Abstract
Objective
To explore the registration of enthesitis among biologic-naïve patients with psoriatic arthritis (PsA) initiating tumour necrosis factor inhibitor (TNFi) treatment across 12 European registries, compare the disease burden and patient-reported outcomes (PROs) between patients with and without enthesitis, and assess the enthesitis treatment response.
Method
Demographics, clinical characteristics, and PROs at first TNFi (TNFi-1) initiation (baseline) were assessed in patients with PsA, diagnosed by a rheumatologist, with versus without assessment of entheses and between those with versus without enthesitis. Enthesitis scores and resolution frequency were identified at follow-up.
Results
Of 10 547 patients in the European Spondyloarthritis (EuroSpA) Research Collaboration Network initiating TNFi, 1357 underwent evaluation for enthesitis. Eight registries included a validated scoring system for enthesitis. At baseline, 874 patients underwent entheses assessment [Maastricht Ankylosing Spondylitis Enthesitis Score (MASES) 485 patients, Spondyloarthritis Research Consortium of Canada (SPARCC) 389 patients]. Enthesitis was detected by MASES in 170/485 (35%, mean score ± sd 3.1 ± 2.4) and by SPARCC in 236/389 (61%, 4 ± 3.4). Achilles enthesitis was most frequent, by both MASES (unilateral/bilateral 28%/9%) and SPARCC (48%/18%). MASES/SPARCC baseline and follow-up scores for TNFi-1 were available for 100/105 patients. Of these, 63 patients (63%) (MASES) and 46 (43.8%) (SPARCC) achieved resolution of enthesitis. The site-specific enthesitis resolution was overall lower at SPARCC sites (peripheral; 63–80%) than at MASES sites (mainly axial; 82–100%) following TNFi-1. Disease activity and PROs were worse in patients with versus without enthesitis.
Conclusion
Entheseal assessments are only registered in a minority of patients with PsA in routine care. When assessed, enthesitis was common, and a substantial proportion demonstrated resolution following treatment with TNFi-1.
Psoriatic arthritis (PsA), diagnosed in 20–40% of patients with psoriasis, is a chronic, inflammatory disease that is phenotypically diverse, involving peripheral arthritis, axial disease, enthesitis, dactylitis, and skin and nail disease (Citation1–4). Inflammation at and immediately around the site of attachment of tendons, ligaments, or joint capsules to bones is termed enthesitis, a common clinical feature in patients with PsA that may be observed at any time during the disease course (Citation5, Citation6). Depending on the cohort and outcome measurement instrument used, the prevalence of enthesitis in patients with PsA varies widely, between 27% and 66% (Citation7–10). The presence of enthesitis in patients with PsA is associated with higher disease activity and often worse patient-reported outcomes (PROs) (Citation9–11). The Outcome Measures in Rheumatology (OMERACT) has endorsed the assessment of enthesitis as one of the core domains in all clinical trials and longitudinal observational studies involving patients with PsA (Citation12).
Clinical assessment of enthesitis is performed by eliciting tenderness at the peripheral and axial entheses, and validated enthesitis scoring systems addressing predefined anatomical areas of entheses have been developed. These include the Spondyloarthritis Research Consortium of Canada (SPARCC) and the Leeds Enthesitis Index (LEI), assessing the peripheral entheses, and the Maastricht Ankylosing Spondylitis Enthesitis Score (MASES), predominantly assessing the axial entheses (Citation13, Citation14).
Notwithstanding the clinical significance of enthesitis, systematic assessment of entheses is frequently not performed in routine care and data on the burden of enthesitis among patients of PsA from routine clinical care are sparse (Citation8–10). Furthermore, although most biological disease-modifying anti-rheumatic drugs (bDMARDs) demonstrate efficacy across the clinical domains in randomized clinical trials among patients with PsA (Citation15–17), there is a paucity of real-world data on the effectiveness of bDMARDs on enthesitis (Citation18, Citation19).
The European Spondyloarthritis (EuroSpA) Research Collaboration Network (RCN), a network of registries across Europe including patients with PsA, is a potential source of real-world data regarding enthesitis (Citation20, Citation21). However, the registration practice and data availability on enthesitis have not been studied previously. Thus, we aimed to: (i) explore the registration practice and data availability of enthesitis in patients with PsA in routine care; (ii) describe the pattern (distribution of affected entheses) of enthesitis in these patients; (iii) assess the disease burden and PROs in patients with versus without enthesitis at initiation of the first tumour necrosis factor inhibitor (TNFi); and (iv) assess the change in enthesitis from baseline to follow-up after initiation of the first and second TNFi.
