Abstract
Objective
Immunoglobulin G (IgG) autoantibodies in systemic lupus erythematosus (SLE) are considered pathogenic, whereas immunoglobulin M (IgM) autoantibodies may have protective effects. The aim of this study was to identify whether IgG/IgM autoantibody ratios differ between patients with incomplete systemic lupus erythematosus (iSLE), patients with SLE, and healthy controls (HCs), and whether IgG/IgM autoantibody ratios relate to progression from iSLE to SLE.
Method
This prospective cohort study included 34 iSLE patients, 41 SLE patients, and 11 HCs. IgG and IgM anti-dsDNA, anti-Ro52, and anti-Ro60 were measured by fluoro-enzyme immunoassay in serum samples obtained at baseline in all groups and in follow-up samples of up to 5 years for iSLE patients. Correlations between IgG/IgM autoantibody ratios, interferon signature, and clinical parameters were also assessed.
Results
At baseline, IgG anti-dsDNA, anti-Ro52, anti-Ro60, and IgM anti-dsDNA were elevated in iSLE and SLE patients. IgG/IgM anti-dsDNA and anti-Ro52 ratios were similar between groups, while IgG/IgM anti-Ro60 ratios were significantly elevated in iSLE and SLE patients compared to HCs. IgG/IgM autoantibody ratios were not correlated with interferon signature or clinical parameters. IgG/IgM ratios at baseline were similar and remained relatively stable during a median follow-up of 18 months in non-progressors and six iSLE patients who progressed to SLE.
Conclusion
IgG anti-dsDNA, anti-Ro52, anti-Ro60, and IgM anti-dsDNA were elevated in iSLE and SLE patients, which was not apparent from the respective IgG/IgM ratios only. IgG/IgM autoantibody ratios remained relatively stable over up to 5 years in iSLE non-progressors and six patients who progressed to SLE.
Systemic lupus erythematosus (SLE) is a heterogeneous autoimmune disease mainly affecting women of reproductive age, and can manifest with different symptoms including skin rash, arthritis, and life-threatening organ involvement. Despite substantial discoveries regarding the pathogenesis of SLE, little is known about the early stages of SLE (Citation1). Patients with incomplete systemic lupus erythematosus (iSLE) have SLE-like symptoms but not enough to establish the diagnosis (Citation2). Some of these patients will progress to SLE within several years, while others will continue to have mild symptoms or develop other autoimmune diseases (Citation1, Citation2).
Early diagnosis of SLE is highly important to prevent organ damage (Citation3). Therefore, new biomarkers are needed to identify iSLE patients with a high risk of progression to SLE. In previous studies, risk factors for progression to SLE have been identified, such as a high interferon signature, high autoantibody diversity, and complement consumption (Citation1, Citation4–6). However, there is no biomarker available that can predict progression.
It has been shown that the presence of autoantibodies can precede onset of SLE by years (Citation7, Citation8). In particular, anti-Sjögren’s syndrome antigen A (anti-Ro/SSA), anti-phospholipid, and anti-double-stranded DNA (anti-dsDNA) antibodies can be detected in blood several years before the diagnosis of SLE (Citation7, Citation8). Whereas immunoglobulin G (IgG) autoantibodies are associated with disease flares, there is evidence that autoantibodies of the immunoglobulin M (IgM) isotype may have beneficial and even protective effects in SLE (Citation9, Citation10). It has been suggested that IgM autoantibodies are involved in clearing cell debris and thereby limit exposure to autoantigens in SLE (Citation11, Citation12). In contrast, IgG autoantibodies are considered pathogenic, leading to the formation of immune complexes and tissue damage (Citation13). IgG autoantibodies are produced after prolonged exposure to autoantigens, and may therefore indicate a more advanced disease state than IgM autoantibodies (Citation11). This hypothesis is further supported by a previous study showing that patients with iSLE have more IgM and fewer IgG autoantibodies compared to SLE patients (Citation14). In addition, anti-dsDNA IgG/IgM autoantibody ratios in SLE patients correlate with disease activity and glomerulonephritis in several studies (Citation15–17).
Therefore, the aim of this study was to evaluate (i) whether IgG/IgM autoantibody ratios are elevated in patients with iSLE compared to healthy controls (HCs); (ii) whether IgG/IgM ratios correlate with interferon score and clinical parameters, such as complement levels; and (iii) whether IgG/IgM ratios relate to progression from iSLE to SLE. The current analysis was limited to anti-dsDNA, anti-Ro60, and anti-Ro52 antibodies, as these antibodies have been shown to be the earliest autoantibodies to appear before SLE diagnosis and are routinely measured in clinical practice (Citation7, Citation8).
