Abstract
Objective
Patients with primary Sjögren’s syndrome (pSS) have an increased risk of lymphoma, especially mucosa-associated lymphoid tissue (MALT) lymphoma of the salivary glands. Risk factors for lymphoma are well known, but there are no studies on screening by imaging. Therefore, we aimed to assess the usefulness and adverse effects of ultrasound of the major salivary glands and neck as lymphoma screening.
Method
A retrospective, single-centre, analysis of imaging studies in pSS patients was conducted. Imaging studies were classified as either screening examinations (asymptomatic patients) or occasion-related (imaging due to signs of lymphoma or at least moderate systemic activity). Results were categorized as: not suspicious; requiring control; triggering tissue sampling with exclusion of lymphoma; or triggering tissue sampling with diagnosis of lymphoma.
Results
The study included 134 patients and covered 1031 patient-years. Lymphoma was diagnosed in 15 patients (11.2%), all of whom had clinical signs of lymphoma at the time of diagnosis. During this period, 569 screening examinations and 179 occasion-related examinations were conducted. None of the screening examinations detected lymphoma, but follow-up imaging was recommended in 17.1% (95% CI 14.2–20.4%) and invasive exclusion of lymphoma was performed in 0.5% (95% CI 0.1–1.5%). In contrast, lymphoma was detected in 6.1% (95% CI 3.5–10.6%) of occasion-related examinations.
Conclusion
pSS patients with neither signs of lymphoma nor increased systemic disease activity did not benefit from screening. In contrast, patients with symptoms of lymphoma or at least moderate systemic activity can benefit from imaging of the neck and major salivary glands.
The association between primary Sjögren’s syndrome (pSS) and an increased risk of B-cell non-Hodgkin lymphoma (B-NHL) is well known (Citation1). Population-based studies have shown a seven- to nine-fold increased risk of developing B-NHL, while hospital cohorts have reported an increased risk by a factor of 16–38 compared to the general population (Citation2, Citation3). This puts the absolute risk of an individual pSS patient being diagnosed with B-NHL within 20 years of diagnosis at up to 18% (Citation4).
The most frequent lymphomas in the context of pSS are mucosa-associated marginal zone lymphomas [mucosa-associated lymphoid tissue (MALT)], mainly manifesting in the parotid glands, other marginal zone lymphomas, and diffuse large B-cell lymphomas (DLBCLs) (Citation5).
Reported clinical risk factors for the development of lymphoma are lymphadenopathy, enlargement of the major salivary glands (MSGs), skin vasculitis, and splenomegaly. With regard to laboratory assessment, lymphopenia, anaemia, C4 hypocomplementemia, monoclonal gammopathy, cryoglobulinemia, and positivity for anti-Sjögren’s syndrome antigen A/B (anti-SSA/B) or anti-La/SSB are independently associated with the occurrence of lymphoma (Citation6). However, there is a high degree of variability between studies. Reasons for this variability are that clinical features such as enlarged lymph nodes and MSGs occur in both systemically active pSS and lymphoma, and that studies on this topic mainly have a retrospective design (Citation4, Citation7).
Although knowledge of risk factors is available, recommendations for the management of patients at risk are scarce (Citation8). Of the current guidelines, only the British guideline on the management of pSS, published in 2017, comments on this. This guideline recommends a regular clinical and laboratory monitoring of patients at risk. New suspicious lesions should be investigated by ultrasound (US). However, the evidence for these recommendations is only low to moderate (Citation9). There are no data on whether people with pSS without risk factors benefit from regular screening by imaging (Citation10).
The possible advantages of screening examinations are earlier diagnosis and possibly better treatment outcomes. These must be weighed against the possible disadvantages, such as false-positive or borderline findings, requiring further (invasive) diagnostics. This causes distress to the patients and puts additional pressure on healthcare systems. Over the past decade, the risks and harms of diagnostic procedures have been increasingly addressed, especially through the Choosing Wisely initiative (Citation11). In line with this, these unfavourable effects will become even more important if, as recently discussed, repeated US of the MSGs is introduced into clinical practice to monitor disease activity (Citation12).