Method
Patients and study design
Prospectively collected data on patients with PsA included in registries participating in the EuroSpA RCN were analysed (Citation20, Citation21). Biologic-naïve, adult patients with a clinical diagnosis of PsA, aged ≥ 18 years at initial diagnosis, who initiated a TNFi (adalimumab, certolizumab pegol, etanercept, golimumab, or infliximab) between 2010 and 2020, were identified from the following clinical registries (countries): AmSpA (Netherlands), ATTRA (Czechia), BSBR-AS (UK), biorx.si (Slovenia), DANBIO (Denmark), GISEA (Italy), ICEBIO (Iceland), ROB-FIN (Finland), RRBR (Romania), Reuma.pt (Portugal), SCQM (Switzerland), and TURKBIO (Turkey). The organization of and data collection practices across the EuroSpA registries have been described previously (Citation21).
Enthesitis
Enthesitis was defined as registration of the domain either as (i) a clinical feature (ever/never), without any knowledge of time-point, or (ii) tenderness at any of the entheses included in the validated enthesitis scoring systems, including the SPARCC and/or MASES (), with an enthesitis score from a clinical visit, i.e. with known time-point (Citation13).
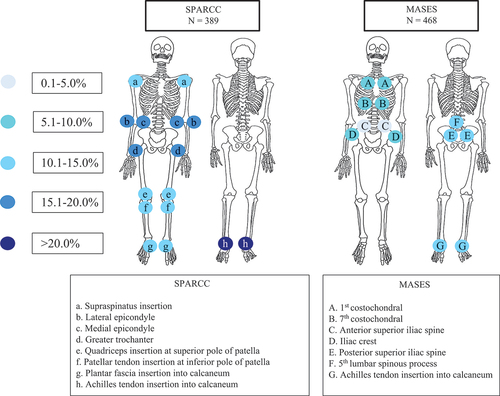
Figure 1. Distribution of enthesitis among patients evaluated with SPARCC and MASES at the initiation of the first tumour necrosis factor inhibitor (TNFi). SPARCC, Spondyloarthritis Research Consortium of Canada enthesitis scoring system; MASES, Maastricht Ankylosing Spondylitis Enthesitis Score. The colours of the circles indicate the proportion (percentage) of examined entheses with enthesitis at the initiation of the first TNFi. The letters in the circles refer to the individual entheses.
Resolution of enthesitis at the patient level was defined as an enthesitis score of 0 at follow-up, in patients who had an enthesitis score > 0 at baseline using either MASES or SPARCC. Resolution of enthesitis at the location-specific level was defined as the registration of the absence of enthesitis at follow-up in an enthesis registered with the presence of enthesitis at baseline. A change in the enthesitis score (increase or decrease in MASES or SPARCC score) was calculated at follow-up.
During data processing, all data managers were queried to ensure that missing entheseal assessment could be distinguished from active registration of no enthesitis.
Data sources
Study variables and statistical analyses for the study were planned a priori in a study protocol including a statistical analysis plan that was approved by investigators from all participating registries. Data sets from each registry were anonymized and uploaded securely to a common EuroSpA server. Analyses were conducted on the pooled data as well as by registry. For analyses stratified by registry, only those registries that had a minimum of 50 patients with enthesitis scores were included.
Time-point definitions
The baseline date was defined as the registered date for the start of treatment with TNFi. A patient could contribute more than one TNFi treatment series. Available data were collected for the first three TNFi treatment series (termed TNFi-1, TNFi-2, and TNFi-3). The baseline visit for each treatment was a registered visit from 30 days before to 30 days after the baseline date. In case more visits occurred within the given time interval, priority was given to a prior visit as close as possible in time to the baseline date. Follow-up visits for each treatment were registered visits at 6, 12, and 24 months, with the corresponding time windows being 90–270, 271–450, and 631–810 days from baseline.
Considering varying availability of data at the follow-up visits, follow-up data were combined to construct a ‘combined follow-up visit’ to maximize the available follow-up data. (i) If follow-up data were available only at the 6 or 12 or 24 month visit, the available visit was used for the combined follow-up visit. (ii) If data were available at all three visits (6, 12, and 24 months), or 6 and 12 months or 12 and 24 months, the data at the 12 month visit were used for the combined follow-up visit. (iii) If follow-up visits were available only at 6 and 24 months, the visit at 6 months was prioritized. Only data from the same TNFi treatment series were combined. For each treatment, visits were included until 2 weeks after a registered stop date of the relevant TNFi.
Study outcomes
Primary outcomes included: (i) availability of clinical data on enthesitis, as defined above, in the participating clinical registries; and (ii) sites and distribution of enthesitis at baseline in patients with PsA who were initiated on TNFi-1.