Method
Study design and population
Patients were recruited into a prospective, longitudinal cohort study at the University Medical Centre Groningen in the Netherlands from 2016 until 2021. The cohort study consisted of a baseline cross-sectional part with iSLE patients, SLE patients, and HCs, and a longitudinal cohort of iSLE patients. Patients were classified as having iSLE if they had a disease duration of less than 5 years, an anti-nuclear antibody (ANA) titre equal to or higher than 1:80, and at least one clinical criterion but fewer than four of the Systemic Lupus International Collaborating Clinics (SLICC) criteria (Citation18). Patients with iSLE who were being treated with immunosuppressive medication were excluded, while treatment with hydroxychloroquine was not an exclusion criterion.
In addition, SLE patients and HCs were included as control groups. SLE patients were eligible if they met the SLICC classification criteria and had a disease duration of less than 10 years. HCs were eligible in case of a negative history of autoimmune diseases and no symptoms of upper respiratory tract infection at the time of inclusion. The study was approved by the local ethics committee (METc 2015/313) and all participants gave written consent.
Study procedures
At baseline, clinical assessments were performed by a qualified physician, and blood samples were collected from HCs, iSLE patients, and SLE patients. In addition, iSLE patients received follow-up visits every 6 months comprising clinical assessment and blood sampling. Follow-up was ceased if iSLE patients progressed to classified SLE according to SLICC criteria. All visits took place at the University Medical Centre in Groningen, which is a tertiary referral centre.
Analysis of autoantibodies
Autoantibodies were analysed in baseline serum samples from HCs and SLE patients, and in baseline and follow-up serum samples from iSLE patients. For iSLE patients who did not progress to SLE, annual samples were analysed, and for iSLE patients who were later identified as progressors, samples were analysed twice a year until disease progression was determined.
Serum was collected after centrifugation of blood that was drawn into serum separating tubes (BD, Plymouth, United Kingdom) and stored at −80°C. Total IgG or IgM levels were measured spectrophotometrically, directly after sampling in lithium heparin plasma on a Cobas8000-C502 instrument (Roche, Mannheim, Germany), and were expressed in g/L. For patients with total IgG or IgM values missing at baseline (n = 5), the nearest available value within a 1 year window was used. IgG and IgM anti-dsDNA, anti-Ro52, and anti-Ro60 levels were measured in serum by automated fluorescence enzyme immunoassay (FEIA) (EliA) on a Phadia 250 instrument (Thermo Fisher Scientific, Nieuwegein, The Netherlands). These samples were stored at −80°C and were measured after collection of all samples within 1 week. IgG anti-dsDNA was expressed in IU/mL and IgG anti-Ro52 and anti-Ro60 IgG in AU/mL, as defined by the manufacturer. For IgG anti-dsDNA, values above 15 IU/mL, and for IgG anti-Ro52 and anti-Ro60, values above 10 AU/mL were considered positive, according to the manufacturer’s instruction.
For IgM anti-dsDNA, anti-Ro52, and anti-Ro60, arbitrary response units as reported by the Phadia 250 system (FEIA response units) were used. For IgM anti-dsDNA, anti-Ro52, and anti-Ro60, levels higher than the mean + two standard deviations of the HC group were considered elevated. To calculate IgG/IgM ratios, we used FEIA response units for both IgG and IgM anti-dsDNA, anti-Ro52, and anti-Ro60 to increase comparability.
Interferon
Expression of 12 interferon inducible genes was measured with quantitative polymerase chain reaction in whole blood collected into PAXgene RNA tubes (Qiagen, Hombrechtikon, Switzerland), and relative expression (RE) was calculated according to the following formula: RE = 2−(CtTestgene − CtGAPDH). We calculated two interferon scores. One score was based on three commonly assessed interferon-related genes (IFN 3 score; IFI44L, LY6E, and MX1) and the other was based on 12 interferon-related genes (IFN 12 score; CXCL10, IFI44L, IFIT3, LY6E, MX1, SERPING1, IFITM1, IRF7, STAT1, C1QA, IFI16, and IRF9). The scores were calculated as follows, using log-transformed relative expression levels: ∑(REsubject − MeanHC)/SDHC (Citation19).
