Anti-synthetase syndrome (ASS) is characterized by auto-antibodies (aAbs) against cytoplasmic aminoacyl-t-RNA-synthetases and the triad of myositis, arthritis, and interstitial lung disease (ILD) (Citation1). Here, we report a case of ASS diagnosed during pregnancy.
A 36-year-old female was referred to our tertiary care high-risk pregnancy clinic for dyspnea, polyarthritis, and myalgia, with onset at 7 weeks’ gestation. She was pregnant for the sixth time. Three of her past pregnancies had resulted in live births, although complicated by pre-eclampsia (33–36 weeks) and intrauterine growth restriction (IUGR). She had also had two medically terminated pregnancies for personal reasons.
The patient was assessed at 15 weeks’ gestation and was hospitalized for further investigations. Clinical examination revealed polyarthritis, proximal muscle weakness [Medical Research Council (MRC)-5 scale 4/5], and lung crackles. Creatinine kinase (CK) levels reached 4244 IU/L. Serological work-up demonstrated negative anti-nuclear antibodies (ANAs) and anti-phospholipid aAbs. She had positive anti-Jo1 (3+) and anti-Ro52 (3+) aAbs on a myositis panel (Euroimmun). Magnetic resonance imaging of the thighs demonstrated T2/STIR hypersignals. Nailfold videocapillaroscopy (NVC) showed dystrophic capillaries. A previous computed tomography (CT) scan of the thorax performed at 7 weeks’ gestation, when the pregnancy was unknown, had revealed ILD with a non-specific interstitial pneumonia pattern. Pulmonary function tests (PFTs) showed a restrictive pattern [forced expiratory volume in 1 s (FEV1) 44%, forced vital capacity (FVC) 44%, diffusing capacity of the lungs for carbon monoxide (DLCO) 48% of predicted value]. After multidisciplinary assessment, muscle biopsy was not performed in this pregnant patient as there was a correlation between the clinical phenotype and serology results. ASS was diagnosed, with moderate ILD, polyarthritis, and myositis.
The patient wished to pursue her pregnancy and was treated with prednisone 20 mg/day, hydroxychloroquine 5 mg/kg/day, azathioprine 2 mg/kg, and monthly intravenous immunoglobulin 2 g/kg. The arthritis and myositis improved significantly. PFTs at 19 weeks remained stable (FEV1 46%, FVC 47%, DLCO 44% of predicted value). She received aspirin 160 mg/day to prevent pre-eclampsia, and insulin as she had developed gestational diabetes. Foetal ultrasound was normal, with no signs of neonatal lupus. At 21 weeks’ gestation, an emergency caesarean section (C-section) was performed for acute placental abruption complicated by haemorrhagic shock. Foetal autopsy revealed IUGR and placental abruption secondary to a 4 × 4 cm clot.
In the immediate post-partum period, the patient experienced a disease flare with relapsing polyarthritis, myalgia, and proximal weakness. CK reached 2998 IU/L. ILD remained stable, although severe. After multidisciplinary discussion, prednisone was increased to 1 mg/kg/day and azathioprine was switched to mycophenolate mofetil. At 8 months post-partum, the patient is now stable on progressive tapering of prednisone currently at 15 mg/day, mycophenolate mofetil 1500 mg bid, and hydroxychloroquine 5 mg/kg/day. Intravenous immunoglobulins have successfully been tapered. Polyarthritis is in remission and proximal muscle weakness has resolved, with the last CK at 527 IU/L. PFTs have improved (FEV1 61%, FVC 61%, DLCO 54% of predicted value) and thorax CT showed a clear improvement of ILD. The obstetrician provided counselling regarding future pregnancies and the patient is now on highly effective long-acting reversible contraception.
We searched for cases of anti-Jo1-positive ASS in pregnancy (PubMed, MEDLINE, Embase) and summarized the data in . Ten anti-Jo1-positive ASS cases have been reported, including seven diagnosed before pregnancy and three diagnosed during pregnancy. Multidisciplinary management of ASS in pregnancy is discussed in .