To improve the evidence on this issue, we investigated the incidence of lymphoma and its manifestations in a defined cohort of pSS patients at a single centre. In addition, we wanted to find out whether screening by US in a low-risk group of patients (defined as absence of clinical signs of lymphoma and not more than a low systemic activity) can detect lymphomas. Moreover, the possible negative effects of screening were determined by recording the frequencies of invasive measures and follow-up examinations.
Method
The data analysis was based on the electronic patient chart of the rheumatology department of the University Hospital of Würzburg (RheMIT/EMIL by itc-ms.de, Marburg, Germany, and SAP SE, Walldorf, Germany). All patients with the term ‘Sjögren’s’ in their diagnosis were selected. Only those for whom the following conditions were all met were included for further analysis: at least two visits to the department, presence of a pSS according to the 2016 American College of Rheumatology (ACR)/European Alliance of Associations for Rheumatology (EULAR) classification criteria classification criteria, and a positive test for SSA and/or SSB antibodies (Citation13).
The period of time between the patient’s initial visit and their most recent visit was selected as the evaluation period for imaging studies. For patients who developed lymphoma, the evaluation period was defined as the period from the first visit to the department until the time of diagnosis of lymphoma. In cases where lymphoma was diagnosed in other medical institutions but within the definition of the evaluation period, the available information from the patient’s medical record was assessed.
Patient characteristics, including age, sex, disease duration, EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI), presence of cryoglobulins, and immunosuppressive therapy, were analysed with reference to the last visit of the evaluation period (Citation14). When ESSDAI scores had not been calculated at the visit, they were calculated retrospectively based on the available records.
Imaging studies of the neck and/or MSGs, performed during the evaluation period at the hospital, were included for the analysis of cervical imaging. US, computed tomography (CT), and/ magnetic resonance imaging (MRI) were assessed. They were retrospectively classified as ‘occasion-related’ if at least one of the following criteria had been present at the time of imaging: (i) clinical signs of lymphoma, (ii) organ involvement accounting for at least moderate systemic activity of Sjögren’s syndrome, and (iii) the presence of cryoglobulins. If this was not the case, the imaging studies were classified as ‘screening imaging’. Clinical signs of lymphoma were defined as swelling of the lymph nodes and/or MSGs and/or the presence of B symptoms. B symptoms were defined as fever, night sweats or 10% or more unintentional weight loss within 6 months. The presence of organ involvement with at least moderate systemic activity was defined as at least 5 points in the ESSDAI score within one of the following domains: articular, cutaneous, pulmonary, renal, muscular, peripheral nervous system (PNS), or central nervous system (CNS) (Citation14). According to the current EULAR guidelines, an ESSDAI score of 5 corresponds to moderate systemic activity (Citation2). Cryoglobulins have been reported to be one of the strongest predictors of the development of lymphoma (Citation4); therefore, all imaging studies in patients with the current presence of cryoglobulins were also classified as occasion related.
The US examinations were performed by different examiners, mostly in the Department of Internal Medicine, with a few cases in the Department of Radiology. They included the parotid and submandibular glands and lymph-node regions I–V of the neck. Inhomogeneous salivary gland parenchyma is the main US finding in pSS. Focal lesions within the parenchyma are common in pSS, and intraparenchymal and extraparenchymal lymph nodes may be increased in size and number. Focal lesions may present a challenging differential diagnosis, as cysts, lymphomas, or malignant/benign primary salivary gland tumours may show overlapping sonographic features (Citation12, Citation15). The following US criteria were used to evaluate the intraparenchymal and extraparenchymal lymph nodes: abnormal Solbiati index, abnormal enlargement, abnormal perfusion, and missing hilum (Citation16). The findings were interpreted by the treating rheumatologist and classified as not suspicious or as requiring further diagnostics. The results and consequences of the imaging study were retrospectively categorized as: (i) not suspicious, (ii) control imaging, (iii) triggering tissue sampling with exclusion of lymphoma, and (iv) entailing tissue sampling with diagnosis of lymphoma.