Statistical analysis
Descriptive statistics were used to compare baseline characteristics. Continuous data are presented as median (interquartile range) and categorical data are presented as number (percentage). To assess differences in autoantibody ratios at baseline between the three groups, Kruskal–Wallis tests were used. In case of significance, Mann–Whitney U tests were performed for pairwise comparisons. Differences between categorical variables were assessed with Fisher’s exact tests. Spearman rank correlation was used to assess correlations between IgG/IgM autoantibody ratios at baseline and clinical and immunological parameters. A two-sided p-value of <0.05 was considered significant. Boxplots show median and interquartile range. R statistical software version 4.2.0 was used for statistical analysis and graphics. Data were collected and managed using REDCap electronic data capture tools hosted at the University Medical Centre Groningen (Citation20).
Results
Baseline characteristics
In total, 34 iSLE patients, 41 SLE patients, and 11 HCs were included for baseline comparison. At baseline, the median age of iSLE and SLE patients was 39 and 43 years, respectively, and most iSLE and SLE patients were female and Caucasian (). Both iSLE and SLE patients had low disease activity, with a median Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI 2000) (Citation21) of 1.5 for iSLE and 2 for SLE patients. Compared to HCs, smoking was more common among iSLE and SLE patients (0% vs 41% vs 17%, p = 0.007). Hydroxychloroquine was used more frequently by SLE patients than by iSLE patients (85% vs 24%, p < 0.001).
Table 1. Baseline characteristics.
IgG and IgM autoantibody positivity at baseline
There was no significant difference in the proportion of iSLE patients positive for IgG anti-dsDNA or IgG anti-Ro52 compared to SLE patients (21% vs 37%, p = 0.2; 21% vs 10%, p = 0.21) (). In both groups, around one-third of patients were positive for IgG anti-Ro60 (32% iSLE vs 34% SLE, p > 0.99) (). IgG anti-dsDNA/total IgG, anti-Ro52/total IgG, and anti-Ro60/total IgG ratios are shown in supplementary . Fewer iSLE than SLE patients had elevated IgM anti-dsDNA levels (47% vs 76%, p = 0.02), while around one-third of both iSLE and SLE patients had elevated IgM anti-Ro52 and anti-Ro60 levels ().
Figure 1. (A) Total immunoglobulin G (IgG)/immunoglobulin M (IgM) ratios, (B) IgG/IgM anti-double-stranded DNA (anti-dsDNA), (C) anti-Ro52, and (D) anti-Ro60 ratios for healthy controls (HCs), incomplete systemic lupus erythematosus (iSLE), and systemic lupus erythematosus (SLE) patients. Boxplots show median and interquartile range. **p ≤ 0.01; ***p ≤ 0.00; ns, not significant.
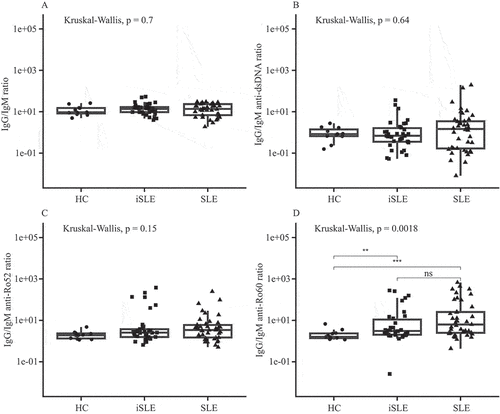
Baseline comparison of IgG/IgM ratios
At baseline, there were no significant differences in total IgG/IgM ratios, IgG/IgM anti-dsDNA, and anti-Ro52 ratios between HCs, patients with iSLE, and patients with SLE (). However, IgG/IgM anti-Ro60 ratios were significantly higher in iSLE and SLE patients than in HCs (HCs vs iSLE, p < 0.01; HCs vs SLE, p < 0.001) ().
Baseline comparison of IgM autoantibody levels
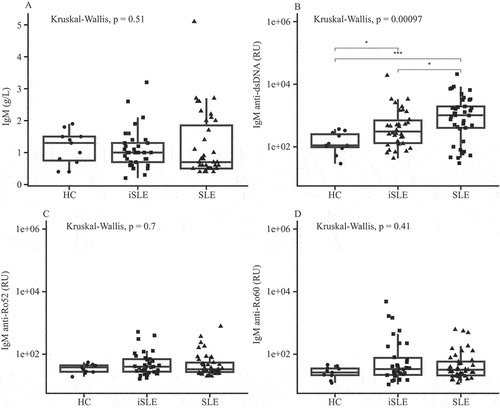
Next, we compared baseline IgM autoantibody levels between groups. Total IgM and IgM anti-Ro52 and anti-Ro60 levels were similar among HCs, iSLE, and SLE patients (), as were IgM anti-Ro52/total IgM and anti-Ro60/total IgM ratios (supplementary Figure 1). In contrast, IgM anti-dsDNA levels () and the IgM anti-dsDNA/total IgM ratio (supplementary Figure 1(E)) were significantly elevated in iSLE and SLE patients compared to HCs (p = 0.02 and p < 0.001; p = 0.01 and p < 0.001).
