Metastatic calcinosis is an uncommon condition characterized by diffuse soft tissue calcification, which may occur with advanced chronic kidney disease (CKD), typically in those undergoing kidney replacement therapy (Citation1, Citation2). Deposits usually appear around large joints such as the elbow, hip, and shoulder. Periarticular deposits may lead to arthralgia, reduced joint mobility, and neurovascular symptoms. Sustained elevation of serum phosphate levels is the main contributor in CKD patients.
A 25-year-old female patient presented with lower limb oedema, arterial hypertension, CKD [estimated glomerular filtration rate of 14 mL/min/1.73 m2 (CKD-EPI)], haematuria, nephrotic proteinuria (5.53 g/24 h), and hypoalbuminaemia. Renal biopsy was not feasible because of the small kidney size. Work-up for immune-mediated, viral aetiologies, and Fabry disease was negative. A next-generation sequencing-based gene panel for nephrotic syndrome identified a rare variant with predicted functional impact in the WT1 gene with substitution of cytosine for guanine in position 1054. The patient was diagnosed with end-stage kidney disease due to nephrotic syndrome secondary to WT1 disorder.
Because of her worsening kidney function and uraemic syndrome, the patient started peritoneal dialysis. Significant disequilibrium in the calcium phosphate metabolism persisted, with hyperparathyroidism (parathyroid hormone-I 610 pg/mL), 25-OH-vitamin D insufficiency (22 mg/L), hyperphosphataemia (6.7–7.5 mg/dL, cut-off < 4.7 mg/dL), and hypercalcaemia (11.4 mg/dL). After 3 months on dialysis, recurrent idiopathic pericarditis, and pleural and pericardial effusion required prednisolone and colchicine treatment.
Polyarthralgia ensued during prednisolone tapering (< 12.5 mg/day), involving the second to fifth proximal interphalangeal (PIP) joints of both hands associated with joint swelling, occasional involvement of elbows, hips, knees, and ankles, and progressive deformation of the elbow extensor surface and fifth right PIP joint. Hand paraesthaesia in the territory of the left cubital nerve was present. No mucocutaneous or gastrointestinal symptoms or other signs or symptoms of connective tissue disease were present.
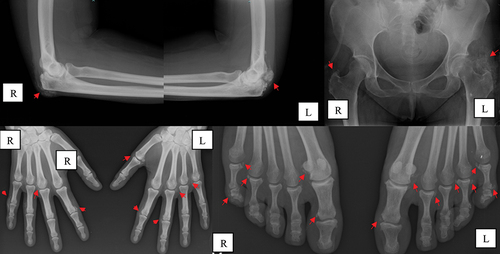
Radiographic evaluation () showed multiple periarticular calcium deposits on the metacarpophalangeal (MCP) and PIP joints of both hands, olecranon process, perifemoral trochanter, and metatarsophalangeal and interphalangeal joints of the toes. Subperiosteal resorption of the radial side of the proximal and intermediate phalanxes on both hands was also noted, compatible with secondary hyperparathyroidism. Hand and wrist ultrasound confirmed the presence of calcifications near the MCP and PIP joints as well as bilateral peri-insertional calcifications of the flexor carpi radialis and flexor carpi ulnaris. Upper limb electromyography revealed a mild neuropraxic lesion of the left ulnar nerve. A cervical–thoracic–abdominal–pelvic computed tomography scan was negative for neoplastic or lymphoproliferative disorders. Parathyroid scintigraphy was normal.
Figure 1. Periarticular calcifications (arrows) in the elbows, hands, and feet, before renal transplantation. L, left side; R, right side.