The imaging studies were conducted between January 2000 and June 2023. Retrospective data analysis was performed between October 2022 and September 2023.
Data analysis and statistics
GraphPad Prism software (version 10.1.1; GraphPad Software, Boston, MA, USA) was used for calculating statistics. The 95% confidence intervals (CIs) for the observed frequency of lymphomas and the frequencies of outcome categories of the studies were calculated using the Wilson/Brown method. The positive predictive value for the diagnosis of lymphoma was determined for the situation in which the imaging procedure triggered the sampling of tissue. The 95% CI for positive predictive value was calculated with the Wilson/Brown method. By analogy with the number needed to treat, we calculated the number needed to be screened (NNBS) (consecutive detection of lymphoma) and the number needed to harm (NNH) (consecutive tissue sampling without diagnosis of lymphoma) for imaging studies. These values were calculated separately for the settings of screening and occasion-related imaging (Citation17). To compare patient characteristics between patients with lymphoma and patients without lymphoma, we performed Mann–Whitney U tests or chi-squared tests with SPSS (version 29; IBM Corp, Armonk, NY, USA). p-Values < 0.05 were considered as significant.
Results
Patient characteristics and incidence/manifestation of lymphoma
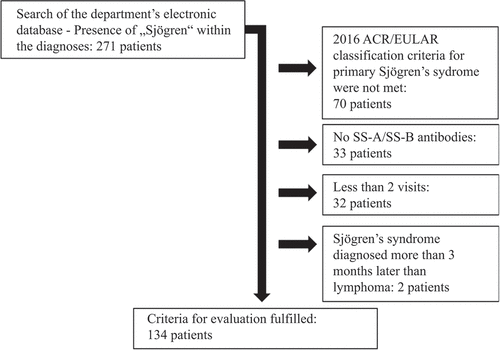
A total of 134 patients met the inclusion criteria (). The evaluation period covered 1031 patient-years. Within the analysed cohort, lymphoma occurred in 15 out of 134 patients. This corresponded to a frequency of 11.2% (95% CI 6.9–17.7%) or one case of lymphoma per 68.6 patient-years.
Figure 1. Flowchart of the patient selection process. ACR, American College of Rheumatology; EULAR, European Alliance of Associations for Rheumatology.
We compared patients without lymphoma and those who developed lymphoma based on data from their last visit in the evaluation period. On average, patients with lymphoma had a shorter duration of pSS (mean of 5.9 vs. 11.4 years). In terms of disease activity, lymphoma patients had a significantly higher total ESSDAI score and demonstrated more frequent activity in the constitutional and glandular domains. By definition, all lymphoma patients had the maximum score value in the lymphadenopathy domain. No significant differences were found in the presence of activity in other ESSDAI domains or the presence of cryoglobulins. Activity in the pulmonary, renal, PNS, and CNS domains, which could potentially lead to very high ESSDAI scores, was rarely present in our cohort at the time of assessment. A comparison of the ESSDAI scores at the initial visit between patients who subsequently developed lymphoma and those who did not revealed no significant difference (Supplementary Table S1). In terms of immunosuppressive therapies used in patients’ medical history, both groups had commonly received prednisolone and disease-modifying anti-rheumatic drugs (DMARDs). However, patients with lymphoma had been treated with methotrexate (MTX) significantly more often, while no clear difference was observed for other DMARDs ().
Table 1. Patient demographics, disease activity, and therapies.
Eleven cases of lymphoma were detected at our hospital. The remaining four cases were diagnosed outside our centre.
At the time of diagnosis, all 15 lymphoma patients had clinical signs of lymphoma. Of these 15 lymphoma cases, eight were MALT lymphomas, four had nodal marginal zone lymphoma, two had DLBCL, and one had Hodgkin’s lymphoma. In five out of 15 patients (33.3%), the diagnoses of lymphoma and pSS were made simultaneously (not more than 12 weeks apart) ().