Figure 1. Key points to consider regarding the management of anti-synthetase syndrome (ASS) in pregnancy. (i) Corticosteroid dosing should be minimized to prevent the risk of infections and maternal–foetal complications, and pregnancy-compatible medication should be used. (ii) Anti-Ro/SSA auto-antibodies (aAbs) increase the risk of interstitial lung disease (ILD) in patients with ASS (Citation16, Citation17). (iii) Anti-Ro/SSA and anti-La/SSB increase the risk of neonatal lupus. Women who test positive for anti-Ro/SSA and/or anti-La/SSB should be screened for neonatal lupus and receive hydroxychloroquine during pregnancy (Citation18). (iv) Anti-phospholipid (APL) aAbs increase the risk of adverse pregnancy outcomes, and prophylactic treatment with low-dose aspirin and/or low molecular weight heparin is recommended (Citation18). (v) Screening for ILD and assessment of severity should be performed with pulmonary function tests (PFTs), as exposure to radiation should be minimized. (vi) Obstetric medicine follow-up is essential to prevent and treat diabetes, pre-eclampsia, and anaemia (Citation19). Obstetric expertise is needed for prevention and screening of prematurity, foetal growth restriction, and neonatal lupus secondary to anti-Ro/SSA aAbs. During childbirth, anaesthesiology should monitor for adrenal and respiratory insufficiency.
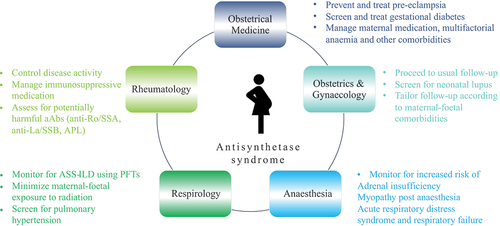
Table 1. Literature review of anti-Jo1-positive anti-synthetase syndrome (ASS) foetal and maternal outcomes in pregnancy.
All cases of anti-Jo1-positive ASS diagnosed during pregnancy were associated with severe maternal disease (ILD or refractory myositis) and foetal death. In cases diagnosed before pregnancy, no foetal deaths occurred. In 2003, Silva et al. suggested that foetal prognosis mirrors the level of maternal disease activity (Citation12).
We hypothesize that adverse foetal prognosis may be secondary to ASS-mediated placental dysfunction. In 2021, Kowitz et al described a pregnant 26-year-old female with ASS (anti-Jo1 and anti-Ro52/SSA aAbs), diagnosed at 21 years of age, who underwent emergency C-section at 24 weeks for IUGR and foetal decelerations (Citation4). Placental histology revealed placental hypoperfusion. As ASS is characterized by vasculopathy (Citation13, Citation14), we hypothesize that it may lead to a decrease in placental blood flow.
One study analysed NVC in 190 non-pregnant ASS patients, 70% of whom were positive for anti-Jo1 aAbs (Citation15). NVC abnormalities were associated with the presence of anti-Jo1 aAbs (p = 0.008) and ILD (p = 0.002). NVC abnormalities that were significantly associated with anti-SSA and anti-Jo1 aAbs were avascular areas, suggesting that ASS may induce vasculopathy. Moreover, concurrent anti-Ro aAbs increase the risk of ILD (Citation16, Citation17).
In conclusion, while favourable outcomes have been described with ASS diagnosed before pregnancy, disease onset during pregnancy was associated with poor outcome in our patient and three other published cases (foetal mortality rate of 100%). Management of ASS in pregnancy is challenging owing to contraindications to several immunosuppressive agents and the increased risk of maternal–foetal complications. Multidisciplinary management is essential to optimize maternal–foetal outcomes.
Author contributions
The authors thank Drs Sophie Grand’Maison, Nazila Bettache, and Vincent Williams, experts in obstetrical medicine at the CHUM, for their contribution in the management of the reported case.
Ethical statement
Approval by our local ethics committee (Comité d’Éthique, Centre de Recherche du CHUM, Université de Montréal) is not required for case reports on fewer than four patients. Nevertheless, written informed consent was obtained from the patient.
Disclosure statement
This case was presented as an abstract at the North American Society of Obstetrical Medicine (NASOM) conference on 14 October 2023 in Quebec City, Canada. No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Opinc AH, Makowska JS. Antisynthetase syndrome - much more than just a myopathy. Semin Arthritis Rheum 2021;51:72–83.