Figure 2. (A) Total immunoglobulin M (IgM), (B) IgM anti-double-stranded DNA (anti-dsDNA), (C) IgM anti-Ro52, and (D) IgM anti-Ro60 levels. Boxplots show median and interquartile range. HC, healthy control; iSLE, incomplete systemic lupus erythematosus; SLE, systemic lupus erythematosus; RU, FEIA response units. *p ≤ 0.05; ***p ≤ 0.001; ns, not significant.
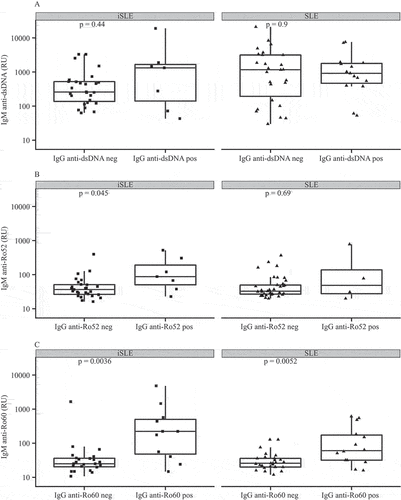
Subsequently, we divided iSLE and SLE patients into IgG anti-dsDNA, anti-Ro52, and anti-Ro60-positive or -negative patients. Patients with autoantibody positivity for IgG anti-dsDNA, anti-Ro52, and anti-Ro60 also showed higher IgG/IgM ratios for the respective autoantibodies (data not shown). Then, we compared IgM autoantibody levels between IgG anti-dsDNA, anti-Ro52, and anti-Ro60-positive and -negative iSLE and SLE patients (). IgM anti-dsDNA levels were similar between IgG anti-dsDNA-positive and -negative iSLE and SLE patients (p = 0.44 and p = 0.9, respectively). IgG anti-Ro52-positive iSLE patients had increased IgM anti-Ro52 levels (p = 0.045), while IgM levels were similar between IgG anti-Ro52-positive and -negative SLE patients (p = 0.69). IgM anti-Ro60 levels were elevated in IgG anti-Ro60-positive iSLE and SLE patients compared to IgG anti-Ro60-negative patients (p = 0.004 and p = 0.005, respectively).
Figure 3. Immunoglobulin M (IgM) autoantibody levels for (A) immunoglobulin G (IgG) anti-double-stranded DNA (anti-dsDNA), (B) anti-Ro52, and (C) anti-Ro60 positive (pos) and negative (neg) incomplete systemic lupus erythematosus (iSLE) and systemic lupus erythematosus (SLE) patients. Boxplots show median and interquartile ranges. RU, FEIA response units.
Correlation analysis
To assess whether baseline IgG/IgM ratios were associated with clinical and immunological parameters such as complement levels and interferon score, correlation analyses were performed for iSLE and SLE patients separately (supplementary Figure 2(A,B)). In SLE patients only, total IgG/IgM ratios were weakly correlated with interferon scores (r = 0.37 p = 0.02 for IgG/IgM ratio and IFN 3 score). For both iSLE patients and SLE patients, there was no significant correlation between IgG/IgM anti-dsDNA, anti-Ro52, and anti-Ro60 ratios, complement levels, SLEDAI 2000 scores, different classification criteria, and interferon scores.
Subsequently, we looked at correlations between IgM anti-dsDNA, anti-Ro52, and anti-Ro60 levels and the above-named variables. IgM anti-Ro52 levels were correlated with interferon scores in iSLE but not in SLE patients (r = 0.44, p = 0.01 for IgM anti-Ro52 and IFN 3 score). In SLE patients, IgM anti-Ro60 levels were correlated with interferon scores (r = 0.31, p = 0.049). In addition, IgM anti-dsDNA levels were negatively correlated with complement component 3 (C3) levels (r = −0.32, p = 0.04) in SLE patients.
Longitudinal analysis of IgG/IgM ratios
IgG/IgM autoantibody ratios were measured in 26 iSLE patients annually, with a median follow-up time of 18 months. During follow-up, six iSLE patients progressed to SLE by acquiring more SLICC criteria. These progressors were younger than non-progressors (23 vs 43 years, p ≤ 0.001, supplementary Table 1) and were all female. One of the progressors was positive for IgG anti-Ro52 at baseline, while none of the progressors was positive for IgG anti-dsDNA or anti-Ro60 at baseline.