Secondary outcomes included: patient characteristics, disease activity measures, and PROs at baseline of the TNFi-1 among patients with PsA with versus without enthesitis, and in those patients with a registration of either absence or presence of enthesitis, versus those who had no such registration. Additional secondary outcomes were an association of disease activity measures and PROs with the enthesitis scores at baseline of TNFi-1, and finally, the proportion of patients and entheses with resolution of enthesitis and change in enthesitis scores from baseline to follow-up for TNFi-1 and TNFi-2. No data from the TNFi-3 treatment series were included owing to low data availability.
Patient characteristics, disease activity measures, and PROs
Baseline data included age, sex, body mass index (BMI), disease duration, smoking status, data on comorbidities (cardiovascular disease, diabetes, and kidney disease) and extra-articular manifestations (uveitis, inflammatory bowel disease, psoriasis, and dactylitis), time from diagnosis to baseline of TNFi-1, and concomitant conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs). Disease activity was assessed by swollen joint count (SJC) (28 and 66 joints), tender joint count (TJC) (28 and 68 joints), Psoriasis Activity and Severity Index (PASI), MASES, SPARCC, composite disease activity indices including Disease Activity Score based on 28-joint count–C-reactive protein (DAS28-CRP) and erythrocyte sedimentation rate (ESR), Disease Activity in Psoriatic Arthritis (DAPSA-28), physician global assessment, and PROs [Health Assessment Questionnaire (HAQ), pain score, patient global score, and patient fatigue score].
Statistical analyses
Analyses were performed on observed data with no imputation of missing data. No statistical tests were performed for any data. For the primary analyses, descriptive statistics (medians with interquartile ranges or mean ± sd, according to the distribution of data) were applied. To analyse the association between enthesitis and functional disability and pain at initiation of TNFi-1, four separate linear regression models with baseline HAQ and pain as independent variables and SPARCC and MASES as explanatory variables were constructed. All models included age, sex, and disease activity indices (SJC, TJC, CRP) as covariates.
Ethics and data protection
All participating registries obtained the necessary approvals in accordance with legal, compliance, and regulatory requirements from national Data Protection Agencies and/or Research Ethics Boards before the data transfer to the EuroSpA coordinating centre. This publication follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (Citation22) and the Declaration of Helsinki.
Results
Registration practice across the participating registries
Of the 12 participating registries, eight included a validated scoring system for enthesitis. Registries in Switzerland, Slovenia, and Finland applied the MASES, and those in Denmark, Iceland, and Turkey used SPARCC to evaluate enthesitis. Both these scoring systems were used in Portugal. The LEI was used in Czechia. The remaining registries had inconsistent methods to register enthesitis, most frequently as a patient-level variable (ever/never).
Data availability regarding enthesitis
Among the 10 548 patients with PsA identified as initiating a TNFi, 4116 (39%) had a registration of either absence or presence of enthesitis recorded as a clinical feature or an enthesitis score at any time-point. Of these, 1357 patients had undergone an assessment of entheses using MASES and/or SPARCC during treatment with a TNFi. Assessment by MASES and SPARCC was recorded in 1021 and 1166 patients, respectively, considering all visits (baseline and 6, 12, and 24 month follow-up). At the baseline of TNFi-1, 485 and 389 patients had recordings of MASES and SPARCC scores, respectively (), and these patients are referred to as the MASES and SPARCC cohorts. Of these, 274 and 197 patients were evaluated at one follow-up visit at least, by MASES and SPARCC, respectively. At the baseline of TNFi-2, the MASES and SPARCC scores were recorded for 114 and 164 patients, respectively. Among these patients, 70 and 73, respectively, were evaluated by MASES and SPARCC at least during one follow-up visit following initiation of TNFi-2. The numbers were lower for the TNFi-3 initiation, and data are therefore not reported.
Table 1. Patient characteristics, disease activity measures, and patient-reported outcomes at initiation of the first tumour necrosis factor inhibitor (TNFi-1) in the cohort stratified by MASES and SPARCC scores.
Patient characteristics, disease activity measures, and PROs in patients with versus without enthesitis at the initiation of TNFi-1
Patient characteristics and disease activity measures were comparable between the patients who underwent assessment of entheses using the MASES or SPARCC at any time-point during treatment with a TNFi and the 9190 patients who did not have an entheseal assessment registered (Supplementary Table S1a).