Table 2. Detailed information on lymphoma patients.
Analysis of cervical imaging
In total, 748 imaging studies could be evaluated. Of these, 722 (96.4%) were US studies, 13 (1.7%) were CT scans, and 13 (1.7%) were MRI studies.
Overall, 569 (76.1%) of the studies were screening examinations. With the exception of one MRI study, all of them were US examinations. The US studies classified as screening were often performed as part of the diagnostic work-up at diagnosis and repeatedly at the discretion of the treating rheumatologist, with a large variability in frequency between patients. The remaining 179 imaging studies were conducted in the presence of symptoms of lymphoma or at least moderate systemic activity or cryoglobulin positivity. MRI studies of the neck and MSGs were requested in cases where US results were inconclusive. CT scans included the thorax and abdomen in the majority of cases and were performed in patients with high suspicion for malignancy who did not have isolated enlargement of an MSG or a neck lymph node.
The majority of screening examinations (82.4%) resulted in findings that were not suspicious for lymphoma. In 17.1% of the screenings, follow-up imaging was scheduled. Three screening examinations (0.5%) resulted in an invasive exclusion of lymphoma. None of the screening examinations finally led to the diagnosis of lymphoma (). The majority of occasion-related examinations (58.7%) also did not show signs of malignancy. However, follow-up imaging was planned in 30.2%, thus more frequently than in screening examinations. Further invasive clarification was markedly more frequent than in screening examinations, as lymphoma could only be excluded by tissue sampling in 5.0% of all occasion-related examinations, whereas 6.1% of all occasion-related examinations resulted in the sampling of tissue establishing the diagnosis of lymphoma ().
Table 3. Imaging studies for screening purposes with respective outcome.
Table 4. Occasion-related imaging studies with respective outcome.
Notably, in one patient with swelling of the cervical lymph nodes, US supported the suspicion of malignancy. Consecutively, tissue sampling was performed, revealing lymph-node metastases from a previously unknown papillary thyroid carcinoma. This occasion-related examination was not included in the analysis of screening imaging for the detection of lymphoma.
In case of an occasion-related imaging with suspicious findings and recommendation for tissue collection, the positive predictive value of imaging for the diagnosis of lymphoma was 55% (95% CI 34–74%). Since no cases of lymphoma were found in screening examinations, no corresponding value could be calculated from our data.
In the screening setting, the NNBS could not be determined because no lymphomas were detected. The corresponding ratio for occasion-related imaging was 16.3 examinations per lymphoma detected. In the context of screening, the NNH for imaging studies with invasive exclusion of lymphoma was 189.7. For occasion-related examinations, the respective NNH was 19.9.
Discussion
We describe a large cohort of patients with pSS, who had received regular imaging (mostly US) as a screening tool for lymphoma detection. In asymptomatic patients with low disease activity and negative cryoglobulins, no lymphoma could be detected through imaging. On the contrary, the imaging led to further diagnostics with invasive measures, which were finally unnecessary (accounting for an NNH of 189.7 for biopsies or surgery). All detected lymphomas occurred in pSS patients with lymphadenopathy, swelling of parotid glands, or B symptoms.
The cumulative incidence of lymphoma in our cohort in the respective observation period was relatively high, with 11.2% of patients or one case of lymphoma per 68.8 patient-years. The main reason for the high incidence in our cohort may be the accumulation of patients with severe pSS, who have an increased risk for lymphoma development, at a university centre (Citation5). This high cumulative incidence of lymphoma is consistent with cohort studies from similar institutions (Citation18). In contrast, population-based cohorts of Sjögren patients report far lower prevalences, with one case of lymphoma per 544.7 patient-years (Citation3, Citation19). Therefore, the risk of the individual patient developing lymphoma should not be overestimated based on our data. MALT lymphomas and nodal marginal zone lymphomas were the most common entities, followed by DLBCL, which is also consistent with the described frequency distribution from larger cohorts (Citation5).