One patient developed sicca symptoms during follow-up and fulfilled the American College of Rheumatology (ACR)/European Alliance of Associations for Rheumatology (EULAR) criteria for primary Sjögren’s syndrome (Citation22). This patient was excluded from further analyses.
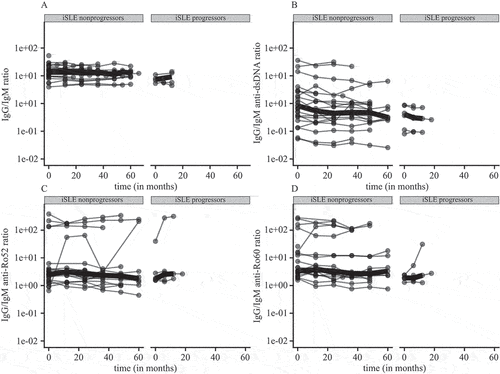
Next, we looked at the course of IgG/IgM ratios over time in iSLE patients. Patients with iSLE were divided into progressors and non-progressors to examine whether an increase in IgG/IgM autoantibody ratios was related to progression. Overall, IgG/IgM ratios remained relatively stable over time in both groups (). In the non-progressor group, several patients showed slight fluctuations and one patient showed a clear increase in the IgG/IgM anti-Ro60 ratio. Among the progressors, one patient had an increase in IgG/IgM anti-Ro52 and anti-Ro60 ratios prior to progression to SLE. This increase was due to an increase in IgG anti-Ro52 and anti-Ro60, while the corresponding IgM antibody level stayed relatively stable (supplementary Figure 3). Similarly to IgG/IgM ratios, IgG and IgM anti-dsDNA, anti-Ro52, and anti-Ro60 levels stayed relatively stable over time in iSLE non-progressors and progressors (supplementary Figure 3). In one of the progressors, a decrease in IgM anti-dsDNA, anti-Ro52, and anti-Ro60 levels could be observed prior to progression.
Figure 4. (A–D) Immunoglobulin G (IgG)/immunoglobulin M (IgM) ratios for incomplete systemic lupus erythematosus (iSLE) non-progressors and systemic lupus erythematosus (SLE) progressors over time. IgG/IgM ratios per patient are depicted as dots and connected by lines. Thick lines show median ratios per group over time.
Discussion
It has been suggested that class switching of autoreactive B-cells from IgM to IgG autoantibodies may drive persistent autoimmunity and could therefore be an indicator of progression from iSLE to SLE (Citation11).
In this study, IgG/IgM anti-dsDNA, anti-Ro52, and anti-Ro60 autoantibody ratios were examined and measured over time in iSLE patients who did and those who did not progress to SLE. At baseline, IgG/IgM anti-Ro60 ratios were elevated in iSLE and SLE patients compared to HCs, while other IgG/IgM ratios did not differ between groups. IgM anti-Ro52 and anti-Ro60 levels themselves were similar between groups, while IgM anti-dsDNA levels were higher in iSLE and highest in SLE patients compared to HCs. In general, IgG/IgM ratios remained stable over time and did not relate to progression from iSLE to SLE.
Baseline autoantibody levels
In this study, the proportion of iSLE and SLE patients positive for IgG anti-dsDNA, anti-Ro52, and anti-Ro60 did not differ significantly, which highlights immunological similarities between these groups despite differences in clinical manifestations. Both IgG and IgM anti-dsDNA antibody levels were elevated in SLE patients, which is concordant with previous reports (Citation15, Citation16, Citation23), and resulted in a similar IgG/IgM anti-dsDNA ratio to that observed in HCs. Remarkably, the same pattern was observed for iSLE patients.
In line with our results, Li et al found similar IgG/IgM anti-dsDNA ratios in iSLE patients compared to healthy controls (Citation14). Furthermore, Li et al reported higher IgG/IgM ratios in SLE than in iSLE patients, whereas we did not find differences in our study. This may be explained by lower disease activity in SLE patients included in our cohort and consequently lower IgG anti-dsDNA levels. Furthermore, Li et al measured autoantibodies using a different method, namely a proteome microarray (Citation14).
IgM anti-dsDNA levels were elevated in both iSLE and SLE patients and were higher in SLE patients than in iSLE patients. There was no difference in IgM anti-dsDNA levels between IgG anti-dsDNA-positive and -negative iSLE and SLE patients. This finding indicates that IgG anti-dsDNA-negative iSLE and SLE patients also show autoreactivity towards dsDNA. Moreover, it does not support the hypothesis that IgM autoantibody levels protect from class switching and from progression to SLE, at least not for this specific autoantibody.