describes the patient characteristics, disease activity measures, and PROs at baseline of TNFi-1 in patients who had an assessment of entheses using MASES and SPARCC, stratified by the presence versus absence of enthesitis. The mean age of patients registered with enthesitis by MASES was 46.9 ± 12.7 years and by SPARCC was 46.6 ± 11.2 years. More women had enthesitis compared to men (MASES: 54.7% vs 45.3%; SPARCC: 58.9% vs 41.1%). The median duration between diagnosis of PsA and initiation of TNFi-1 was shorter among patients with enthesitis (2 years) compared to those without enthesitis (3 years) in the MASES cohort. The most used TNFi-1 in both patients with and without enthesitis was adalimumab (MASES: 37.1% for both; SPARCC: 50% and % and 38.6%, respectively). The use of concomitant NSAIDs at TNFi-1 initiation was also higher in the group with enthesitis (MASES: 78%; SPARCC: 66.7%) compared to those without enthesitis (MASES: 59.2%; SPARCC: 46%). The mean disease activity in PsA (DAPSA) was higher among patients with enthesitis (MASES: 30.74 ± 15; SPARCC: 29.8 ± 13.5) compared to those without enthesitis (MASES: 23.14 ± 12; SPARCC: 25.2 ± 13.2). Similar findings were observed in the individual registries (Supplementary Table S1b). PROs were numerically higher among patients with enthesitis compared to those without in both MASES and SPARCC cohorts ().
Enthesitis scores and distribution of enthesitis among patients at the initiation of TNFi-1
The mean baseline enthesitis scores in the MASES and SPARCC cohorts were 3.1 ± 2.4 and 4 ± 3.4, respectively. Women had higher mean enthesitis scores compared to men, by both MASES (3.7 ± 2.7 vs 2.4 ± 1.6) and SPARCC (4.5 ± 3.6 vs 3.2 ± 2.8). In the registries assessing enthesitis by MASES, Achilles tendon entheses (unilateral 27.6%; bilateral 8.5%), posterior superior iliac spine (unilateral 24.8%; bilateral 10%), and fifth lumbar spinous process (13%) were the most affected. In the registries with SPARCC scores, Achilles tendon (unilateral 47.8%; bilateral 18%), lateral epicondyle (unilateral 35.5%; bilateral 14.7%), and greater trochanter (unilateral 31.7%; bilateral 13.1%) were the most affected entheses ( and ).
Table 2. Distribution of enthesitis by MASES and SPARCC scoring systems among all patients at the initiation of the first tumour necrosis factor inhibitor (TNFi-1).
Correlation between enthesitis scoring systems and PROs
In the linear regression models with MASES as an explanatory variable, HAQ and pain demonstrated significant positive associations with tender joint count (p < 0.0001 for both) and negative associations with male gender (p = 0.0003 and 0.03), while no association between MASES and HAQ or pain was observed. In the models including SPARCC, both HAQ and pain had a significant positive association with baseline SPARCC score (p = 0.007 and 0.008, respectively) and TJC (p = 0.049 and 0.011, respectively), whereas HAQ had a significant negative association with male gender (p = 0.0003) ().
Table 4. Linear regression models for association between clinical variables and patient-reported outcomes with enthesitis scores at baseline.
Effectiveness of TNFi in resolution and change in enthesitis at follow-up
For TNFi-1, assessment of entheses by MASES and SPARCC at baseline was performed in 274 and 197 patients, respectively. Of these, 100 and 105 patients, respectively, had enthesitis. At the follow-up visit, in the MASES and SPARCC cohorts, 63 (63%) and 46 (43.8%) of the patients still on treatment, respectively, demonstrated resolution of enthesitis (). Among patients initiating TNFi-2, enthesitis was observed in 26 of the 70 patients evaluated by MASES and 42 of the 73 patients assessed by SPARCC. Resolution of enthesitis following TNFi-2 was observed in 30.8% and 28.6% of the patients still on treatment, by MASES and SPARCC, respectively. There were no major differences in the resolution of enthesitis at different entheses following TNFi therapy, among either those who underwent assessment by MASES or those assessed by SPARCC (Supplementary Table S2). Among the patients who underwent assessment of entheses using MASES at the initiation of TNFi-1 (n = 266), complete resolution of enthesitis was noted in > 80% of patients at all the entheses, except for the left Achilles tendon insertion (74.3%). Patients who underwent SPARCC assessment of entheses at the initiation of TNFi-1 (n = 197) demonstrated an overall lower rate of site-specific enthesitis resolution (43.8–63%).
Table 3. Effectiveness of the first tumour necrosis factor inhibitor (TNFi) evaluated as change in MASES and SPARCC scores and resolution of enthesitis at follow-up.