Remarkably, in one-third of lymphoma patients in our study, the diagnoses of pSS and lymphoma were made almost simultaneously. Consecutively, patients with lymphoma had a significantly shorter median duration of pSS. In a population-based study, the two diagnoses were made at virtually the same time in 17% of patients. As these patients had reported the onset of their sicca symptoms several years before the lymphoma diagnosis, the authors concluded that undiagnosed Sjögren’s syndrome had been present for some time (Citation20). This assumption is consistent with the prevailing pathophysiological concept that chronic antigen-driven B-cell stimulation is one reason for lymphoma development, and the reported observation that the risk of lymphoma increases with disease duration (Citation21, Citation22). A possible explanation for the discrepancy with our findings could be that the early immunological development of the disease and the onset of symptoms may precede the diagnosis of pSS by years, making it almost impossible to determine the exact duration of the disease (Citation8). In particular, in patients reporting only mild symptoms of mucosal dryness, there can be a long delay in diagnosis.
The cohort of lymphoma patients in our study had significantly increased ESSDAI scores and were more often active in the glandular, lymphadenopathy, and constitutional domains. These findings replicate the risk factors reported in other studies (Citation4, Citation23). The notably higher overall ESSDAI counts in lymphoma patients were mainly attributed to the high ESSDAI scores of B-NHL, which accounts for 12 points in the lymphadenopathy domain (Citation14). Remarkably, the higher ESSDAI counts in lymphoma patients were only caused by the three domains mentioned above. Our study did not confirm other reported risk factors, such as activity in the cutaneous, biological, and haematological domains or positivity for cryoglobulins (Citation24). The study unexpectedly found a higher frequency of MTX use in the medication history of lymphoma patients. In pSS, MTX is typically used in patients with synovitis that is not adequately treated by hydroxychloroquine (Citation2). Thus, one possible explanation for the association in our study is that there may be a link between early joint symptoms and future systemic activity in pSS, which could contribute to the increased risk of lymphoma (Citation25). A study from Japan had suggested an association between the use of MTX and lymphoproliferative diseases (Citation26). However, more recent data from large cohorts of patients with rheumatoid arthritis indicate that there is no such link between the development of lymphoma and previous therapy with MTX (Citation27, Citation28). Data on MTX in pSS and its disease-related adverse events are scarce and, to our knowledge, long-term prospective studies have not been performed (Citation29). It is noteworthy that none of the patients diagnosed with lymphoma had been treated with intensive immunosuppression, namely mycophenolate mofetil, cyclophosphamide, or B-cell-targeting biologicals, prior to their diagnosis. For B-cell-targeting biologicals, which had been administered to 17.6% of the non-lymphoma patients, this difference was almost significant. This prompts the question of whether intensive therapy might have prevented the development of lymphoma. In our study, a confounding factor could have been the shorter disease duration in the lymphoma patients. However, to our knowledge, the effect of the aforementioned medications on the development of lymphoma in pSS has not been investigated to date. Consequently, further research on different immunosuppressive medications in pSS, correcting for possible confounding risk factors of lymphoma, is required.
The finding that, in our cohort, all lymphoma cases manifested with the aforementioned symptoms of lymphoma, and that no lymphoma was detected among the asymptomatic patients, does not exclude the possibility of asymptomatic lymphoma in pSS. However, it raises the question of whether screening for asymptomatic lymphoma should be performed, as asymptomatic lymphomas often have no indication for treatment and a watchful waiting strategy is chosen. Our study supports the hypothesis that screening low-risk pSS groups with imaging of the neck and MSGs will not detect lymphomas requiring treatment.
Our data confirm that screening diagnostics can have negative implications. Specifically, almost one out of five screening studies led to follow-up imaging or invasive tests without benefit for the patient. The consequences of unclear findings in screening diagnostics can range from repeated imaging with contrast agent and radiation exposure to unnecessary biopsies or surgery. This also has negative psychosocial consequences for patients lasting for years, as shown for false-positive findings in mammography screening programmes (Citation30). On the other hand, a possible benefit of screening in pSS could be to educate patients about the possibility of lymph-node swelling or enlargement of MSGs.