IgG/IgM anti-Ro60 ratios were elevated in iSLE and SLE patients in our study, while there was no difference in IgM anti-Ro60 levels between groups. In addition, iSLE patients who were positive for IgG anti-Ro52 or anti-Ro60 also had higher IgM levels of these antibodies. Hence, it can be concluded that differences in anti-Ro60 ratios were mainly driven by IgG anti-Ro60 levels.
Association of IgG/IgM autoantibody ratios and IgM levels with clinical and immunological parameters
In SLE patients, IgM-anti-dsDNA was negatively associated with C3 levels, which are regarded as a marker of disease activity (Citation21). The fact that we did not find this correlation in iSLE patients may be explained by the lower proportion of iSLE patients with low C3 levels. We did not find a correlation between IgG/IgM anti-dsDNA, anti-Ro52, and anti-Ro60 autoantibody ratios and interferon scores. However, our results showed correlations between IgM anti-Ro60 levels and interferon scores in SLE patients and between IgM anti-Ro52 levels and interferon scores in iSLE patients. Along with C3, interferon is regarded as a measure of disease activity in SLE (Citation24). In contrast, Li et al found IgM autoantibodies (taking into account a large number of autoantibodies) to be negatively correlated with interferon scores in patients with iSLE. The authors concluded that higher IgM autoantibody levels are therefore associated with a less severe iSLE phenotype (Citation25). It should be noted that it is difficult to compare results between studies because of the different antibodies studied.
Longitudinal changes in autoantibody levels
In a small cohort study, Olsen et al assessed the levels of around 80 IgG and IgM autoantibodies with a proteome microarray in 22 iSLE patients, of whom three progressed to SLE by accumulation of ACR criteria (Citation5). In line with our longitudinal results, progressors were all female and younger at baseline than non-progressors. All progressors had an increase in IgG autoreactivity prior to progression, including three antibodies directed against SSA/SSB (Citation5). In our study, one iSLE progressor also showed an increase in IgG/IgM anti-Ro52 and anti-Ro60 ratios prior to progression, and this increase was also due to an increase in IgG anti-Ro52 and anti-Ro60 antibodies, whereas the respective IgM levels remained stable. Notably, compared to other reports, progressors in this study were positive for relatively few of the measured autoantibodies (Citation1, Citation7, Citation8).
There is strong evidence that IgG autoreactivity increases before a diagnosis of SLE and that the cumulative number of different IgG autoantibodies is related to progression from iSLE to SLE (Citation4, Citation7, Citation8). Therefore, the IgG autoantibody status is regularly assessed in patients with iSLE and SLE in clinical practice. However, the role of IgM autoantibodies in iSLE and SLE remains unclear. There is evidence from mouse studies indicating that IgM autoantibodies have protective effects (Citation9, Citation10), while studies in humans show more contradictory results (Citation11, Citation26, Citation27). The current hypothesis that IgM autoantibodies in iSLE may be protective is based on early studies in mice and relatively small studies in humans with iSLE (Citation5, Citation11, Citation14, Citation25, Citation28). Furthermore, different methods used to measure IgM autoantibodies, differences in the antibodies studied, and discrepancies in the definition of iSLE make it difficult to compare results.
In addition to autoantibodies of the IgM isotype, natural IgM should be considered. Natural IgM refers to autoreactive IgM that is produced independently of antigen encounter, and has been linked to the clearance of apoptotic cells. Accordingly, lower levels of natural IgM seem to be associated with autoimmunity in mice and in humans (Citation27, Citation29–31). There is evidence implying that, in contrast to natural IgM, IgM autoantibodies that are produced upon antigen encounter may be early indicators of autoimmunity and have pathogenic effects. Examples are bullous pemphigoid caused by IgM antibodies and anti-modified protein IgM antibodies exerting pro-inflammatory effects in rheumatoid arthritis (Citation32, Citation33).
The relatively high anti-Ro52, anti-Ro60, and anti-dsDNA IgM levels in some IgG-negative patients in this study may also be an early indicator of autoimmunity. We did not, however, observe higher IgM autoantibody levels in progressors compared to non-progressors.
Strengths and limitations
This prospective longitudinal study showed changes in autoantibody levels over multiple years in iSLE patients. The study has several limitations, including the relatively low number of iSLE patients. It should also be noted that most SLE patients used immunosuppressive medication, which may influence the results.