Discussion
In this cohort of patients with PsA across 12 European countries, registration of enthesitis was inconsistent both within and across clinical registries. In all registries, it was possible to register enthesitis either as a dichotomous clinical feature, without mentioning a time-point, or as an enthesitis score from a clinical visit. However, only about one-third of patients had any recording of enthesitis and even fewer had entheseal assessment performed using validated scores. Among the patients who underwent assessment of entheses using a validated scoring system at the initiation of a first TNFi, enthesitis was observed in a large proportion. Enthesitis was noted more commonly in women compared to men, and patients with enthesitis initiated TNFi earlier in the disease course than those without enthesitis. Patients with enthesitis demonstrated an overall higher disease activity and poorer PROs compared to those without enthesitis. Baseline HAQ and pain had a positive association with baseline SPARCC enthesitis scores. Complete remission of enthesitis was observed after initiation of TNFi-1 in approximately 63% and 44% of patients assessed by MASES and SPARCC, respectively.
As in the current study, significant gaps in the registration of enthesitis have previously been reported in the Danish DANBIO registry (Citation10). This emphasizes the need for a more rigorous assessment of entheses in routine care by rheumatologists. In our study, we also found a wide variability in the assessment of entheses in the countries participating in the EuroSpA collaboration. Many patients in our study had enthesitis registered as a dichotomous variable, without any time-point. Only eight registries in the EuroSpA research collaboration network applied assessment of entheses using validated scoring indices. In a 2021 systematic review exploring outcome measures used in 27 PsA registries or cohorts, enthesitis was reported in 21 registries, of which 12 had registration using MASES or LEI (Citation23). Clinical trials have also used varied entheseal assessment methods, with some being dichotomous and others using validated indices (Citation24–29). This heterogeneity in assessing enthesitis impedes comparison between trials and cohorts.
The Achilles tendon was the most commonly affected enthesis, both in the patients who underwent assessment with the SPARCC and in those assessed with the MASES. This is in agreement with the Toronto PsA cohort and the Assessment of SpondyloArthritis international Society (ASAS) PerSpA data, but not with the CorEvitas registry data, in which lateral epicondyle was the most affected enthesis (Citation8, Citation30, Citation31). A Turkish multicentre study reported the Achilles tendon, followed by the fifth lumbar spinous process, as the most affected entheses (Citation32). This concurs with findings among patients who underwent entheseal assessment using the MASES in our study.
Women had worse mean MASES and SPARCC scores than men. This difference between the sexes was maintained across individual entheses, depicting an overall higher burden of enthesitis among women, as reported in previous studies (Citation10, Citation33–35). In contrast, a study evaluating the differences between sexes, comparing clinical findings and ultrasound examination at the joints and entheses in patients with PsA, demonstrated women to have more clinical findings of enthesitis than men, whereas men had significantly higher sonographic inflammatory enthesitis scores compared to women (Citation35). This discrepancy between clinical examination and ultrasonography may partly be explained by the well-recognized higher prevalence of fibromyalgia in women and previous studies reporting significantly higher clinical enthesitis scores among patients with concomitant fibromyalgia (Citation36). Data on fibromyalgia were, unfortunately, not available in our study.
Composite disease activity measures were found to be associated with enthesitis scores in our study, i.e. patients with enthesitis had less favourable outcomes than those without enthesitis. A similar association was noted for PROs and enthesitis scores. This is in accordance with the observations from the Psoriatic Arthritis-International Database, in which enthesitis was a significant risk factor for patients not achieving the minimal disease activity state (Citation37). In another cross-sectional survey of 3200 patients with PsA from nine countries comparing clinician- and patient-reported outcomes among those with versus without enthesitis, the former group experienced worse outcomes than the latter. Physicians expressed more dissatisfaction with the overall lack of efficacy and lack of pain control with the treatment provided among patients with enthesitis compared to those without enthesitis (Citation11).
Data on response with treatment in patients with PsA in real-life settings are sparse, as are data on the resolution of peripheral versus axial enthesitis. A study from the Toronto PsA clinic cohort reported resolution of enthesitis in 86% of patients within a year of initiation of therapy, regardless of the medication used (Citation38). In our study, following TNFi-1, a higher rate of overall enthesitis resolution was noted among patients assessed using MASES (63%) compared to those assessed by SPARCC (44%). This was also reflected in the site-specific resolution of enthesitis, wherein axial enthesitis demonstrated a higher rate of resolution than peripheral enthesitis. This may suggest that axial entheses respond better to TNFi compared to peripheral entheses. It is, nevertheless, important to recognize that the patients assessed were different. Moreover, a lower baseline MASES compared to SPARCC may contribute to the observed disparity.