In occasion-related imaging, around one out of three examinations led to follow-up imaging or invasive work-up. However, this must be weighed against the considerably higher probability of the presence of lymphoma, as one lymphoma was detected per 16.3 imaging studies. At the same time, US showed no evidence of malignancy in 58.7% of these studies. This finding lends support to the view that in symptomatic patients, US provides valuable information for further management. However, inconclusive results in US studies often prompted further work-up with cross-sectional imaging.
Our study cannot answer the question of whether different recommendations regarding imaging of the neck should be applied to patients with lymphoma symptoms versus patients with high pSS activity (apart from symptoms of lymphoma), as both groups were pooled in one group in our study. Our primary intention in designing this study was to differentiate these groups from a group of patients with uncomplicated pSS to investigate the value of screening in an uncomplicated course of pSS (Citation31). However, all of our patients diagnosed with lymphoma had associated symptoms, such as MSGs or lymph-node swelling or B symptoms, and none of the lymphoma cases became apparent only because of high ESSDAI scores.
As our study focuses on the value of US for the detection of lymphoma in pSS, it must be emphasized that US of the MSGs and neck can also be used for other indications. In patients with suspected pSS, US of the MSGs has been discussed as part of the diagnostic work-up and as part of the classification criteria (Citation32). In addition, US of the MSGs has been used in clinical trials to monitor the response to immunosuppressive therapy (Citation33). Apart from pSS, US is the primary imaging modality for inflammatory or indolent swelling of the MSGs to differentiate between mechanical, infectious, autoimmune processes and benign and malignant tumours (Citation34).
There are some limitations of our study, including the single-centre design, the retrospective character, with the possibility of missing values and selection bias, and the long analysis period, as guidelines and diagnostic technologies have changed over time. Furthermore, the high number of examiners probably had a negative impact on the consistency of the US examinations. Nevertheless, we think that our study reflects the real-world setting in patient care at a university hospital and offers unique data on the advantages and disadvantages of repetitive US in pSS.
Conclusion
In summary, our study showed no benefit of screening for lymphoma by imaging of the neck and MSGs in a low-risk group of Sjögren’s syndrome patients. At the same time, negative consequences of screening occurred in a relevant proportion of patients. Accordingly, in asymptomatic pSS patients, US of the neck and MSGs should only be performed cautiously, with specific questions being asked to avoid overdiagnosis. Hence, our data support the use of US to detect lymphoma in situations where there are clinical signs of lymphoma or increased systemic activity.
Supplemental Material
Download PDF (23.7 KB)Disclosure statement
Sebastian Hüper has received travel grants or speaker’s fess from AbbVie, UCB, and Janssen-Cilag. Lea Nagler has received travel grants from AbbVie, Medac, and UCB. Patrick P Strunz has received speaker’s fees and travel grants from Janssen-Cilag, Galapagos, Eli Lilly, Boehringer/Ingelheim, and AbbVie (less than $10 000 each), as well as research funding from Chugai (25 000 USD). Matthias Froehlich has received travel grants, compensation for advisory boards, or speaker’s fees from AbbVie, Janssen, Novartis, and Eli Lilly. Hannah Labinsky has received travel grants, compensation for advisory boards, or speaker’s fees from Janssen, Pfizer, UCB, Boehringer Ingelheim, and AbbVie. Marc Schmalzing has received speaker’s fees, travel grants, research funding, or compensation for consultancies or board memberships from AbbVie, Actelion, Amgen, AstraZeneca, BMS, Boehringer/Ingelheim, Celgene, Chugai/Roche, Eli Lilly, EUSA Pharma, Galapagos, Genzyme, Gilead, Hexal/Sandoz, Janssen-Cilag, MSD, Mylan, Novartis, Pfizer, Sanofi Pasteur, Takeda (Shire), and UCB (less than $10 000 each). Michael Gernert has received travel grants, compensation for advisory boards, or speaker’s fees from AbbVie, AstraZeneca, Lilly, Hexal, Janssen, Novartis, Pfizer, Takeda, and UCB.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/03009742.2024.2370109
References
- Kassan SS, Thomas TL, Moutsopoulos HM, Hoover R, Kimberley RP, Budmann DR, et al. Increased risk of lymphoma in sicca syndrome. Ann Internal Med 1978;89:888–92.