- Alshwairikh LA, Babay Z. A 25-year-old Saudi woman with a 2-year history of antisynthetase syndrome with interstitial lung disease who commenced azathioprine treatment in the third trimester of pregnancy and had a successful birth at term. Am J Case Rep 2022;23:e936833–1.
- Dumitrascu CI, Olsen DA, Arendt KW, Rose CH, Sharpe EE. Antisynthetase syndrome with severe interstitial lung disease in pregnancy. Case Rep Anesthesiol 2021;2021:1–4.
- Kowitz M, Chakradeo K, Hennessey A, Wolski P. Premature live birth in a woman with antisynthetase syndrome following recurrent miscarriages. BMJ Case Rep 2021;14:e240929.
- Mayu S, Isojima S, Miura Y, Nishimi S, Hatano M, Tokunaga T, et al. Polymyositis-dermatomyositis and interstitial lung disease in pregnant woman successfully treated with cyclosporine and tapered steroid therapy. Case Rep Rheumatol 2019;2019:1–4.
- Green LJ, O’Neill L, Frise CJ. Antisynthetase syndrome in pregnancy: a case and review of the literature. Obstet Med 2020;13:96–100.
- Dalmau-Carolà J. Childbirth in a woman with antisynthetase syndrome and severe lung disease on long-term rituximab therapy. Indian J Rheumatol 2016;11:117.
- Váncsa A, Ponyi A, Constantin T, Zeher M, Dankó K. Pregnancy outcome in idiopathic inflammatory myopathy. Rheumatol Int 2007;27:435–9.
- Okada R, Miyabe Y-S, Kasai S, Hashimoto K, Yamauchi S, Yoshikawa M, et al. Successful treatment of interstitial pneumonia and pneumomediastinum associated with polymyositis during pregnancy with a combination of cyclophosphamide and tacrolimus: a case report. Nihon Rinsho Meneki Gakkai Kaishi 2010;33:142–8.
- Tojyo K, Sekijima Y, Hattori T, Tsuyuzaki J, Nakamura A, Kita N, et al. A patient who developed dermatomyositis during the 1st trimester of gestation and improved after abortion. Rinsho Shinkeigaku 2001;41.
- Satoh M, Ajmani AK, Hirakata M, Suwa A, Winfield JB, Reeves WH. Onset of polymyositis with autoantibodies to threonyl-tRNA synthetase during pregnancy. J Rheumatol 1994;21.
- Silva CA. Pregnancy outcome in adult-onset idiopathic inflammatory myopathy. Rheumatology 2003;42:1168–72.
- Sylaja PN, Divya KP, Sukumaran S, Sreedharan S. Antisynthetase syndrome with stroke. Neurol India 2013;61:83.
- Tarabishy AB, Khan M, Bunyard M, Lowder CY. Retinal vasculitis associated with the anti-synthetase syndrome. Ocul Immunol Inflamm 2010;18:16–18.
- Sebastiani M, Triantafyllias K, Manfredi A, González-Gay MA, Palmou-Fontana N, Cassone G, et al. Nailfold capillaroscopy characteristics of antisynthetase syndrome and possible clinical associations: results of a multicenter international study. J Rheumatol 2019;46:279–84.
- La Corte R, Lo Mo Naco A, Locaputo A, Dolzani F, Trotta F. In patients with antisynthetase syndrome the occurrence of anti-Ro/SSA antibodies causes a more severe interstitial lung disease. Autoimmunity 2006;39:249–53.
- Mileti LM, Strek ME, Niewold TB, Curran JJ, Sweiss NJ. Clinical characteristics of patients with anti-Jo-1 antibodies: a single center experience. J Clin Rheumatol 2009;15:254–5.
- Sammaritano LR, Bermas BL, Chakravarty EE, Chambers C, Clowse MEB, Lockshin MD, et al. American College of Rheumatology guideline for the management of reproductive health in rheumatic and musculoskeletal diseases. Arthritis Care Res (Hoboken) 2020;72:461–88.
- Magee LA, Smith GN, Bloch C, Côté A-M, Jain V, Nerenberg K, et al. Guideline no. 426: hypertensive disorders of pregnancy: diagnosis, prediction, prevention, and management. J Obstet Gynaecol Can 2022;44:547–571.e1.