Several clinical trials of TNFi in patients with PsA have explored the effect of therapy on enthesitis as either a secondary or an exploratory endpoint. The only study designed with enthesitis as a primary endpoint was the ACHILLES trial, in which 204 patients with active axial spondyloarthritis or PsA and heel enthesitis were randomized to receive secukinumab or placebo, followed by secukinumab. The study, however, did not demonstrate the superiority of secukinumab over placebo, according to the percentage of patients achieving clinical resolution of Achilles tendon enthesitis in the affected foot assessed by the LEI (approximately 42% vs 31%, respectively; not statistically significantly different) (Citation38). In the SEAM-PsA trial, resolution of enthesitis was noted in 53% of 173 patients on etanercept monotherapy, which is comparable to the findings in this current study (Citation26). The GO-VIBRANT study of intravenous golimumab in PsA demonstrated enthesitis resolution in around 63% of patients assessed by the LEI, at week 52 (Citation39). In the CRESPA trial of golimumab involving patients with very early peripheral spondyloarthritis, a 25% drop in enthesitis assessed by modified MASES, along with a significant reduction in enthesitis scores among patients on golimumab compared to placebo, was demonstrated at weeks 8, 16, and 24 (Citation40).
A major strength of this study is the real-world analysis of prospectively collected data on the treatment of enthesitis with TNFi across different countries in Europe, providing important evidence for the effectiveness of TNFi on enthesitis from a real-world setting. The results must, however, be interpreted considering the limitations of this study. One of the major limitations is the high number of patients with no data on the evaluation of entheses. The patients who underwent a detailed evaluation of entheses could have had more severe symptoms, consequently leading to a selection bias. This missingness of data can potentially lead to misrepresentation of the true burden of this clinical domain. Nevertheless, the patients who underwent assessment of entheses seemed to be a representative sample of the overall study population in this study, as shown in Supplementary Table S1. The fallacies associated with clinical assessment of enthesitis add to this uncertainty. Competent awareness and training among clinicians on the importance of this clinical domain may result in a more consistent assessment of enthesitis in patients with PsA. Registries should facilitate the evaluation and registration of enthesitis at each visit. An important limitation is the lack of data on coexisting fibromyalgia, as the presence of fibromyalgia or nociplastic pain can distort the clinical examination findings significantly. This limitation could potentially have been overcome if data from imaging modalities such as ultrasound or magnetic resonance imaging had been available. Imaging can be very helpful in differentiating true enthesitis from tenderness due to other causes (Citation41). Unfortunately, no data on imaging or coexistent fibromyalgia were available in the registries. The effectiveness of TNFi in the investigated cohort may not be representative of all patients treated in clinical practice. Furthermore, limited data on enthesitis and its resolution hindered a robust analysis of effectiveness following the initiation of the second and third TNFi.
Conclusion
This study highlights the deficiencies in clinical assessment and treatment of enthesitis in patients with PsA in the real-world setting. Based on our data, enthesitis appears to be present in a high proportion of patients with PsA and the presence of enthesitis is associated with a higher overall disease burden. Axial entheses are affected in a lower proportion of patients with PsA compared to peripheral entheses. Following initiation of a first TNFi, a sizable proportion of PsA patients with enthesitis achieves complete resolution.
Supplemental Material
Download MS Word (65.8 KB)Acknowledgements
The EuroSpA Research Collaboration Network was financially supported by Novartis Pharma AG. Novartis did not influence data collection, statistical analyses, manuscript preparation or decision to submit the manuscript. The outline of was drawn by Mr Rajkumar, Department of Anatomy, Christian Medical College, Vellore, India.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Additional supplementary material may be found in the online version of this article. Please note that the editors are not responsible for the content or functionality of any supplementary material supplied by the authors. Any queries should be directed to the corresponding author.
Additional information
Funding
References
- Alinaghi F, Calov M, Kristensen LE, Wu JJ, Thyssen JP, Egeberg A. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol 2019;80:251–65.
- Rech J, Sticherling M, Stoessel D, Biermann MHC, Häberle BM, Reinhardt M. Psoriatic arthritis epidemiology, comorbid disease profiles and risk factors: results from a claims database analysis. Rheum Adv Pract 2020;4:rkaa033.
- FitzGerald O, Ogdie A, Chandran V, Coates LC, Kavanaugh A, Tillett W, et al. Psoriatic arthritis. Nat Rev Dis Primers 2021;7:59.
- Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med 2017;376:957–70.
- Kaeley GS, Eder L, Aydin SZ, Gutierrez M, Bakewell C. Enthesitis: a hallmark of psoriatic arthritis. Semin Arthritis Rheum 2018;48:35–43.
- Freeston J, Coates LC, Helliwell PS, Hensor EMA, Wakefield RJ, Emery P, et al. Is there subclinical enthesitis in early psoriatic arthritis? A clinical comparison with power doppler ultrasound. Arthritis Care Res 2012;64:1617–21.
