course of experimental autoimmune encephalomyelitis in mice. Front Physiol 2021;12:770820.
- Kobayashi S, Wakeyama T, Ono S, Ikeda Y, Omura M, Oda T, et al. A multicenter, randomized, double-blind, controlled study to evaluate the efficacy and safety of dantrolene on ventricular arrhythmia as well as mortality and morbidity in patients with chronic heart failure (SHO-IN trial): rationale and design. J Cardiol 2020;75:454–61.