- Ramos-Casals M, Brito-Zerón P, Bombardieri S, Bootsma H, De Vita S, Dörner T, et al. EULAR recommendations for the management of Sjögren’s syndrome with topical and systemic therapies. Ann Rheum Dis 1978;89:888–92.
- Weng MHY, Huang, Y. T., Liu M, Liu M-F, Lu T-H, et al. Incidence of cancer in a nationwide population cohort of 7852 patients with primary Sjögren’s syndrome in Taiwan. Ann Rheum Dis 2012;71:524–7.
- Nishishinya MB, Pereda CA, Muñoz-Fernández S, Pego-Reigosa JM, Rúa-Figueroa I, Andreu JL, et al. Identification of lymphoma predictors in patients with primary Sjögren’s syndrome: a systematic literature review and meta-analysis. Rheumatol Int 2015;35:17–26.
- Brito-Zerón P, Kostov B, Fraile G, Caravia-Durán D, Maure B, Rascón FJ, et al. Characterization and risk estimate of cancer in patients with primary Sjögren syndrome. J Hematol Oncol 2017;10:90.
- Fragkioudaki S, Mavragani CP, Moutsopoulos HM. Predicting the risk for lymphoma development in Sjogren syndrome: an easy tool for clinical use. Medicine (Baltimore) 2016;95:e3766.
- De Vita S, Isola M, Baldini C, Goules AV, Chatzis LG, Quartuccio L, et al. Predicting lymphoma in Sjögren’s syndrome and the pathogenetic role of parotid microenvironment through precise parotid swelling recording. Rheumatology 2022;62:1586–93.
- Nocturne G, Mariette X. Sjögren syndrome-associated lymphomas: an update on pathogenesis and management. Br J Haematol 2015;168:317–27.
- Price EJ, Rauz S, Tappuni AR, Sutcliffe N, Hackett KL, Barone F, et al. The British Society for Rheumatology guideline for the management of adults with primary Sjögren’s syndrome. Rheumatology (Oxford) 2017;56:1643–7.
- Stefanski AL, Tomiak C, Pleyer U, Dietrich T, Burmester GR, Dörner T. The diagnosis and treatment of Sjögren’s syndrome. (In German) Dtsch Arztebl Int 2017;114:354–61.
- American Board of Internal Medicine Foundation. Choosing Wisely initiative. 2011 [ cited 2024 Mar 14]. Available from: https://www.choosingwisely.org/
- Lorenzon M, Spina E, Tulipano Di Franco F, Giovannini I, De Vita S, Zabotti A. Salivary gland ultrasound in primary Sjögren’s syndrome: current and future perspectives. Open Access Rheumatol 2022;14:147–60.
- Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome. Ann Rheum Dis 2017;76:9.
- Seror R, Bowman SJ, Brito-Zeron P, Theander E, Bootsma H, Tzioufas A, et al. EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI): a user guide. RMD Open 2015;1:e000022.
- Mantsopoulos K, Koch M, Fauck V, Schinz K, Schapher M, Constantinidis J, et al. Primary parotid gland lymphoma: pitfalls in the use of ultrasound imaging by a great pretender. Int J Oral Maxillofac Surg 2021;50:573–8.
- Prativadi R, Dahiya N, Kamaya A, Bhatt S. Chapter 5 Ultrasound characteristics of benign vs malignant cervical lymph nodes. Semin Ultrasound CT MR 2017;38:506–15.
- Bender R. Calculating confidence intervals for the number needed to treat. Control Clin Trials 2001;22:102–10.