Conclusion
More mechanistic and clinical studies are needed to identify the effects of autoreactive IgM in early forms of autoimmune diseases, and to discover whether measuring IgM autoantibodies next to IgG autoantibodies is of added value in clinical practice. Ideally, international consensus on how to measure IgM autoantibodies and how to define iSLE for scientific research in the future will increase the applicability of studies.
Authors’ contributions
Svenja Henning: methodology, formal analysis, investigation, data curation, writing – original draft, visualization, and project administration; Johanna Westra: conceptualization, methodology, writing – review and editing, supervision, and project administration; Caroline Roozendaal: conceptualization, methodology, and writing – review and editing; Greet Haarsma-de Boer: formal analysis, investigation, data curation, and writing – review and editing; Juan J Fierro: writing – review and editing, and visualization; Hendrika Bootsma and Barbara Horvath: writing – review and editing; Karina de Leeuw: conceptualization, methodology, investigation, writing – review and editing, supervision, project administration, and funding acquisition.
Supplemental Material
Download MS Word (1 MB)Acknowledgement
We would like to thank Dr Wietske Lambers for her valuable contribution regarding collection of clinical data.
Disclosure statement
Svenja Henning: speaker for Thermo Fisher Scientific, for which fees were paid to the institution. No potential conflict of interest was reported by the remaining authors.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/03009742.2024.2321700.
Additional information
Funding
References
- Sciascia S, Roccatello D, Radin M, Parodis I, Yazdany J, Pons-Estel G, et al. Differentiating between UCTD and early-stage SLE: from definitions to clinical approach. Nat Rev Rheumatol 2021;18:9–21.
- Lambers WM, Westra J, Jonkman MF, Bootsma H, de Leeuw K. Incomplete systemic lupus erythematosus: what remains after application of American College of Rheumatology and Systemic Lupus International Collaborating Clinics criteria? Arthritis Care Res 2020;72:607–14. doi:10.1002/acr.23894
- Samnaliev M, Barut V, Weir S, Langham J, Langham S, Wang X, et al. Health-care utilization and costs in adults with systemic lupus erythematosus in the United Kingdom: a real-world observational retrospective cohort analysis. Rheumatol Adv Pract 20216;5:rkab071. doi:10.1093/rap/rkab071
- Lambers WM, Westra J, Bootsma H, de Leeuw K. From incomplete to complete systemic lupus erythematosus; A review of the predictive serological immune markers. Semin Arthritis Rheum 2021:51:43–8. doi:10.1016/j.semarthrit.2020.11.006
- Olsen NJ, Li Q-Z, Quan J, Wang L, Mutwally A, Karp DR. Autoantibody profiling to follow evolution of lupus syndromes. Arthritis Res Ther 2012;14:R174. doi:10.1186/ar3927
- Vilá LM, Mayor AM, Valentín AH, García-Soberal M, Vilá S. Clinical outcome and predictors of disease evolution in patients with incomplete lupus erythematosus. Lupus 2000;9:110–15. doi:10.1191/096120300678828073
- Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med 2003;349:1526–33. doi:10.1056/NEJMoa021933
- Eriksson C, Kokkonen H, Johansson M, Hallmans G, Wadell G, Rantapää-Dahlqvist S. Autoantibodies predate the onset of systemic lupus erythematosus in northern Sweden. Arthritis Res Ther 2011;13:R30. doi:10.1186/ar3258
- Boes M, Schmidt T, Linkemann K, Beaudette BC, Marshak-Rothstein A, Chen J. Accelerated development of IgG autoantibodies and autoimmune disease in the absence of secreted IgM. Proc Natl Acad Sci U S A 2000;97:1184–9. doi:10.1073/pnas.97.3.1184
- Ehrenstein MR, Cook HT, Neuberger MS. Deficiency in serum immunoglobulin (Ig) M predisposes to development of IgG autoantibodies. J Exp Med 2000;191:1253–8.
- Olsen NJ, Karp DR. Autoantibodies and SLE: the threshold for disease. Nat Rev Rheumatol 2014;10:181–6. doi:10.1038/nrrheum.2013.184
- Witte T. IgM antibodies against dsDNA in SLE. Clin Rev Allergy Immunol 2008;34:345–7. doi:10.1007/s12016-007-8046-x
- Pisetsky DS. Anti-DNA antibodies - Quintessential biomarkers of SLE. Nat Rev Rheumatol 2016;12:102–10. doi:10.1038/nrrheum.2015.151
- Li Q-Z, Zhou J, Wandstrat AE, Carr-Johnson F, Branch V, Karp DR, et al. Protein array autoantibody profiles for insights into systemic lupus erythematosus and incomplete lupus syndromes. Clin Exp Immunol 2007;147:60–70. doi:10.1111/j.1365-2249.2006.03251.x
- Villalta D, Bizzaro N, Bassi N, Zen M, Gatto M, Ghirardello A, et al. Anti-dsDNA antibody isotypes in systemic lupus erythematosus: IgA in addition to IgG anti-dsDNA help to identify glomerulonephritis and active disease. PLoS One 2013;8:e71458.