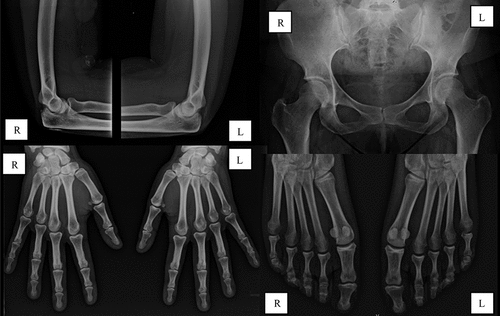
The diagnosis of metastatic calcinosis secondary to calcium phosphate metabolism imbalances in a patient with end-stage renal disease was established. Phosphate-binding therapy was optimized with sucroferric oxyhydroxide titrated up to 3000 mg/day, with improvement of serum parathormone (274 pg/mL) and serum phosphate (4.6 mg/dL). A slight resorption of calcifications was observed after 6 months. Shortly after this, the patient underwent renal transplantation, resulting in sustained normalization of these parameters. Symptomatic improvement was sustained even under minimal dose maintenance corticosteroids (prednisolone 5 mg/day). Almost complete reabsorption of calcifications was observed on radiographic reassessment 9 months post-transplantation (), and the patient remained free of joint pain after 12 months of follow-up.
Figure 2. Radiographic assessment 9 months after renal transplantation. L, left side; R, right side.
Calcinosis cutis in a young female patient may be due to several causes; namely, trauma, infection, conditions associated with phosphate or calcium balance, and autoimmune disorders (Citation3). The location of calcifications in this patient was slightly atypical, as it predominantly affected peripheral small joints (Citation4), thus implying the exclusion of differential diagnosis for several rheumatic diseases with peripheral calcific predilection (Citation5). Chronic tophaceous gout was considered unlikely as the patient had serum levels of uric acid < 5 mg/dL under allopurinol and trochanteric calcification would only be seen after a long period of uncontrolled disease, and even so rarely (Citation6). In this case, the CKD aetiology was not linked directly to calcinosis, but rather to the end-stage renal disease itself. Several pathogenic variants in the WT1 gene have been associated with congenital and steroid-resistant nephrotic syndromes (Citation7), and recurrence after renal transplantation has not been described (Citation8, Citation9). Disease onset in adults, especially without external genitalia abnormalities, is exceptional (Citation10).
Overall, in this patient, CKD-related hyperphosphataemia was considered the main mechanism for periarticular calcification, which was corroborated by an improvement after controlling phosphataemia and, ultimately, the reversal of calcinosis after kidney transplantation.
This case highlights metastatic calcinosis as a possible, albeit infrequent cause of arthralgia in patients with end-stage renal disease, especially in patients undergoing dialysis with hyperphosphataemia who are resistant to nutritional and pharmacological measures. Despite ill-defined targets for these parameters, this case reinforces the need to adequately control mineral and bone disorders associated with CKD and reflects the superiority of kidney transplantation in reversing disease complications.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Sankar L, Norfolk E, Kalra K. Tumoral calcinosis in an end stage kidney disease patient on peritoneal dialysis. Am J Med Sci 2023. Published online: 14 October 2023. doi:10.1016/j.amjms.2023.10.005
- Binnani P, Aggarwal V, Bahadur MM, Fulara N. Tumoral calcinosis (Teutschlander disease) in a dialysis patient. Indian J Nephrol 2008;18:122.
- Jiménez-Gallo D, Ossorio-García L, Linares-Barrios M. Calcinosis cutis and calciphylaxis. Actas Dermosifiliogr 2015;106:785–94.
- Bartolomeo K, Tan XY, Fatica R. Extraosseous calcification in kidney disease. Cleve Clin J Med 2022;89:81–90.
- Elahmar H, Feldman BM, Johnson SR, Johnson S. Management of calcinosis cutis in rheumatic diseases. J Rheumatol 2022;49:980–9.
- Dalbeth N, Gosling AL, Gaffo A, Abhishek A. Gout. Lancet 2021;397:1843–55.
- Dong L, Pietsch S, Englert C. Towards an understanding of kidney diseases associated with WT1 mutations. Kidney Int 2015;88:684.
- Roca N, Munõz M, Cruz A, Vilalta R, Lara E, Ariceta G. Long-term outcome in a case series of Denys-Drash syndrome. Clin Kidney J 2019;12:836–9.
- Chen H, Zhang M, Lin J, Lu J, Zhong F, Zhong F, et al. Genotype-phenotype correlation of WT1 mutation-related nephropathy in Chinese children. Front Pediatr 2023;11:1192021.
- Gribouval O, Boyer O, Hummel A, Dantal J, Martinez F, Sberro-Soussan R, et al. Identification of genetic causes for sporadic steroid-resistant nephrotic syndrome in adults. Kidney Int 2018;94:1013–22.