- Zufferey P, Meyer OC, Grossin M, Kahn MF. Primary Sjögren’s syndrome (SS) and malignant lymphoma: a retrospective cohort study of 55 patients with SS. Scand J Rheumatol 1995;24:342–5.
- Johnsen SJ, Brun JG, Gøransson LG, Småstuen MC, Johannesen TB, Haldorsen K, et al. Risk of non-Hodgkin’s lymphoma in primary Sjögren’s syndrome: a population-based study. Arthritis Care Res (Hoboken) 2013;65:816–21.
- Vasaitis L, Nordmark G, Theander E, Backlin C, Smedby KE, Askling J, et al. Population-based study of patients with primary Sjögren’s syndrome and lymphoma: lymphoma subtypes, clinical characteristics, and gender differences. Scand J Rheumatol 2020;49:225–32.
- Routsias JG, Goules JD, Charalampakis G, Tzima S, Papageorgiou A, Voulgarelis M. Malignant lymphoma in primary Sjögren’s syndrome: an update on the pathogenesis and treatment. Semin Arthritis Rheum 2013;43:178–86.
- Teixeira Mendes LS, Wotherspoon A. Marginal zone lymphoma: associated autoimmunity and auto-immune disorders. Best Pract Res Cl Ha 2017;30:65–76.
- Sebastian A, Madej M, Sebastian M, Butrym A, Woytala P, Hałoń A, et al. Prevalence and clinical presentation of lymphoproliferative disorder in patients with primary Sjögren’s syndrome. Rheumatol Int 2020;40:399–404.
- Retamozo S, Brito-Zerón P, Ramos-Casals M. Prognostic markers of lymphoma development in primary Sjögren syndrome. Lupus 2019;28:923–36.
- Fauchais A-L, Ouattara B, Gondran G, Lalloué F, Petit D, Ly K, et al. Articular manifestations in primary Sjögren’s syndrome: clinical significance and prognosis of 188 patients. Rheumatology 2010;49:1164–72.
- Hoshida Y, Xu J-X, Fujita S, Nakamichi I, Ikeda J-I, Tomita Y, et al. Lymphoproliferative disorders in rheumatoid arthritis: clinicopathological analysis of 76 cases in relation to methotrexate medication. J Rheumatol 2007;34:322–31.
- Hellgren K, Baecklund E, Backlin C, Sundstrom C, Smedby KE, Askling J. Rheumatoid arthritis and risk of malignant lymphoma: is the risk still increased? Arthritis Rheumatol 2017;69:700–8.
- Schmalzing M, Strangfeld A, Tony HP. Drug therapy for rheumatoid arthritis with a history of malignancy. (In German) Z Rheumatol 2016;75:22–31.
- Skopouli FN, Jagiello P, Tsifetaki N, Moutsopoulos HM. Methotrexate in primary Sjögren’s syndrome. Clin Exp Rheumatol 1996;14:555–8.
- Brodersen J, Siersma VD. Long-term psychosocial consequences of false-positive screening mammography. Ann Fam Med 2013;11:106–15.
- Witte T. Longstanding Sjögren’s syndrome without heightened risk of lymphoma. (In German) Z Rheumatol 2020;79:571–3.
- van Nimwegen JF, Mossel E, Delli K, van Ginkel MS, Stel AJ, Kroese FGM, et al. Incorporation of salivary gland ultrasonography into the American College of Rheumatology/European League Against Rheumatism criteria for primary Sjögren’s syndrome. Arthritis Care Res (Hoboken) 2020;72:583–90.
- Diekhoff T, Fischer T, Schefer Q, Posch MG, Dörner T, Laurent D, et al. Ianalumab (VAY736) in primary Sjögren’s syndrome: assessing disease activity using multi-modal ultrasound. Clin Exp Rheumatol 2020;38:228–36.
- Koch M, Sievert M, Iro H, Mantsopoulos K, Schapher M. Ultrasound in inflammatory and obstructive salivary gland diseases: own experiences and a review of the literature. J Clin Med 2021:10:3547.