- Pittam B, Gupta S, Harrison NL, Robertson S, Hughes DM, Zhao SS. Prevalence of extra-articular manifestations in psoriatic arthritis: a systematic review and meta-analysis. Rheumatology (Oxford) 2020;59:2199–206.
- Polachek A, Li S, Chandran V, Gladman D. Clinical enthesitis in a prospective longitudinal psoriatic arthritis cohort: incidence, prevalence, characteristics and outcome. Arthritis Care Res (Hoboken) 2017;69:1685–91.
- Mease PJ, Karki C, Palmer JB, Etzel CJ, Kavanaugh A, Ritchlin CT, et al. Clinical characteristics, disease activity, and patient-reported outcomes in psoriatic arthritis patients with dactylitis or enthesitis: results from the corrona psoriatic arthritis/spondyloarthritis registry. Arthritis Care Res (Hoboken) 2017;69:1692–9.
- Mathew AJ, Glintborg B, Krogh NS, Hetland ML, Østergaard M. Enthesitis in patients with psoriatic arthritis and axial spondyloarthritis – data from the Danish nationwide DANBIO registry. Semin Arthritis Rheum 2022;52:151948.
- Orbai A-M, Birt JA, Holdsworth EA, Booth N, Malatestinic WN, Sprabery AT, et al. Impact of enthesitis on psoriatic arthritis patient-reported outcomes and physician satisfaction with treatment: data from a multinational patient and physician survey. Rheumatol Ther 2020;7:937–48.
- Orbai A-M, de Wit M, Mease PJ, Callis Duffin K, Elmamoun M, Tillett W, et al. Updating the psoriatic arthritis (PsA) core domain set: a report from the PsA workshop at OMERACT 2016. J Rheumatol 2017;44:1522–8.
- Mease PJ. Enthesitis in psoriatic arthritis (Part 3): clinical assessment and management. Rheumatology (Oxford) 2020;59:i21–i28.
- Mease PJ, Van den Bosch F, Sieper J, Xia Y, Pangan AL, Song I-H. Performance of 3 enthesitis indices in patients with peripheral spondyloarthritis during treatment with Adalimumab. J Rheumatol 2017;44:599–608.
- Simons N, Degboé Y, Barnetche T, Cantagrel A, Ruyssen-Witrand A, Constantin A. Biological DMARD efficacy in psoriatic arthritis: a systematic literature review and meta-analysis on articular, enthesitis, dactylitis, skin and functional outcomes. Clin Exp Rheumatol 2020;38:508–15.
- Kerschbaumer A, Smolen JS, Dougados M, de Wit M, Primdahl J, McInnes I, et al. Pharmacological treatment of psoriatic arthritis: a systematic literature research for the 2019 update of the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis 2020;79:778–86.
- McInnes IB, Sawyer LM, Markus K, LeReun C, Sabry-Grant C, Helliwell PS. Targeted systemic therapies for psoriatic arthritis: a systematic review and comparative synthesis of short-term articular, dermatological, enthesitis and dactylitis outcomes. RMD Open 2022;8:e002074.
- Colombo D, Frassi M, Mariano GP, Fusaro E, Lomater C, Del Medico P, et al. Real-world evidence of biologic treatments in psoriatic arthritis in Italy: results of the CHRONOS (EffeCtiveness of biologic treatments for psoriatic artHRitis in Italy: an observatioNal lOngitudinal study of real-life clinical practice) observational longitudinal study. BMC Rheumatol 2022;6:57.
- Mathew AJ, Sutton M, Pereira D, Gladman DD, Chandran V. Effectiveness of disease-modifying antirheumatic drugs for enthesitis in a prospective longitudinal psoriatic arthritis cohort. J Rheumatol 2022;49:1020–5.
- Brahe CH, Ørnbjerg LM, Jacobsson L, Nissen MJ, Kristianslund EK, Mann H, et al. Retention and response rates in 14261 PsA patients starting TNF inhibitor treatment – results from 12 countries in EuroSpA. Rheumatology (Oxford) 2020;59:1640–50.
- Linde L, Ørnbjerg LM, Rasmussen SH, Love TJ, Loft AG, Závada J, et al. Commonalities and differences in set-up and data collection across European spondyloarthritis registries – results from the EuroSpA collaboration. Arthritis Res Ther 2023;25:205.
- Mulder MLM, Wenink MH, Vriezekolk JE. Being overweight is associated with not reaching low disease activity in women but not men with psoriatic arthritis. Rheumatology (Oxford) 2022;61:770–4.
- Aouad K, Moysidou G, Rakotozafiarison A, Fautrel B, Gossec L. Outcome measures used in psoriatic arthritis registries and cohorts: a systematic literature review of 27 registries or 16,183 patients. Semin Arthritis Rheum 2021;51:888–94.