- Förger F, Matthias T, Oppermann M, Becker H, Helmke K. Clinical significance of anti-dsDNA antibody isotypes: IgG/IgM ratio of anti-dsDNA antibodies as a prognostic marker for lupus nephritis. Lupus 2004;13:36–44.
- Jia Y, Zhao L, Wang C, Shang J, Miao Y, Dong Y, et al. Anti-double-stranded DNA isotypes and anti-C1q antibody improve the diagnostic specificity of systemic lupus erythematosus. Dis Markers 2018;2018:4528547. doi:10.1155/2018/4528547
- Petri M, Orbai AM, Alarcõn GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86. doi:10.1002/art.34473
- Kirou KA, Lee C, George S, Louca K, Papagiannis IG, Peterson MGE, et al. Coordinate overexpression of interferon-alpha-induced genes in systemic lupus erythematosus. Arthritis Rheum 2004;50:3958–67. doi:10.1002/art.20798
- Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208. doi:10.1016/j.jbi.2019.103208
- Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2002;29:288–91.
- Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis 2017;76:9–16. doi:10.1136/annrheumdis-2016-210571
- Witte T, Hartung K, Sachse C, Matthias T, Fricke M, Deicher H, et al. IgM anti-dsDNA antibodies in systemic lupus erythematosus: negative association with nephritis. Rheumatol Int 1998;18:85–91. doi:10.1007/s002960050063
- Psarras A, Wittmann M, Vital EM. Emerging concepts of type I interferons in SLE pathogenesis and therapy. Nat Rev Rheumatol 2022;18:575–90. doi:10.1038/s41584-022-00826-z
- Q-Z L, Zhou J, Lian Y, Zhang B, Branch VK, Carr-Johnson F, et al. Interferon signature gene expression is correlated with autoantibody profiles in patients with incomplete lupus syndromes. Clin Exp Immunol 2010;159:281–91.
- Bootsma H, Spronk PE, Ter Borg EJ, Hummel EJ, De Boer G, Limburg PC, et al. The predictive value of fluctuations in IgM and IgG class anti-dsDNA antibodies for relapses in systemic lupus erythematosus. A prospective long term observation. Ann Rheum Dis 1997;56:661–6. doi:10.1136/ard.56.11.661
- Amendt T, Yu P. TLR7 and IgM: dangerous partners in autoimmunity. Antibodies (Basel) 2023;12:4. doi:10.3390/antib12010004
- Chong BF, Tseng L-C, Lee T, Vasquez R, Li QZ, Zhang S, et al. IgG and IgM autoantibody differences in discoid and systemic lupus patients. J Invest Dermatol 2012;132:2770–9.
- Ehrenstein MR, Notley CA. The importance of natural IgM: scavenger, protector and regulator. Nat Rev Immunol 2010;10:778–86. doi:10.1038/nri2849
- Grönwall C, Hardt U, Gustafsson JT, Elvin K, Jensen-Urstad K, Kvarnström M, et al. Depressed serum IgM levels in SLE are restricted to defined subgroups. Clin Immunol 2017;183:304–15. doi:10.1016/j.clim.2017.09.013
- Silverman GJ, Vas J, Grönwall C. Protective autoantibodies in the rheumatic diseases: lessons for therapy. Nat Rev Rheumatol 2013;9:291–300. doi:10.1038/nrrheum.2013.30
- Reijm S, Kissel T, Stoeken-Rijsbergen G, Slot LM, Wortel CM, van Dooren HJ, et al. Cross-reactivity of IgM anti-modified protein antibodies in rheumatoid arthritis despite limited mutational load. Arthritis Res Ther 2021;23:230. doi:10.1186/s13075-021-02609-5
- Hirano Y, Iwata H, Tsujuwaki M, Mai S, Mai Y, Imafuku K, et al. Super-resolution imaging detects BP180 autoantigen in immunoglobulin M pemphigoid. J Dermatol 2022;49:374–8. doi:10.1111/1346-8138.16260