- Ramiro S, Smolen JS, Landewé R, van der Heijde D, Gossec L. How are enthesitis, dactylitis and nail involvement measured and reported in recent clinical trials of psoriatic arthritis? A systematic literature review. Ann Rheum Dis 2018;77:782–3.
- Antoni C, Krueger GG, de Vlam K, Birbara C, Beutler A, Guzzo C, et al. Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann Rheum Dis 2005;64:1150–7.
- Mease PJ, Gladman DD, Ritchlin CT, Ruderman EM, Steinfeld SD, Choy EHS, et al. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum 2005;52:3279–89.
- Mease PJ, Gladman DD, Collier DH, Ritchlin CT, Helliwell PS, Liu L, et al. Etanercept and methotrexate as monotherapy or in combination for psoriatic arthritis: primary results from a randomized, controlled phase III trial. Arthritis Rheumatol 2019;71:1112–24.
- Kavanaugh A, van der Heijde D, McInnes IB, Mease P, Krueger GG, Gladman DD, et al. Golimumab in psoriatic arthritis: one-year clinical efficacy, radiographic, and safety results from a phase III, randomized, placebo-controlled trial. Arthritis Rheum 2012;64:2504–17.
- Mease PJ, Fleischmann R, Deodhar AA, Wollenhaupt J, Khraishi M, Kielar D, et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann Rheum Dis 2014;73:48–55.
- Lopez-Medina C, Chevret S, Molto A, Sieper J, Duruöz T, Kiltz U, et al. Identification of clinical phenotypes of peripheral involvement in patients with spondyloarthritis, including psoriatic arthritis: a cluster analysis in the worldwide ASAS-PerSpA study. RMD Open 2021;7:e001728.
- Mease PJ, Liu M, Rebello S, Hua W, McLean RR, Hur P, et al. Disease characteristics, quality of life, and work productivity by enthesitis site: real-world data from the US Corrona psoriatic arthritis/spondyloarthritis registry. J Rheumatol 2021;48:367–75.
- Sunar I, Ataman S, Nas K, Kilic E, Sargin B, Kasman SA, et al. Enthesitis and its relationship with disease activity, functional status, and quality of life in psoriatic arthritis: a multi-center study. Rheumatol Int 2020;40:283–94.
- Orbai A-M, Perin J, Gorlier C, Coates LC, Kiltz U, Leung YY, et al. Determinants of patient-reported psoriatic arthritis impact of disease: an analysis of the association with sex in 458 patients from fourteen countries. Arthritis Care Res (Hoboken) 2020;72:1772–9.
- de Vlam K, Steinfeld S, Toukap AN, de Vlam K, van den Bosch F, Joos R, et al. The burden of psoriatic arthritis in the biologics era: data from the Belgian epidemiological psoriatic arthritis study. Rheumatology (Oxford) 2021;60:5677–85.
- Furer V, Wollman J, Levartovsky D, Aloush V, Elalouf O, Sarbagil-Maman H, et al. Sex-based differences in sonographic and clinical findings among patients with psoriatic arthritis. J Rheumatol 2023;50:197–203.
- Polachek A, Furer V, Zureik M, Nevo S, Mendel L, Levartovsky D, et al. Role of ultrasound for assessment of psoriatic arthritis patients with fibromyalgia. Ann Rheum Dis 2021;80:1553–8.
- Bakirici S, Solmaz D, Al Osaimi N, Dalkilic E, Can M, Erden A, et al. What are the main barriers to achieve minimal disease activity in psoriatic arthritis in real life? Clin Exp Rheumatol 2019;37:808–12.
- Baraliakos X, Sewerin P, de Miguel E, Pournara E, Kleinmond C, Wiedon A, et al. Achilles tendon enthesitis evaluated by MRI assessments in patients with axial spondyloarthritis and psoriatic arthritis: a report of the methodology of the ACHILLES trial. BMC Musculoskelet Disord 2020;21:767.
- Husni ME, Kavanaugh A, Murphy F, Rekalov D, Harrison DD, Kim L, et al. Efficacy and safety of intravenous golimumab through one year in patients with active psoriatic arthritis. Arthritis Care Res (Hoboken) 2020;72:806–13.
- Carron P, Varkas G, Cypers H, Van Praet L, Elewaut D, Van den Bosch F, et al. Anti-TNF-induced remission in very early peripheral spondyloarthritis: the CRESPA study. Ann Rheum Dis 2017;76:1389–95.
- Kaeley GS. Enthesitis in psoriatic arthritis (Part 2): imaging. Rheumatology (Oxford) 2020;Suppl_1:15–20.
